Abstract
Recombinant adeno-associated viruses (AAV) are promising gene therapy vectors. We have recently identified a bovine adeno-associated virus (BAAV) that demonstrates unique tropism and transduction activity compared to primate AAVs. To better understand the entry pathway and cell tropism of BAAV, we have characterized the initial cell surface interactions required for transduction with BAAV vectors. Like a number of AAVs, BAAV requires cell surface sialic acid groups for transduction and virus attachment. However, glycosphingolipids (GSLs), not cell surface proteins, were required for vector entry and transduction. Incorporation of gangliosides, ceramide-based glycolipids containing one or more sialic acid groups, into the cytoplasmic cell membranes of GSL-depleted COS cells partially reconstituted BAAV transduction. The dependency of BAAV on gangliosides for transduction was further confirmed by studies with C6 cells, a rat glioma cell line that is deficient in the synthesis of complex gangliosides. C6 cells were resistant to transduction by BAAV. Addition of gangliosides to C6 cells prior to transduction rendered the cells susceptible to transduction by BAAV. Therefore, gangliosides are a likely receptor for BAAV.
Cell surface carbohydrates play an important role in virus entry and infection. Due to the broad array of structural motifs possible with carbohydrates compared to proteins, many viruses and pathogens utilize carbohydrates as cell attachment receptors. For example, JC virus and polyomavirus bind sialic acid but have different specificities (4, 17). Furthermore, studies of cell entry by polyomavirus and simian virus 40 (SV40) suggest that sialic acid, linked to specific gangliosides, functions as the receptor for these viruses and mediates virus binding and transport from the plasma membrane to the endoplasmic reticulum (10, 11, 26).
Heparan sulfate proteoglycans (HSPGs) serve as initial receptors for the binding of both herpes simplex virus (HSV) type 1 and HSV type 2 to cell surfaces. However, each virus recognizes different structural features of the HS and, as a result, has a different epidemiology and cell tropism (13). HSPG also serves as a receptor for dengue virus. In this case, the degree of sulfonation of HS in the liver is thought to be a determinant of viral tropism for the liver (5, 14).
Adeno-associated viruses are members of the genus Dependovirus, a small group of viruses that were classified based on similar size and structure and dependence upon a helper virus for replication. This genus appears to utilize a diverse array of cell surface carbohydrates for attachment and infection. Like HSV and dengue virus, adeno-associated virus type 2 (AAV2) has been shown to bind HSPGs on the cell surface (25). Competition experiments have demonstrated that soluble heparin can block virus binding and transduction. Furthermore, differentiated airway lung epithelial cells, which express very little HSPG on their apical surfaces, are poorly transduced.
Not all AAVs interact with HSPGs. For example, AAV4 and AAV5 both use different forms of sialic acid for cell attachment. While both AAV4 and AAV5 required the α2-3 form of sialic acid for cell attachment and transduction (15, 27), treatment of cells with specific glycosylation inhibitors and resialation experiments with neuraminidase-treated erythrocytes demonstrated that AAV4 preferentially attached to an α2-3 sialic acid present on the O-linked carbohydrate core, while AAV5 attached to the N-linked type.
AAVs have also been reported in other mammalian species, including canines, bovines, ovines, and equines (2). Previously, we reported the cloning of bovine adeno-associated virus (BAAV), which is serologically distinct from primate AAVs (23). The bovine AAV genome is similar in size and organization to those of other AAV isolates. The Rep open reading frame and inverted terminal repeats are very similar to those of AAV5, and BAAV is the only reported isolate that will cross-complement the replication and packaging of AAV5. The capsid open reading frame is most similar to that of AAV4, but molecular modeling and tropism comparison have demonstrated that BAAV has a distinct binding and entry activity compared to AAV4 or any other reported isolate. Furthermore, BAAV was not neutralized by antisera raised against other AAVs. Overall, these data suggest that vectors based on BAAV could be useful for gene transfer applications. We have recently demonstrated that BAAV can efficiently deliver genes to hair cells in the inner ear, an important target cell for the treatment of deafness and balance disorders (9).
To better understand the entry pathway and cell tropism of BAAV, we have characterized the initial cell surface interactions that are required for transduction with BAAV vectors. Our results show that, like other AAVs, BAAV requires cell surface sialic acid for transduction. However, in contrast to other AAVs, terminal sialic acid groups are not required for cell attachment and BAAV does not utilize proteins for binding and entry. Instead, like SV40 and polyomavirus (26), BAAV requires gangliosides for internalization and infection.
MATERIALS AND METHODS
Cell culture and virus propagation.
COS (simian kidney) and C6 (rat glioma) cells, obtained from the American Type Culture Collection (Manassas, VA), were cultured in RPMI 1640 medium (Biosource, Camarillo, CA) supplemented with 5% fetal bovine serum (HyClone, Logan, UT), 2 mM l-glutamine, 100 U of penicillin/ml, and 0.1 mg of streptomycin/ml (Invitrogen, Carlsbad, CA). A CHO cell line (Pro5) and a Pro5 mutant (Lec2) were obtained from the American Type Culture Collection. The cells were cultured in minimal essential medium (Biosource) supplemented with 10% fetal calf serum, 100 mg of penicillin/ml, and 100 U of streptomycin/ml. The cells were maintained at 37°C under a humidified 5% CO2 atmosphere. Recombinant viruses based on AAV5 and BAAV expressing a nuclear-localized green fluorescent protein (GFP), rAAV5-GFP and rBAAV-GFP, were produced as described previously (23). The cells were transduced with recombinant AAV in medium supplemented with 1% horse serum (HyClone).
Enzymatic removal of cell surface sialic acid.
COS cells were seeded at a density of 5,000 cells/well in a 96-well plate. Twenty-four hours after being seeded, the cells were incubated for 30 min with broad-spectrum neuraminidases from Arthrobacter ureafaciens or Vibrio cholerae or a 2-3 linkage-specific neuraminidase from Staphylococcus pneumoniae (Calbiochem, La Jolla, CA, and Glyko, San Leandro, CA). The cells were then washed with medium, and 1 × 108 particles each of rAAV5-GFP and rBAAV-GFP were added for 1 h to the cells. Twenty-four hours after transduction, the cells were analyzed for GFP expression by flow cytometry with the Guava PCA-96 (Guava Technologies, Hayward, CA).
Lectin competition assay.
COS cells were seeded at a density of 10,000 cells/well in a 96-well plate. After 16 h, the cells were preincubated for 15 min at 4°C with 1 μg/ml and 10 μg/ml wheat germ agglutinin (WGA) or 200 μg/ml Griffonia simplicifolia Lectin I (Vectorlabs, Burlingame, CA). Subsequently, the cells were washed and transduced for 60 min at 4°C with 1 × 108 particles of rAAV5-GFP and rBAAV-GFP in medium supplemented with lectins at the above concentrations. The cells were assayed for virus binding immediately after transduction and at 24 hours after transduction for GFP expression by flow cytometry.
Binding assay.
Cells were chilled for 30 min at 4°C in medium and then incubated for 60 min at 4°C with 1 × 108 recombinant AAV5-GFP and BAAV-GFP particles. The cells were then washed twice with cold medium and once with phosphate-buffered saline (PBS) and lysed in 50 μl PCRnGo buffer (Pierce, Rockford, IL). To measure cell-associated virus genomes, 0.1 μl of cell lysates was assayed by quantitative real-time PCR using the TaqMan system (Applied Biosystems, Foster City, CA) with probes specific to the cytomegalovirus promoter, which is part of the GFP expression cassette.
Protease treatment.
COS cells were cultured in a 15-cm-diameter culture dish until the cells were 80% confluent. The cells were then washed twice with PBS, scraped, resuspended in 10 ml PBS, and divided into four aliquots (approximately 2 × 106 cells). The aliquots were treated with 0.05% trypsin (Biosource) or 2 U/ml dispase (Roche, Basel, Switzerland) or mock treated (untreated control) for 15 min at 37°C. The cells were then washed twice with medium and seeded at a density of 10,000 cells/well in a 96-well dish. After 1 h of culture at 37°C, the cells were transduced at a multiplicity of infection of 2 × 104 for 1 h with rAAV5-GFP and rBAAV-GFP. Virus binding was assayed immediately after transduction. Transduction efficiency was determined 24 h later by GFP expression detection with a fluorescent-cell counter (Guava Technologies).
Inhibition of glycolipid synthesis.
COS cells were plated at a density of 5 × 103/well in a flat-bottom 96-well plate. Eight hours after being seeded, the cells were incubated for 40 h with the glucosylceramide synthase inhibitors dl-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP) or (±)-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol hydrochloride (PDMP) (Sigma, St. Louis, MO). The cells were then washed with medium and transduced with 2 × 108 particles each of rAAV5-GFP and rBAAV-GFP for 1 h. GFP expression was analyzed 48 h after transduction by detection with a fluorescent-cell counter (Guava Technologies).
DNA uptake measurement.
COS cells were plated at a density of 5 × 103 cells/well in a flat-bottom 96-well plate and incubated for 40 h with 5 μM PPMP or mock treatment. The cells were then transduced with 2 ×108 particles of rAAV5-GFP and rBAAV-GFP for 1 h in medium, washed with medium, and cultured for 3 h. The culture medium was then removed, and the cells were washed once with medium and PBS. Cell-associated but noninternalized virus was removed by treating the cells for 30 min with 50 mU/ml V. cholerae neuraminidase, followed by a 15-min incubation with 0.05% trypsin (Biosource) and 0.2 mg/ml proteinase K (Roche), and then washed with PBS. The cells were then lysed in 50 μl PCRnGo (Pierce) buffer, and the DNA copy numbers of cell-internalized vector genomes were determined by quantitative real-time PCR as described above.
Ganglioside treatment.
C6 cells were plated at a density of 5,000 cells/well in a 96-well plate and cultured for 12 h in medium supplemented with 25 μg/ml bovine brain gangliosides (Sigma) or 25 μg/ml neutral glycosphingolipids (GSLs) (Alexis, Lausen, Switzerland) or mock treated (19). Gangliosides were incorporated into the cytoplasmic membranes of PPMP-treated COS cells by liposome transfection. Liposomes were generated by dissolving 16 mg phosphatidylcholine and 8 mg cholesterol (Sigma) with or without the addition of 25 μg gangliosides in 2 ml ethanol. The mixture was evaporated at room temperature under an N2 stream, resuspended in 1 ml PBS, and sonicated at room temperature for 30 min in a G112SP1 water bath sonicator (Laboratory Supplies, Hicksville, NY). Five microliters of liposomes was added to PPMP-treated or untreated cells and incubated for 4 h. After removal of the ganglioside and liposome reagents from the cells and a wash step with medium, the C6 and COS cells were then transduced with 2 ×108 each rAAV5-GFP and rBAAV-GFP particles for 1 h, cultured for 24 h, and then analyzed for GFP expression by flow cytometry.
RESULTS
Cell surface sialic acid is required for rBAAV transduction.
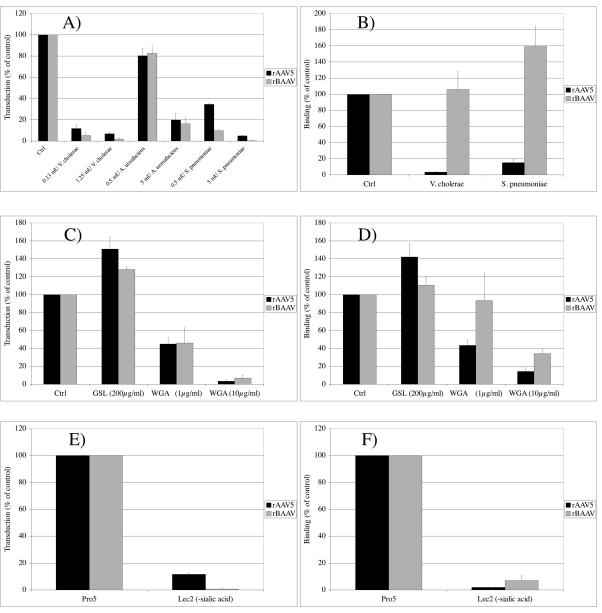
BAAV shows the highest homology to two primate AAVs, AAV4 and AAV5. Both of these AAV serotypes require sialic acid as a coreceptor for cell binding and transduction (15, 27). The importance of sialic acid in BAAV transduction was analyzed by enzymatic removal of terminal surface sialic acid groups from glycoproteins and glycolipids with neuraminidases (Fig. 1A). Treating COS cells with different exoneuraminidases resulted in a dose-dependent inhibition of rBAAV transduction. The broad-spectrum exoneuraminidases from A. ureafaciens and V. cholerae, as well as α2-3-specific neuraminidase from S. pneumoniae, all inhibited rBAAV-mediated gene transfer up to 84%, 98%, and 99%, respectively, compared to the untreated control. As previously reported, rAAV5 was also inhibited (15, 27). This result suggests that BAAV depends on cell-associated α2-3-linked sialic acid for transduction. The dependency of rBAAV on sialic acid for transduction was confirmed with lectin competition assays. Lectins are proteins that recognize and bind oligosaccharides conjugated to proteins and lipids and can be used to block virus binding (27). WGA, a lectin that recognizes a number of carbohydrate structures, including sialic acid, inhibited transduction of rAAV5 and rBAAV dose dependently, which is in agreement with our previous results (Fig. 1C). GSL, a lectin specific for α-N-acetylgalactosamine, was used as a negative control and demonstrated no inhibitory potential. Furthermore, transduction of a sialic acid-deficient CHO cell line (Lec2) was reduced for rAAV5 and rBAAV by 89% and 99%, respectively, compared to the parental CHO cell line (Pro5) (Fig. 1E). These results demonstrated that BAAV transduction is sialic acid dependent.
FIG. 1.
BAAV transduction and cell attachment are sialic acid dependent. The effects of enzymatic removal of terminal cell surface sialic acid groups from COS cells on transduction with rAAV5-GFP and rBAAV-GFP (A) and binding (B) were studied by pretreatment of the cells with neuraminidases from A. ureafaciens, V. cholerae, or S. pneumoniae. The dependence of rBAAV transduction and cell attachment on terminal and nonterminal sialic acid groups was studied by lectin competition assays (C and D) with the sialic acid-specific lectin WGA and by comparing transduction (E) and binding (F) of recombinant virus to the sialic acid-deficient CHO mutant Lec2 or the parental Pro5 cells. Virus binding was determined by quantitative PCR after 1 h of transduction at 4°C. Transduction efficiency was analyzed 24 hours after transduction by flow cytometry. The values are means from three experiments; the error bars represent standard deviations.
Sialic acid is a cell attachment factor for BAAV.
To investigate the role of α2-3-linked sialic acid in the transduction pathway of BAAV, we analyzed whether rBAAV, like AAV4 and AAV5, required α2-3-linked sialic acid for cell binding. While the removal of terminal cell surface sialic acids with exoneuraminidases from S. pneumoniae or V. cholerae, which are either specific or show strong preference for the hydrolysis of α2-3-linked sialic acid, resulted in 85% and 96% reduction in cell binding of rAAV5, no inhibition of the rBAAV cell interaction was observed (Fig. 1B). This demonstrated that terminal 2-3-linked sialic acid groups mediated the binding of rAAV5, but not rBAAV. To study the role of nonterminal sialic acid groups in BAAV cell attachment, we analyzed virus binding to a sialic acid-deficient CHO cell mutant (Lec2) and performed lectin competition assays. Competition with the sialic acid-specific lectin inhibited rAAV5 and rBAAV binding dose dependently (Fig. 1D). Cell attachment in the presence of 10 μg/ml WGA was reduced for rAAV5 and rBAAV by 86% and 66%, respectively. Binding to the sialic acid-deficient CHO mutant, Lec2, was reduced by 98% and 93% compared to the parental cell line, CHO Pro5 (Fig. 1F). These results suggest that nonterminal sialic acid residues are essential for BAAV cell binding.
The BAAV receptor is protease resistant.
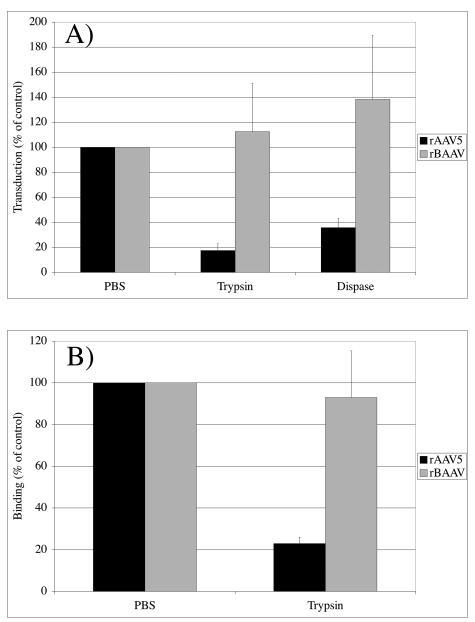
Sialic acid is a common terminal structure on both glycoproteins and GSLs. To analyze whether proteins are part of the BAAV cell entry pathway, we analyzed the effect of proteolytic digestion of the cell membrane on rBAAV transduction and binding. When COS cells were subjected to protease treatment with either trypsin or dispase prior to transduction, we observed no change in BAAV transduction activity (Fig. 2A). In contrast, with rAAV5, which uses the platelet-derived growth factor receptor for binding and entry (8), transduction activity was inhibited by 81% and 62% upon pretreatment with trypsin or dispase, respectively, compared to a PBS-treated control. Trypsin pretreatment also resulted in a 77% reduction of rAAV5 binding, whereas rBAAV cell attachment was not significantly reduced (Fig. 2B). This finding indicates that, unlike AAV5, cell surface proteins either do not appear to be required for BAAV binding and transduction or are protease resistant.
FIG. 2.
BAAV transduction and cell binding are protease resistant. COS cells were treated with trypsin or dispase prior to transduction with rAAV5-GFP and rBAAV-GFP. Transduction (A) and binding (B) with these cells were compared to those with untreated cultures. The values are means from three experiments; the error bars represent standard deviations.
Glycolipid inhibitors block rBAAV transduction.
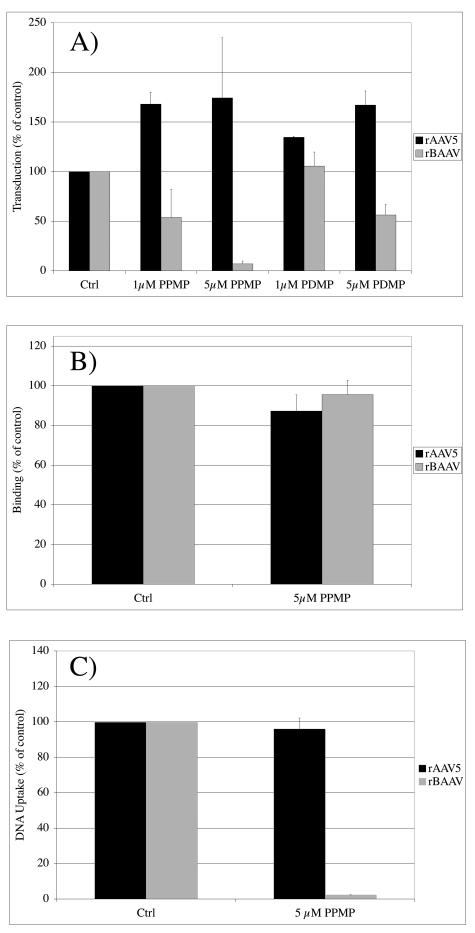
In addition to glycoproteins, glycolipids are reported to act as receptors for virus entry. PPMP and PDMP are glucosylceramide synthase inhibitors, which act to deplete GSLs from the cell membrane (16). COS cells were treated for 40 h with 1 μM or 5 μM of either PPMP or PDMP prior to transduction with rBAAV or rAAV5. While both GSL inhibitors did not inhibit rAAV5-mediated gene transfer, rBAAV transduction was blocked in a dose-dependent manner (Fig. 3A). Treatment of cells with 5 μM PPMP or PDMP reduced rBAAV transduction by 93% and 46%, respectively. This result suggests that unlike AAV5, BAAV transduction requires glycolipids.
FIG. 3.
Glycosphingolipid synthesis inhibitors block BAAV transduction and cell entry, but not cell attachment. (A) Transduction. COS cells were pretreated with PPMP or PDMP before transduction with rAAV5-GFP and rBAAV-GFP, and transduced cells were counted 24 h after vector addition by flow cytometry. (B) Binding. COS cells were pretreated with 5 μM PPMP, chilled, and incubated with rAAV5-GFP and rBAAV-GFP at 4°C. After the cells were washed, bound virus was measured by quantitative PCR (QPCR). (C) Cell entry. COS cells were pretreated with 5 μM PPMP prior to transduction with rAAV5-GFP and rBAAV-GFP at 37°C. Four hours after transduction, cell-associated virus was removed by a combination of wash steps, neuraminidase treatment, and proteolytic digestion of intact cells, and internalized vector was quantitated by QPCR. The values are means from three experiments; the error bars represent standard deviations.
Glycosphingolipids, while not required for BAAV cell binding, are required for internalization.
To analyze whether GSLs act as attachment factors and facilitate binding of BAAV to the cell or are essential for a later step in transduction, we compared the binding of BAAV in cells treated with PPMP versus control cells. Treatment of cells with 5 μM PPMP was sufficient to block over 90% of rBAAV transduction (Fig. 3A) but had no effect on rBAAV cell binding (Fig. 3B). In contrast, PPMP-induced GSL depletion had no inhibitory effect on rAAV5 transduction or binding. These results suggest that while BAAV does require GSLs for transduction, they are not required for cell binding.
Since GSLs appear not to be essential for BAAV cell binding, we hypothesized that the PPMP-induced inhibition of BAAV transduction is due to an inhibition of virus internalization. To test this hypothesis, we compared rAAV5 or rBAAV DNA uptake 4 h after transduction of cells that were either untreated or treated for 24 h with 5 μM PPMP. Internalization of rAAV5 was unaffected by PPMP, while rBAAV uptake was reduced by 98% (Fig. 3C). The PPMP-induced reduction in rBAAV uptake corresponded to the observed reduction in transduction, suggesting that PPMP acts on the level of BAAV cell entry. Therefore, the role of GSLs in BAAV transduction appears to be at the level of internalization.
Gangliosides restore BAAV gene transfer in PPMP-treated cells.
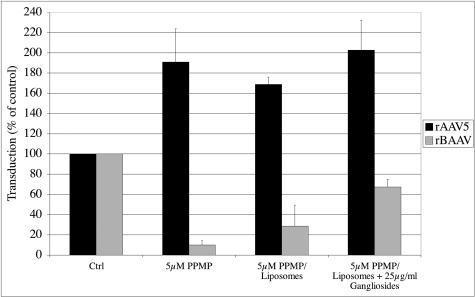
Our results indicate that BAAV requires both sialic acid and GSLs for vector internalization and transduction. Both of these requirements would be fulfilled in gangliosides, which are ceramide-based glycolipids that contain one or more sialic acid groups. We therefore investigated whether this subgroup of GSLs is involved in BAAV transduction. We analyzed whether incorporation of gangliosides into PPMP-treated COS cells could restore the BAAV permissive phenotype inhibited by PPMP treatment. For these experiments, PPMP-treated COS cells were transfected with ganglioside-containing liposomes prior to transduction with rBAAV or rAAV5 vectors. While liposome transfection alone did increase BAAV transduction of PPMP-treated cells, ganglioside incorporation into the cell membrane restored 67% of the BAAV transduction activity to PPMP-treated cells compared with control cells (Fig. 4). Transfection with either liposomes or ganglioside-containing liposomes did not inhibit rAAV5 transduction (Fig. 4). These results suggest that the PPMP-induced inhibition of BAAV is likely due to the depletion of gangliosides from the cell.
FIG. 4.
Ganglioside incorporation corrects the PPMP-induced inhibition of BAAV transduction. COS cells were pretreated with 5 μM PPMP before ganglioside-loaded liposomes were added to the cells. The cells were cultured in the presence of PPMP and liposomes before they were transduced with rBAAV-GFP or rAAV5-GFP. Twenty-four hours after transduction, the cells were analyzed for GFP expression by flow cytometry. The values are means from three experiments; the error bars represent standard deviations.
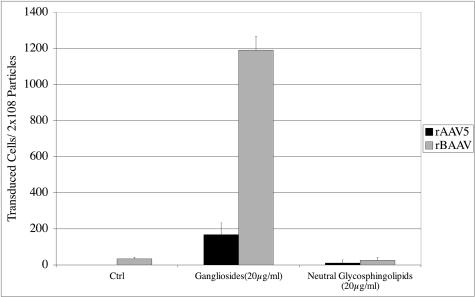
Gangliosides enhance transduction of C6 cells.
To further investigate the function of gangliosides during BAAV transduction, we studied rBAAV transduction in C6 cells, a rat glioma cell line that is deficient in the biosynthesis of complex gangliosides (24). Gene transfer by rBAAV, as well as AAV5, was very poor in these cells. Gangliosides can be incorporated in the cell membranes of C6 cells by culturing the cells in the presence of ganglioside micelles (10, 11). When C6 cells were incubated with ganglioside micelles and then transduced with either BAAV or AAV5, a significant increase in BAAV transduction was observed (Fig. 5). To control for nonspecific effects of this lipid transfection, C6 cells were also incubated with neutral GSL micelles. Addition of the neutral GSLs to the C6 cells had no effect on rBAAV transduction. In contrast to rBAAV, rAAV5 transduction activity increased only slightly with either micelle preparation (Fig. 5). Thus, incorporation of gangliosides into the C6 plasma membrane significantly increased rBAAV gene transfer in these cells. This finding further confirms that gangliosides are essential for BAAV transduction and suggests that, as with SV40 and polyomavirus (26), gangliosides function as receptors for BAAV transduction.
FIG. 5.
Gangliosides addition renders nonpermissive C6 cells permissive for BAAV transduction. C6 cells were cultured in the presence of 20 μg/ml ganglioside micelles isolated from the bovine brain or neutral glycosphingolipids or mock treated prior to transduction with rAAV5-GFP and rBAAV-GFP. Twenty-four hours after transduction, the cells were analyzed for GFP expression by flow cytometry. The values are means from three experiments; the error bars represent standard deviations.
DISCUSSION
Sialic acids, monosaccharide derivatives of N-acetyl-neuramic acid, are frequently found in oligosaccharide chains of glycoproteins and glycolipids. Previously, we documented that terminal cell surface 2-3-linked sialic acid is important for AAV4, AAV5, AAV6, AAV(VR-195), and AAV(VR-355) binding and transduction (15, 22, 27). In contrast, BAAV cell attachment is unaffected by the enzymatic removal of terminal 2-3-linked sialic acid, while transduction is blocked. The observed inhibition of rBAAV cell attachment in the presence of the sialic acid-specific lectin WGA and the reduced binding of rBAAV to the sialic acid-deficient CHO cell mutant Lec2 compared to the parental Pro5 cells suggest that nonterminal sialic acid groups mediate BAAV cell binding while terminal sialic acid groups are essential for transduction.
Infection by several viruses and toxins is proposed to occur by a two-step process. The first step is cell binding via a high-abundance, low-affinity receptor, followed by cell entry via a low-abundance high-affinity receptor (7, 18). Likewise, BAAV cell attachment and entry appear to be two distinct processes. Enzymatic removal of terminal cell surface sialic acid groups and depletion of GSLs resulted in a loss of transduction, while cell binding was unaffected. This suggests that BAAV transduction involves a two-step cell entry pathway in which cell attachment is required but not sufficient for cell entry.
Since sialic acid can be linked to proteins and lipids, we studied the effect of protease digestion of the cell surface, as well as inhibitors that mediated depletion of glycolipids. The AAV5 receptor has been identified as the integral membrane protein platelet-derived growth factor receptor (8). As expected, protease digestion of cells blocked gene transfer of AAV5, whereas BAAV-mediated gene transfer was unaffected by pretreating the cells with either trypsin or dispase. This indicates that the BAAV receptor is not a protein or that it is more protease resistant than the AAV5 receptor. Depletion of GSLs with PPMP and PDMP resulted in a dose-dependent inhibition of BAAV cell entry and transduction. The inhibition of BAAV uptake correlated with the reduction of transduction. Therefore, the observed reduction in transduction in GSL-depleted cells appears to be caused by an inhibition of cell entry rather than affecting a later step, such as intracellular trafficking or conversion of the single-stranded DNA genome to double-stranded DNA. PPMP and PDMP both deplete GSLs via inhibition of the UDP-glucose ceramide glucosyltransferase and block the synthesis of glycosphingolipids, which are derived from glucosylceramide (20). PPMP has a longer fatty acid chain than PDMP, is more effectively taken up by eukaryotic cells, and results in stronger GSL depletion (1), which explains the stronger inhibition of BAAV transduction by PPMP than by PDMP. These results indicate that GSLs are directly involved in BAAV cell entry, potentially as cell entry receptors or as part of a virus uptake complex.
Globoside, a neutral GSL, functions as a receptor for the human autonomous parvovirus B19 (3, 6). While globoside was essential for B19 infection, it was not sufficient (3, 28). Globoside, therefore, may be part of a more complex B19 receptor structure. Likewise, an additional interaction is important for BAAV cell attachment and may indicate the requirement for a more complex cell surface structure for virus binding and entry.
Gangliosides are reported to function as receptors for the murine polyomavirus, SV40, and rotavirus, as well as several toxins, including cholera and Shiga toxins (21, 26). Interestingly, our findings parallel recent studies with polyomavirus and rotavirus. Gangliosides enhanced infection of C6 cells by polyomavirus, while cell binding was unchanged (10). Furthermore, infection of rhesus monkey epithelial cell line MA104 cells with rotaviruses requiring sialic acid and gangliosides for infection was inhibited by PDMP, whereas cell attachment was not affected (12).
Incorporation of gangliosides that were isolated from the bovine brain in the cytoplasmic cell membrane of GSL-depleted COS cells partially reconstituted BAAV transduction but did not affect a control AAV isolate. While the addition of liposomes alone had some effect on BAAV transduction, it was minor compared to the ganglioside-loaded liposomes. The effect of liposomes alone could be at the level of membrane integrity and could increase viral uptake via a nonspecific route.
The dependency of BAAV on gangliosides for transduction was further confirmed by studies with C6 cells, a rat glioma cell line that is deficient in the synthesis of complex gangliosides. C6 cells were resistant to transduction by BAAV and AAV5. Addition of gangliosides to C6 cells prior to transduction (10, 19) renders the cells susceptible to transduction by BAAV, while a mixture of neutral glycosphingolipids had no effect. AAV5 transduction was only slightly increased by the addition of either neutral GSLs or ganglioside micelles. As noted earlier, the increase in AAV5 transduction could be the result of a change in the membrane integrity that results in an increase in nonspecific uptake. Alternatively, it is possible that AAV5 can utilize the sialic acid on gangliosides as a secondary pathway for either binding or uptake. In contrast to BAAV, AAV5 does not depend on gangliosides for transduction. Since the addition of gangliosides alone was sufficient to restore BAAV susceptibility in GSL-depleted cells, it is very likely that they serve as receptors for this AAV isolate.
Acknowledgments
We thank the NCI Fellows Editorial Board for editorial assistance. We thank Roberta Knox and Karen Knight for administrative assistance and the reviewers for their helpful suggestions.
This research was supported by the Intramural Research Program of the NIH and NIDCR.
REFERENCES
- 1.Abe, A., J. Inokuchi, M. Jimbo, H. Shimeno, A. Nagamatsu, J. A. Shayman, G. S. Shukla, and N. S. Radin. 1992. Improved inhibitors of glucosylceramide synthase. J. Biochem. 111:191-196. [DOI] [PubMed] [Google Scholar]
- 2.Berns, K. I., M. Bergoin, M. Bloom, M. Lederman, N. Muzyczka, G. Siegl, J. Tal, and P. Tattersall. 1994. Parvoviridae, p. 166. In F. A. Murphy, C. M. Faquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Martelli, M. A. Mayo, and M. D. Summers (ed.), Virus taxonomy. Fifth report of the International Committee on Taxonomy of Viruses. Springer-Verlag, Vienna, Austria.
- 3.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 4.Cahan, L. D., and J. C. Paulson. 1980. Polyoma virus adsorbs to specific sialyloligosaccharide receptors on erythrocytes. Virology 103:505-509. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 6.Cooling, L. L., T. A. Koerner, and S. J. Naides. 1995. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect. Dis. 172:1198-1205. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Pasquale, G., B. L. Davidson, C. S. Stein, I. Martins, D. Scudiero, A. Monks, and J. A. Chiorini. 2003. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9:1306-1312. [DOI] [PubMed] [Google Scholar]
- 9.Di Pasquale, G., A. Rzadzinska, M. E. Schneider, I. Bossis, J. A. Chiorini, and B. Kachar. 2005. A novel bovine virus efficiently transduces inner ear neuroepithelial cells. Mol. Ther. 11:849-855. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert, J., and T. Benjamin. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, J., J. Dahl, C. Riney, J. You, C. Cui, R. Holmes, W. Lencer, and T. Benjamin. 2005. Ganglioside GD1a restores infectibility to mouse cells lacking functional receptors for polyomavirus. J. Virol. 79:615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herold, B. C., S. I. Gerber, B. J. Belval, A. M. Siston, and N. Shulman. 1996. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J. Virol. 70:3461-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgard, P., and R. Stockert. 2000. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 32:1069-1077. [DOI] [PubMed] [Google Scholar]
- 15.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs, P., M. Pinter, and G. Csaba. 2000. Effect of glucosphingolipid synthesis inhibitor (PPMP and PDMP) treatment on Tetrahymena pyriformis: data on the evolution of the signaling system. Cell Biochem. Funct. 18:269-280. [DOI] [PubMed] [Google Scholar]
- 17.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 72:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez, S., and C. F. Arias. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271-278. [DOI] [PubMed] [Google Scholar]
- 19.Moss, J., P. H. Fishman, V. C. Manganiello, M. Vaughan, and R. O. Brady. 1976. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc. Natl. Acad. Sci. USA 73:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radin, N. S., J. A. Shayman, and J. Inokuchi. 1993. Metabolic effects of inhibiting glucosylceramide synthesis with PDMP and other substances. Adv. Lipid Res. 26:183-213. [PubMed] [Google Scholar]
- 21.Rolsma, M. D., T. B. Kuhlenschmidt, H. B. Gelberg, and M. S. Kuhlenschmidt. 1998. Structure and function of a ganglioside receptor for porcine rotavirus. J. Virol. 72:9079-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt, M., E. Grot, P. Cervenka, S. Wainer, C. Buck, and J. A. Chiorini. 2006. Identification and characterization of novel adeno-associated virus isolates in ATCC virus stocks. J. Virol. 80:5082-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 78:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sottocornola, E., I. Colombo, V. Vergani, G. Taraboletti, and B. Berra. 1998. Increased tumorigenicity and invasiveness of C6 rat glioma cells transfected with the human alpha-2,8 sialyltransferase cDNA. Invasion Metastasis 18:142-154. [DOI] [PubMed] [Google Scholar]
- 25.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 28.Weigel-Kelley, K. A., M. C. Yoder, and A. Srivastava. 2001. Recombinant human parvovirus B19 vectors: erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. J. Virol. 75:4110-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]