Abstract
We present the first detailed expression profiles of nonprimate-derived adeno-associated viruses, namely, bovine adeno-associated virus (B-AAV) and avian adeno-associated virus (A-AAV), which were obtained after the infection of cell lines derived from their natural hosts. In general, the profiles of B-AAV and A-AAV were quite similar to that of AAV5; however, both exhibited features found for AAV2 as well. Like adeno-associated virus type 5 (AAV5), B-AAV and A-AAV utilized an internal polyadenylation site [(pA)p]; however, it was used to greater relative levels by B-AAV than by A-AAV. Similar to AAV5, >99% of B-AAV RNAs generated from upstream promoters were polyadenylated at (pA)p and hence not spliced. In contrast, ca. 50% of the A-AAV RNAs generated from upstream promoters read through (pA)p, as seen for AAV2. However, A-AAV generated lower levels of spliced P5 and P19 products than does AAV2, suggesting that A-AAV generates lower relative levels of Rep 68 and Rep 40. An additional difference in the expression profile of these viruses was that B-AAV generated a greater level of ITR-initiated RNAs than did A-AAV or AAV5. In addition, we demonstrate that, like AAV2, transactivation of transcription of the capsid-gene promoter of B-AAV required both adenovirus and targeting of its Rep protein to the transcription template; however, expression of the capsid-gene promoter of A-AAV was, like AAV5, largely independent of both adenovirus and its Rep proteins.
Adeno-associated viruses (AAVs) are members of the genus Dependovirus in the family Parvoviridae (29). They contain a single-strand linear DNA genome of ∼4.7 kb flanked by identical inverted terminal repeats (ITRs) at both ends. The ITRs contain cis-acting elements required for the replication, integration, packaging, and, in some cases, the transcriptional activation of the viral genome (16, 22, 28, 44, 51). AAVs are small, nonpathogenic nonenveloped icosahedral DNA viruses that are dependent on a larger DNA helper virus, such as adenovirus and herpesvirus, for efficient replication (reviewed in references 4, 29, and 48).
Many different AAV genomes have been identified in human and nonhuman primate tissue (2, 3, 18, 21, 25, 43). Of the original isolates, AAV1, -2, -3, -4, and -6 were isolated from adenovirus stocks (2, 21, 25, 43), whereas AAV5 was isolated from human tissue (3). The genomes of AAV7, -8, and -9 were later identified after amplification from monkey tissue (17, 18); however, isolation of these viruses has not yet been reported. Recently, additional AAVs have been identified from human patients, and a number of these have been isolated and cloned (6, 46). AAVs have also been isolated from animal adenovirus stocks; these include avian AAV (A-AAV) (12, 53), bovine AAV (B-AAV) (11, 23, 30), snake AAV (15), ovine AAV (10), and caprine AAV (1).
Of the AAVs, the genetic maps of only AAV2 and AAV5 have been characterized in detail (29, 39, 49, 50). These viruses each have a large Rep protein-encoding an open reading frame (ORF) in the left-hand end of the genome and a large Cap protein-encoding ORF in the right-hand end, which are separated by a small intron. Both utilize three similarly placed promoters to express their genomes; however, their transcription profiles are quite different. All AAV2 transcripts extend to the polyadenylation site in the right-hand end of the genome. Unspliced AAV2 transcripts from the P5 and P19 promoter encode Rep 78 and Rep 52, respectively; however, a significant portion of these RNAs are spliced and encode Rep 68 and Rep 40. In contrast, for AAV5, the majority of transcripts generated from the P7 and P19 promoter are polyadenylated at an internal polyadenylation site, termed (pA)p, within the central intron (38, 39), which precludes further splicing, and thus for AAV5 only Rep 78 and Rep 52 are produced (39). The significance of this difference in the viral life cycles is not yet clear. U1 snRNP binding to the intron donor of the AAV5 P41 capsid gene transcripts inhibits polyadenylation at (pA)p, which allows read-through of these RNAs into the capsid ORF (41). Thus, AAV5 P41, like AAV2 P40, generates two alternatively spliced mRNAs that encode the viral capsid proteins. In both cases, the major spliced RNAs encode VP2 and VP3, and the minor spliced RNAs encode VP1 (39, 49). The maps of AAV1, -3, -4, and -6 have been suggested to be similar to that of AAV2 (42).
A-AAV and B-AAV have been fully sequenced and have been developed as useful recombinant viral vectors (5, 14, 45). The genome of A-AAV has been reported to be 4,694 nucleotides (nt) in length with inverted repeats of 142 nt. The ORF encoding the Rep proteins of A-AAV displays only ∼50% identity with that of other AAVs. B-AAV has been reported to have a genome at a size of 4,693 nt, which includes ITRs of 173 nt. The Rep protein ORF and the ITRs of B-AAV have high homology to the Rep ORF and ITRs of AAV5 (89 and 96%, respectively) (7, 45). The capsid ORF of B-AAV is most homologous to the capsid ORF of the AAV4 (76%). The A-AAV genome (both rep and cap) is equally divergent from the human AAVs and goose parvovirus (GPV), another avian parvovirus of the Dependovirus genome, which shows a transcription profile that exhibits features of both the Dependovirus and the Parvovirus genera (37).
We present here for the first time the detailed expression profile of B-AAV and A-AAV, which we obtained after the infection of cells derived from the natural hosts of these viruses. The expression profiles of B-AAV and A-AAV, the first presented for any nonprimate-derived AAV, are quite similar to that of AAV5; however, the A-AAV map exhibited some significant differences. All three viruses utilized an internal polyadenylation site; however, it was used to greater levels by AAV5 and B-AAV than by A-AAV. For AAV5 and B-AAV, >99% of RNAs generated from upstream promoters are polyadenylated at (pA)p and hence not spliced. In contrast, for A-AAV, ca. 50% of RNAs generated from upstream promoters read through (pA)p, as seen for AAV2; however, A-AAV generated lower relative levels of spliced P5 and P19 products than does AAV2, suggesting that it generates lower relative levels of Rep 68 and Rep 40. An additional difference in the expression profile of these viruses lay in the abundance of the ITR-generated RNAs. B-AAV generated a greater level of these RNAs than did A-AAV or AAV5. Finally, we demonstrate that, like AAV2, transactivation of transcription of the capsid-gene promoter of B-AAV requires both adenovirus and targeting of its Rep protein to the transcription template; however, the expression of the capsid-gene promoter of A-AAV is, like AAV5, largely independent of both adenovirus and its Rep protein.
MATERIALS AND METHODS
Cells and virus.
293 human kidney cells (ATCC CRL-1573), MDBK (bovine kidney) cells (ATCC CCL-22), and HeLa (human cervix) cells (ATCC CCL-2) were maintained in Dulbecco modified Eagle medium with 10% fetal calf serum at 37°C in 5% CO2. Primary chicken kidney cells were prepared from 2 week-old specific-pathogen-free chicken embryos (Charles River Laboratories). Bovine adenovirus type 1 (BAd1; ATCC VR-313) and fowl adenovirus 1 (ATCC VR-432) were purchased from the American Type Culture Collection (Manassas, VA). Human adenovirus type 5 (Ad5) was propagated and assayed in 293 cells as previously reported (40). A-AAV was generated by transfection of an infectious clone (pA-AAV; obtained from J. Chiorini, National Institutes of Health [NIH]) in 293 cells (5).
Plasmids. (i) Production of B-AAV infectious clone.
A B-AAV RepCap clone (pMTVRepCap; nt 339 to 4454 [45]) was a gift from Jay Chiorini, NIH. All nucleotide B-AAV nucleotide numbers refer to GenBank accession number AY388617. The 5′ end of the B-AAV RepCap sequence was extended to the SfiI site at nt 89, and the 3′ end was extended to the SfiI site at the right end (nt 4633) by PCR using primers with BAd1 virus as a template. The final B-AAV construct (nt 81 to 4637), which contained half of the ITR sequence, which included the putative Rep-binding element (RBE), bearing SfiI sites at both ends, was inserted into KpnI/HindIII-digested pBluescript SK(+) (Stratagene), in which sequences between EcoRI and SacII were deleted. This plasmid was named B-AAV[1/2]TRRepCap. The remaining half of the ITR sequences (nt 1 to 80 at the left-hand side and nt 4648 to 4693 at the right) were amplified from the corresponding complementary sequences (nt 4545 to 4637 and nt 81 to 173, respectively) from B-AAV[1/2]TRRepCap and subsequently inserted into SacII/EcoR I and KpnI/HindIII sites of SK(+), respectively. This plasmid was named skB-AAV[1/2]TRs. The large internal B-AAV fragment (nt 81 to 4637), taken as an SfiI fragment from B-AAV[1/2]TRRepCap, was then inserted into SfiI-digested skB-AAV[1/2]TRs to generate the full-length clone pB-AAV. pHelper (Stratagene) is a plasmid that expresses all Ad5 gene products necessary to support AAV replication.
(ii) Constructs used in transcription activation studies. (a) B-AAV plasmids.
B-AAVRepCap[1/2]TR was constructed by deletion of the left-half ITR (nt 81 to 173) in B-AAV[1/2]TRRepCap. Further deletion of the right-hand half ITR in B-AAVRepCap[1/2]TR generated a plasmid of B-AAVRepCap. Knockout of the Rep ORF by blunting the EcoRI site at nt 541 in B-AAVRepCap[1/2]TR and B-AAVRepCap resulted in plasmids of B-AAVRep(−)Cap[1/2]TR and B-AAVRep(−)Cap, respectively. Deletion of the P7 promoter (nt 174 to 367) and the P41 upstream region (nt 174 to 1544) in B-AAVRep(−)Cap[1/2]TR and B-AAVRep(−)Cap generated plasmids of B-AAVΔP7Rep(−)Cap, B-AAVΔP7Rep(−)Cap[1/2]TR and of B-AAVP41Cap, B-AAVP41Cap[1/2]TR, respectively. The Rep supplemental plasmid (B-AAVRepSM) was constructed by the introduction of multiple silent mutations in the region of nt 1841 to 2039 into B-AAVRepCap, in which the capsid region (nt 2345 to 4520) was deleted.
(ii) Constructs used in transcription activation studies. (b) A-AAV plasmids.
The infectious clone of pA-AAV was a gift from Jay Chiorini, NIH (5). The plasmid A-AAV[1/2]TRRepCap[1/2]TR was constructed after insertion of an A-AAV ApaI fragment (nt 53 to 4621) into the ApaI site of SK(+). Deletion of the right-hand half ITR (nt 4560 to 4694) resulted in the plasmid A-AAV[1/2]TRRepCap. Blunting of the Aat II at nt 288 generated A-AAV[1/2]TRRep(−)Cap. The A-AAV plasmid designed to supply Rep in trans (A-AAVRepSM) was constructed so as not to interfere with the RNase protection probe RP. To generate A-AAVRepSM, multiple silent mutations within the region covered by the probe (nt 1690 to 1885) were introduced into A-AAV[1/2]TRRepCap, in which, additionally, the capsid region (nt 2461 to 4560) had been deleted.
(iii) ITR-luciferase constructs.
To generate the luciferase-expressing constructs diagrammed in Fig. 9, the luciferase ORF and simian virus 40 polyadenylation signal from plasmid pGL3-Basic (Promega) were inserted between the various AAV ITRs as indicated.
FIG. 9.
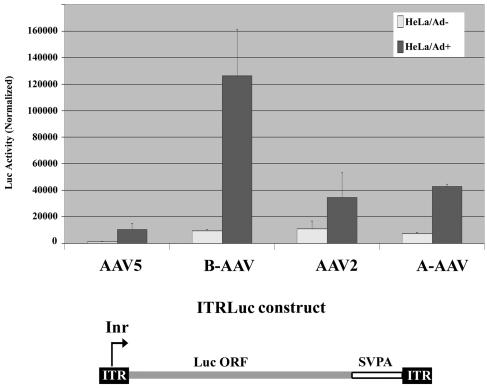
Comparison of the transcription activities of AAV ITRs. The luciferase ORF and the simian virus 40 poly(A) signal were inserted between the ITRs of AAV5, B-AAV, AAV2, and A-AAV, as diagrammed. These ITR-luciferase plasmids were cotransfected with a Renilla luciferase-expressing control plasmid into HeLa cells in the presence (dark gray bar) or absence (light gray bar) of Ad5. The cells were harvested at 36 to 40 h posttransfection. Luciferase activity was quantified and standardized relative to the Renilla luciferase transfection control. The values shown are the average with standard deviations from three independent plasmid transfections done in a single experiment.
(iv) Clones used to generate probes for RPA.
To map the transcription profile of B-AAV, RNase protection assay (RPA) probes, PP7, SB, RP, and DH were utilized, and they were constructed by cloning the following regions of B-AAV into BamHI-HindIII-digested pGEM4Z (Promega): nt 305 to 400 (PP7), nt 801 to 1040 (SB), nt 1841 to 2039 (RP), and nt 2001 to 2344 (DH). To map the transcription profile of A-AAV, RPA probes, PP5, SB, RP, and DH were constructed by cloning the following regions of A-AAV into BamHI-HindIII-digested pGEM4Z (Promega): nt 90 to 320 (PP5), nt 721 to 880 (SB), nt 1690 to 1885 (RP), and nt 2081 to 2400 (DH).
All of the DNA constructs were sequenced at the DNA core facility of the University of Missouri-Columbia to ensure that they were as predicted.
RNA isolation and RPA.
Total RNA was isolated by using guanidine isothiocyanate and purified by CsCl ultracentrifugation as previously described (47). RPAs were performed by using 10 μg of total RNA as previously described (32, 47). Probes were generated from linearized templates by in vitro transcription. RNA hybridizations were done in substantial probe excess, and signals were quantified with Fuji FLA 3000 and Fuji Multi Gauge v2.3 software (FUJIFILM Medical Systems U.S.A., Inc.). Relative molar ratios of individual species of RNAs were determined after adjustment for the number of 32P-labeled uridines in each protected fragment as previously described (47).
Northern blot analysis.
Northern analyses were done as previously described (35), using mRNA purified from 10 μg of total RNA (39). The 32P-labeled B-AAV DNA probes—repcap, rep, cap, and ITR—spanned B-AAV genome nt 174 to 4480, nt 174 to 1920, nt 3921 to 4480, and nt 81 to 320, respectively, as diagrammed in Fig. 2. The 32P-labeled A-AAV DNA probes repcap, rep, and cap spanned A-AAV genome sequences nt 53 to 4621, nt 90 to 1760, and nt 2721 to 4560, respectively, as diagrammed in Fig. 5. The probes for AAV2 repcap were described previously (37).
FIG. 2.
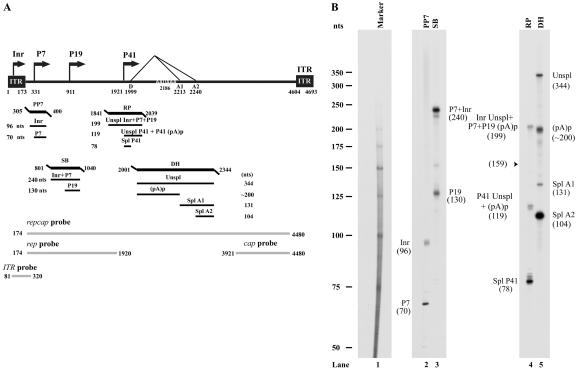
RPA analysis of B-AAV RNA generated from B-AAV-infected MDBK cells. (A) Schematic diagram of the B-AAV genome and the probes used for RPAs and Northern blots. The landmarks of transcription—the promoters (within the ITR [Inr], P7, P9, and P41), the splice donor site (D), the acceptor sites (A1 and A2), and the internal polyadenylation signal (pA)p—are shown. The RPA probes PP7 (nt 305 to 400), SB (nt 801 to 140), RP (nt 1841 to 2039), and DH (nt 2001 to 2344) are shown, along with the bands they are expected to protect and their predicted sizes. The probes repcap (nt 174 to 4480), rep (nt 174 to 1920), cap (nt 3921 to 4480), and ITR (nt 81 to 320), which are used for the Northern blot analysis in Fig. 3, are also shown. (B) Mapping of the B-AAV transcription units by RPA. A total of 10 μg of total RNA isolated 36 to 40 h after infection was protected by PP7, SB, RP, and DH probes, in individual reactions as indicated. Lane 1, 32P-labeled RNA markers (39), with sizes indicated to the left. The origins of the protected bands in lanes 2 and 5 are indicated. Spl, spliced RNAs; Unspl, unspliced RNAs; (pA)p, RNAs polyadenylated at the (pA)p site; Inr, RNAs generated from the initiator within the left ITR.
FIG. 5.
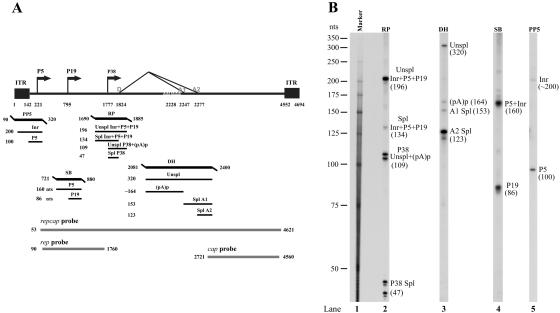
RPA analysis of A-AAV RNA. (A) Schematic diagram of the B-AAV genome and the probes used for RPAs and Northern blots. The landmarks of transcription—the promoters [within the ITR (Inr), P5, P19, and P38], the splicing donor site (D) and the acceptor sites (A1 and A2), and the internal poly(A) signal (pA)p—are shown. RPA probes PP5 (nt 90 to 320), SB (nt 721 to 880), RP (nt 1690 to 1885), and DH (nt 2081 to 2400) are shown together with the bands expected to be protected by A-AAV RNA. Probes repcap (nt 53 to 4621), rep (nt 90 to 1760), and cap (nt 2721 to 4560), are those used for Northern blot analysis in Fig. 6. (B) Mapping of the A-AAV transcription units by RPA. A total of 10 μg of total RNA isolated 36 to 40 h after the infection of chicken primary kidney cells was protected by the PP7, SB, RP, and DH probes, as indicated. Lane 1, 32P-labeled RNA maker (39), with sizes indicated to the left. The origins of the protected bands in lanes 2 to 5 are indicated.
Real-time PCR quantification of B-AAV virus titer (genome copy/milliliter).
Quantitative PCR was performed essentially as previous described (36). The amplicon and the TaqMan MGB probe were designed by Primer Express 2.0.0 (Applied Biosystems). Their sequences were as follows: forward primer, 5′-TGCCGAAGGTCCAACCA-3′ (B-AAV, nt 831 to 847); reverse primer, 5′-GGCCAATTTATACTCTTCGAGGTTAG-3′ (B-AAV, nt 892 to 867); and MGB probe, 5′-AGCTTCAGTGGGCGTG-3′ (B-AAV, nt 849 to 864). The TaqMan Universal PCR master mix (Applied Biosystems) was used for amplification using the ABI Prism 7500 Real-Time PCR System, using the protocol provided by the manufacturer.
Plaque assay to titrate bovine adenovirus.
MDBK cells were used for plaque assay of B-Ad utilizing a protocol essentially identical to that previously described for MVM titration (31).
Luciferase assays.
A total of 0.5 μg of firefly luciferase-expressing constructs (AAV2TRLuc, AAV5TRLuc, B-AAVTRLuc, and A-AAVTRLuc) was transfected into HeLa cells on 12-well plates with a Renilla-luciferase expression plasmid (0.05 μg) cotransfected as an internal control. In some experiments, Ad5 was added at a multiplicity of infection (MOI) of 10. Two days later, cells were lysed, and the activities of the firefly and Renilla luciferases were measured by using the protocol provided by the manufacturer (Promega, Madison, WI). All firefly luciferase activities reported were normalized to the internal control of Renilla expression.
Immunofluorescence assay to confirm generation of the infectious clone of B-AAV.
A total of 2 μg of pB-AAV was transfected into 293 cells with coinfection of Ad5 at 5 PFU/cell in 60-mm-diameter plates. Cells and media taken 2 days after transfection were combined, frozen, and thawed three times, and the lysate was further treated for 30 min at 56°C to inactivate adenovirus. Then, 10 μl of this lysate was used to infect 293 or MDBK cells on chamber slides (Lab-Tek; Nunc, Inc.), with Ad5 coinfection (5 PFU/cell). Immunofluorescence analyses were performed as previously described (39), using the anti-Rep monoclonal antibody 303.9, which was raised to an amino-terminally truncated AAV2 Rep78 (52) (American Research Products, Inc., Belmont, Mass.), and a fluorescein isothiocyanate-conjugated anti-mouse secondary antibody (ICN Biochemicals, Irvine, Calif.). Images were generated on an Olympus IX70 microscope with a ×20 objective lens. All images were obtained at the same exposure time.
RESULTS
Construction of an infectious clone of B-AAV.
Quantitative PCR determined that an initial stock of BAd1 received from the American Type Culture Collection (VR-313) contained a high level of B-AAV (4.3 × 10 × genome copies/ml; Table 1). If we assume there were 103 genome copies per PFU for this parvovirus (36), there was considerably more B-AAV than BAd1 in the ATCC BAd1 stock. Passage of this stock in MDBK cells at an MOI of 0.001 significantly boosted the titers of both BAd1 and B-AAV (to 3.3 × 108 PFU/ml, and 9.9 × 1012 genome copies/ml, respectively; BAdP1, Table 1). Upon subsequent passage of BAd1 stocks in MDBK cells at an MOI of 0.01, the titers of BAd1 and B-AAV remained at approximately 107 to 108 PFU/ml and 1 × 1012 to 5 × 1012 genome copies/ml, respectively (BAdP2 to -5, Table 1), suggesting that BAd1 and B-AAV grow well together and did not interfere with each other in the course of coinfection.
TABLE 1.
Quantification of B-AAV particles in passages of BAd
| BAd passage | BAd (PFU/ml) | B-AAV (gc/ml)a |
|---|---|---|
| BAd (ATCC) | 1.6 × 105 | 4.3 × 109 |
| BAdP1 | 3.3 × 108 | 9.9 × 1012 |
| BAdP2 | 2.8 × 107 | 2.6 × 1012 |
| BAdP3 | 8.7 × 107 | 1.4 × 1012 |
| BAdP4 | 4.6 × 107 | 3.1 × 1012 |
| BAdP5 | 7.4 × 107 | 4.8 × 1012 |
gc, genome copies.
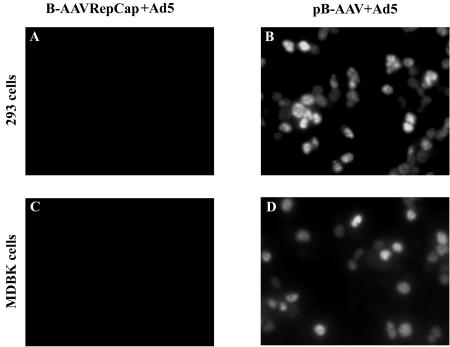
Because a pure B-AAV stock was not available and because our initial attempts to obtain pure stocks by CsCl gradient purification were unsuccessful (data not shown), we generated an infectious molecular clone of B-AAV from the initial BAd1 stock, as described in Materials and Methods. Transfection of this full-length clone (pB-AAV), but not a B-AAV-derived RepCap construct, into 293 cells with either Ad5 coinfection (Fig. 1) or pHelper plasmid cotransfection (Stratagene; data not shown) resulted in B-AAV virus production, as demonstrated by successful reinfection of 293 cells or MDBK cells in the presence of Ad5 coinfection (as monitored by anti-Rep immunofluorescence; Fig. 1, compare panels B and D to panels A and C).
FIG. 1.
Infectivity of the pB-AAV clone. 293 cells or MDBK cells in Lab-Tek two-chambers slides were infected with 2 μl of cell lysates from pB-AAV-transfected 293 cells, plus Ad5 at an MOI of 5 (panel B and D), or 2 μl of cell lysates from B-AAVRepCap (a nonreplicating plasmid)-transfected 293 cells, plus Ad5 at an MOI of 5 (panel A and C). Immunofluorescence assays were performed at 36 to 40 h postinfection, using an AAV anti-Rep monoclonal antibody that recognizes B-AAV Rep proteins.
Transcription profile of B-AAV.
The BAd1P1 stock described above was used to infect MDBK cells. Three days later, total RNA was isolated and subjected to Northern analysis, RNase protection analysis, and reverse transcription-PCR using B-AAV specific probes and primers to generate a comprehensive transcription profile of B-AAV RNA.
RPAs.
Because of their sequence similarity, we suspected that the transcription profile of B-AAV was likely going to be similar to that previously determined for AAV5. Thus, we made a set of four antisense probes (PP7, SB, RP, and DH) designed to assess transcripts generated from key areas of the genome. The locations of these probes relative to a schematic diagram of the B-AAV genome derived from published sequence data are shown in Fig. 2A.
(i) RP probe.
The RP probe, which spans the putative P41 promoter and the 5′ splice site (donor), was protected by B-AAV RNA to generate three bands of approximately 199 nt and 119 nt (which appear as a doublet) and 78 nt (Fig. 2B, lane 4). The band of 199 nt was consistent with protection by unspliced RNA generated in total by the upstream promoters within the ITR (Inr) at P7 and P19. If spliced versions of these RNAs were present, they would protect a band of 159 nt; however, such a band was not detected in these assays. The bands of 119 and 78 nt were consistent with protection by unspliced and spliced RNAs, respectively, generated from the P41 promoter. These assays suggest that the major initiation site of P41 is at the A residue at nt 1921, a position analogous to that found for AAV5, and the donor site of the central intron is at nt 1999. The distance between the P41 initiation site and the donor is 78 nt, similar to that of AAV5, and somewhat longer than this distance in AAV2 (53 nt). Interestingly, although B-AAV pre-mRNAs all share the same intron, under steady-state evaluations the ratio of spliced to unspliced P41 generated RNAs (the ratio of the 78-nt band versus the 119-nt band) was approximately 6:1, while virtually all RNAs generated from the upstream Inr, P7, and P19 promoters were found to be unspliced (compare the 199-nt band to the location at 159 nt; Fig. 2A, lane 4). These assays also showed that ca. 80% of the steady-state levels of B-AAV RNAs generated in these infections were capsid-encoding P41-spliced mRNAs.
(ii) DH probe.
Because B-AAV shares such high homology with AAV5, it seemed likely that a significant portion B-AAV-generated RNA, like AAV5-generated RNA, would be polyadenylated at the putative internal site (pA)p, within the intron. The DH probe, which spans (pA)p and the intron acceptor sites A1 and A2, protected bands at approximately 344, 200, 131, and 104 nt (Fig. 2B, lane 5). The 131- and 104-nt bands are consistent with acceptor site A1 and A2 being at nt 2213 and nt 2240, respectively. The 200-nt band is consistent with internal cleavage and polyadenylation downstream of the (pA)p AAUAAA signal. Because the majority of P41-generated RNAs were spliced, and the majority of RNA generated by the upstream promoters (Inr, P7, and P41) were unspliced across the region protected by the RP probe (Fig. 2, lane 4), the capsid-encoding RNAs, similar to the case for AAV5, must primarily read through the internal polyadenylation site into the capsid coding ORF and are subsequently spliced, whereas the vast majority of RNAs generated by the upstream promoters were polyadenylated at (pA)p. This was confirmed by Northern analysis described below. Unspliced RNA across the region protected by the DH probe (primarily contributed by P41) was reflected by the 344-nt band in this assay, and its relative abundance is consistent with this interpretation. Of the RNAs that are spliced, >95% utilize the A2 acceptor, which removes the AUG that initiates translation of the VP1 capsid protein.
(iii) SB probe.
Protection of the SB probe, which spans the putative P19 promoter, by B-AAV RNA generated bands at approximately 240 and 130 nt, a finding consistent with the presence of RNAs generated from the upstream Inr and P7 promoters and from the P19 promoter, respectively. Thus, the initiation site for P19-generated RNA was mapped to nt 911. Nearly 40% of the steady-state RNA spanning this region of the rep gene was generated from the P19 promoter.
(iv) PP7 probe.
Protection of the PP7 probe, which spans the putative P7 promoter, generated bands of approximately 96 and 70 nt. The band at 69 nt was consistent with a P7-generated RNA initiating at nt 331. The band at 96 nt, which essentially protected the whole probe, reflects RNA generated by an upstream promoter within the ITR, as has been described previously for both AAV2 and AAV5 (16, 19, 39). Interestingly, ca. 40% of the RNAs protected by this probe were generated from the upstream region, which is a significantly higher percentage than that seen previously for either AAV2 or AAV5 (16, 19, 39).
Northern blot analysis.
Northern blot analysis of B-AAV RNA exhibited a pattern similar to that of AAV5 (39). Hybridization to bead-purified, poly(A)-containing B-AAV-generated mRNA, both with the repcap probe and cap probe, detected two main bands, an abundant band of approximately 2.3 kb and a second, less-abundant band of approximately 4.4 kb (Fig. 3A, lanes 4 and 6). The 2.3-kb band is the size expected from the main spliced capsid protein-encoding mRNA, and the 4.4-kb band is the expected size of a near-full-length transcript. The repcap probe also detected minor bands at 1.9 and 1.3 kb, which likely are P7- and P19-generated RNAs polyadenylated at the internal site (pA)p, as described more fully below.
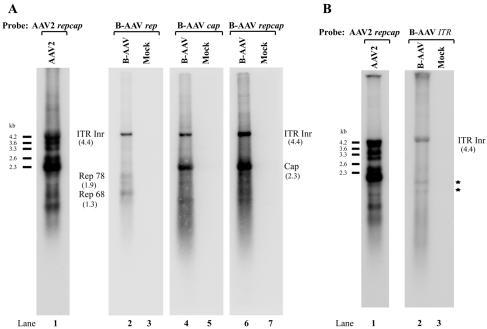
FIG. 3.
Northern blot analysis of B-AAV RNA. Total RNA was isolated 36 to 40 h postinfection of MDBK cells or from uninfected MDBK cells (Mock), and mRNA purified from 10 μg of total RNA was used for Northern analysis. The blot was hybridized to either a whole B-AAV repcap probe (A, lanes 2 and 3), rep probe (A, lanes 4 and 5), cap probe (A, lanes 6 and 7), or TR probe (B, lanes 2 and 3), which are diagrammed in Fig. 2B and described in Materials and Methods. The identities of bands protected by B-AAV RNA are shown with their respective sizes in parentheses. AAV2 RNA from AAV2-infected 293 cells was used as size markers (A and B, lane 1). Bands indicated with an asterisk (B, lane 2) were likely degraded RNAs.
Hybridization with a probe exclusively from the rep gene detected three main bands of 1.9, 1.3, and 4.4 kb (Fig. 3A, lane 2). The 1.9-kb band is the size expected from an RNA generated from the P7 promoter and polyadenylated at (pA)p, the 1.3-kb band is the size expected from an RNA generated from the P19 promoter and polyadenylated at (pA)p, and the band at 4.4 kb is the size expected of an RNA generated from within the ITR. The 1.9- and 1.3-kb bands were also detected by the full repcap gene probe (Fig. 3A, lane 6) but, as expected from this interpretation, not by the cap gene probe (Fig. 3A, lane 4). An ITR-based probe, whose limits were fully upstream of P7, also hybridized with the 4.4-kb band, confirming that this poly(A)-containing RNA was transcribed from the left-hand ITR (Fig. 3B, lane 2). No RNAs transcribed from the right-hand ITR were detected either by Northern blot or RPA (data not shown).
A summary of the transcription profile of B-AAV RNA generated in MDBK cells is shown in Fig. 4A. RPAs determined that ca. 90% of the transcripts detected in total RNA preparations at steady-state levels late in infection were generated from P41. Approximately 90% of P41-generated RNAs were spliced, and approximately 40 times more RNAs were detected that utilized the A2 acceptor, used to generate mRNAs encoding VP2 and VP3, than utilized A1, which retains the VP1 AUG. P7-derived RNAs were approximately as abundant as those generated by P19, and together these amounted to some 7 to 8% of the total RNA detected. Virtually all P7- and P19-generated RNAs were polyadenylated at (pA)p. A significant amount (ca. 2%) of RNA was detected that was initiated within the left-hand ITR. These molecules were polyadenylated at the distal polyadenylation site (pA)d and remained unspliced. The major transcription initiation and termination sites, as well as the splice junctions, were also confirmed by reverse transcription-PCR (data not shown), as described previously (39). The general map of B-AAV is similar to that of AAV5; however, RNA transcripts initiated from the B-AAV ITR were significantly more abundant than those previously detected from the AAV5 ITR. In addition, similar to AAV5, and as predicted from the RNA profile, after infection the only Rep proteins detected by Western blotting were the Rep 78 and Rep 52 proteins (data not shown).
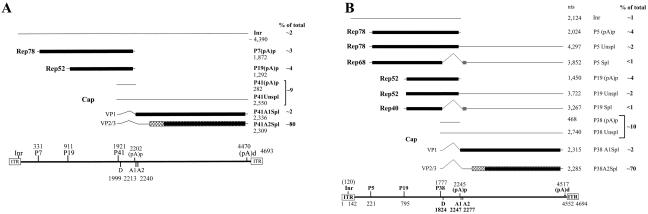
FIG. 4.
Transcription maps of B-AAV and A-AAV. (A) Transcription map of B-AAV. The genome of B-AAV is diagrammed, with transcription landmarks shown to scale, including the ITR, P7 promoter, P19 promoter, P41 promoter, splice donor (D), and acceptor (A1 and A2) sites, the internal polyadenylation site [(pA)p], and the distal polyadenylation site [(pA)d]. All of the RNA species detected in our assays are diagrammed with an indication of their identities and respective sizes. The only Rep-encoding transcripts generated by B-AAV were the Rep 78 and Rep 68 mRNAs, which were polyadenylated at the (pA)p site. RNA initiation within the ITR was from multiple start sites (data not shown). The approximate relative accumulated levels of each transcript type in total RNA is given as a percentage. (B) Transcription map of A-AAV. The genome of A-AAV is shown with transcription landmarks shown to scale, including the ITR, P5 promoter, P19 promoter, P38 promoter, splice donor (D) and acceptor (A1 and A2) sites, the internal polyadenylation site [(pA)p], and the distal polyadenylation site [(pA)d]. All of the RNA species are shown with their respective sizes. In contrast to B-AAV and AAV5, a significant percentage of P5- and P19-generated mRNAs read through the internal (pA)p site and are found either as unspliced (unspl) or spliced (spl) species. The approximate relative accumulated levels of each transcript type in total RNA as determined by RPAs is given as a percentage.
Transcription profile of A-AAV.
A-AAV virus was generated after transfection of an infectious A-AAV clone (obtained from J. Chiorini) into 293 cells in the presence of Ad5, as previously described (39). Purified A-AAV was then used to infect primary chicken kidney cells, together with coinfection by fowl adenovirus type 1 (ATCC VR-432). Total RNA was isolated and subjected to RPA and Northern blot analysis, which was similar to the analysis described above for B-AAV.
RPAs.
A schematic diagram of the A-AAV genome and the four antisense probes (PP5, SB, RP, and DH) used for this analysis is shown in Fig. 5A.
(i) RP probe.
The RP probe, which spans the putative P38 promoter and the 5′ splice site (donor), was protected by A-AAV to generate four species of approximately 196, 134, 109, and 47 nt. The bands of 196 and 134 nt were consistent with protection by unspliced and spliced RNAs, respectively, generated in total by P5, by P19, and from activity from within the ITR (Inr), as described below. The bands at 109 nt (appearing as a doublet in this assay) and at approximately 47 nt (also appearing as multiple bands in this assay) were consistent with protection by unspliced and spliced RNAs, respectively, generated from the P38 promoter. These assays suggest that whereas there may be an additional nearby initiation site, the major RNA initiation site of the P38 promoter is the A residue at nt 1777, and the donor site is at nt 1824. The distance between the A-AAV P38-generated RNA initiation site and the intron donor is only 47 nt, which is smaller than the analogous region for other AAVs thus far tested (37, 39, 40). Splicing of the P38-generated capsid protein-encoding RNAs was relatively poor: the steady-state accumulated ratio of spliced to unspliced RNAs was only approximately 3:1, which is significantly less than the ratio see for other AAVs during permissive infection. Whether this ratio is also seen for cytoplasmic poly(A)-containing mRNA is currently under investigation. In contrast to B-AAV, a significant amount of RNAs generated from the upstream ITR, P5, and P19 promoters is spliced. However, as is the case for the AAV2-like viruses, excision of the A-AAV intron from pre-RNAs generated from the upstream ITR, P5, and P19 promoters was considerably less efficient (ca. 5% of steady-state RNA) than it was from P38-generated RNAs (Fig. 5B, lane 2). In addition, the relative level of splicing of RNAs generated by the upstream promoters of A-AAV was less than previously observed for RNAs generated by the upstream promoters of AAV2. These results suggest that A-AAV produces relatively less Rep 68 and Rep 40 during infection than does AAV2.
(ii) DH probe.
To examine usage of the internal polyadenylation site within the A-AAV intron [(pA)p], we used a probe (the DH probe; Fig. 5A) that spanned the putative intron acceptor sites and (pA)p, similar to the DH probe used for the analysis of B-AAV RNA. This probe was protected by A-AAV RNA to generate bands at approximately 320, 164, 153, and 123 nt. The bands of 153 and 123 nt were consistent with acceptor site A1 and A2 being at 2247 and nt 2277, respectively. The band at 164 nt is consistent with internal cleavage and polyadenylation at approximately at nt 2245. As for B-AAV, acceptor site A2 was used to a significantly greater extent than A1. However, in contrast to other examples, the A-AAV internal polyadenylation site was used to a significantly lesser extent than it is found for either B-AAV, AAV5 (39), or GPV (37). This is consistent with the observation that a significant (albeit low) level of RNAs from upstream promoters are spliced, presumably after extension through (pA)p and polyadenylation at the 3′ (pA)d site.
(iii) SB probe.
To map the P19 promoter and to quantify the level of accumulated P19-generated RNA, we used a P19-spanning probe (the SB probe; Fig. 5B), similar to that used for the analysis of B-AAV above. When protected by A-AAV RNA, this probe generated two bands of approximately 160 and 86 nt. This protection mapped the P19-generated RNA initiation site to approximately nt 795 and demonstrated that P19-generated RNAs were present at a level approximately equal, on a molar basis, to RNAs generated by upstream promoters. The presence of the P19 promoter is a hallmark of the AAVs, and its presence in A-AAV is in contrast to the distantly related avian dependovirus, GPV, which has been shown to lack a P19 promoter and rely on alternative splicing to generate small Rep-encoding mRNAs (37).
(iv) PP5 probe.
The PP5 probe (Fig. 5A) was used to map and quantify transcripts from the P5 promoter and from within the ITR. A-AAV RNA protected two bands of approximately 200 and 100 nt. The band at 100 nt mapped the P5 initiation site to nt 221. The band of approximately 200 nt mapped the initiation site for a transcript within the ITR to approximately nt 120, which would be within the terminal resolution site in the ITR. (The ITR-generated transcript of AAV5 also maps to the TRS site of that ITR [39].) However, the A-AAV ITR-generated RNA is present at a much lower level relative to the P5-generated transcript (approximately 1:10 [Fig. 5B, lane 5]) than it is for B-AAV.
Northern blot analysis.
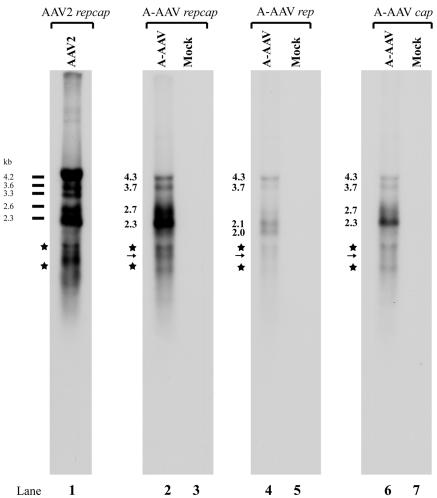
Northern blot analysis of A-AAV RNA revealed many similarities to both AAV5 and B-AAV. Hybridization to total A-AAV-generated RNA with either the repcap probe or the cap probe detected four primary bands of approximately 4.3, 3.7, 2.7, and 2.3 kb (Fig. 6, lanes 2 and 6). The 4.3- and 3.7-kb bands are the sizes expected of unspliced RNAs generated from the P5 and P19 promoters, respectively, that extended to the polyadenylation site at the right-hand end of the genome (pA)d. The 2.7- and 2.3-kb bands were the sizes expected from the unspliced and spliced RNA from generated from the P38 promoter, with the spliced P38-generated RNA being considerably more abundant. Hybridization with a probe exclusively from the rep gene detected four main bands of approximately 4.3, 3.7, 2.1, and 2.0 kb. As described above, the 4.3- and 3.7-kb bands are the sizes expected of unspliced RNAs generated from the P5 and P19 promoters, respectively. The 2.0-kb band is the size expected of an RNA generated from the P5 promoter that had been polyadenylated at the internal site (pA)p. The 2.1-kb band is the size expected of an RNA initiated from the ITR and polyadenylated at (pA)p. RNAs generated from the P19 promoter and polyadenylated at (pA)p would be expected to be approximately 1.5 kb. A band of this size was detected using the rep and repcap probes (marked with an arrow in Fig. 6, lanes 2 and 4); however, this band comigrated in these gels with nonspecific degradation products (Fig. 6, lanes 2, 4, and 6), and so the presence and abundance of such RNAs cannot be accurately quantified in these assays.
FIG. 6.
Northern blot analysis of A-AAV RNA. Total RNA was isolated 36 to 40 h postinfection from A-AAV-infected chicken primary kidney cells. mRNA purified from 10 μg of total RNA was subjected to Northern analysis; uninfected mRNA was used as a mock control. The Northern blot was hybridized with either a whole A-AAV repcap probe (lanes 2 and 3), a rep probe (lanes 4 and 5), or a cap probe (lanes 6 and 7), which are diagrammed in Fig. 5. The sizes of different A-AAV RNA species are shown. AAV2 RNA from AAV2-infected 293 cells was used as size markers. Because the bands marked with an asterisk appear with all probes, it is likely that they are degradation products. P19-generated RNAs polyadenylated at (pA)p would be expected to run in this region, and a putative band detected with the repcap and rep probe, but not with the cap probe, is marked with an arrow; however, as described in the text, the appearance of degradation products in this area of the gel precluded their accurate assessment.
Summary of the transcription profile of A-AAV and comparison of the transcription profiles of B-AAV and A-AAV.
The overall transcription profile of A-AAV is presented in Fig. 4B. In general, the A-AAV map is similar to both AAV5 and B-AAV; however, there are some significant differences. For A-AAV, RPAs determined that ca. 80% of the transcripts detected in total RNA preparations at steady-state levels late in infection were generated from P38, rather than the 90% determined for B-AAV. As was the case for B-AAV, the great majority (almost 90%) of P41 RNAs were spliced, primarily at the A2 acceptor. A-AAV P5-derived RNAs were approximately as abundant as those generated by P19, but together these amounted to almost 15% of the total RNA detected, which was approximately twice as much as was generated by B-AAV P7 and P19 at steady-state conditions. A-AAV, B-AAV, and AAV5 all utilize an internal polyadenylation site; however, in our analysis, we found that it was used to a greater extent by B-AAV than by A-AAV. For B-AAV, >99% of RNAs generated from upstream promoters were polyadenylated at (pA)p and hence not spliced, similar to AAV5 (39). In contrast, for A-AAV, ca. 50% of RNAs generated from upstream promoters read through (pA)p; however, only a small proportion of these RNAs (<2%) were subsequently spliced. Thus, whereas AAV5 and B-AAV generated only two Rep proteins (Rep 78 and Rep 52), A-AAV, like AAV2, would be predicted to generate the full complement of four Rep proteins, Rep 78, Rep 68, Rep 52, and Rep 40. However, because A-AAV generated much lower levels of spliced P5 and P19 products than does AAV2, it would be expected to generate lower relative levels of Rep 68 and Rep 40. This could not be directly tested because the available antibodies do not react with the A-AAV Rep proteins.
An additional difference between these virus expression patterns lay in the abundance of the ITR-generated RNAs. B-AAV generated a greater level of these RNAs than did A-AAV or AAV5. This will be addressed further below.
Transactivation of the B-AAV and A-AAV capsid gene promoters.
The constitutive activity in either 293 or HeLa cells of the AAV2 P40 promoter is very weak. The AAV2 large Rep protein is a positive transactivator of transcription of P40, and its activity in this regard is dependent upon targeting to an RBE in either the P5 promoter region or the ITR (26, 27, 33, 34). In contrast, the constitutive activity in 293 cells of the P41 promoter of AAV5 within an AAV5 RepCap construct is strong independently of AAV5 Rep or additional Ad5 gene products (39; C. Ye, J. Qiu, and D. J. Pintel, unpublished data).
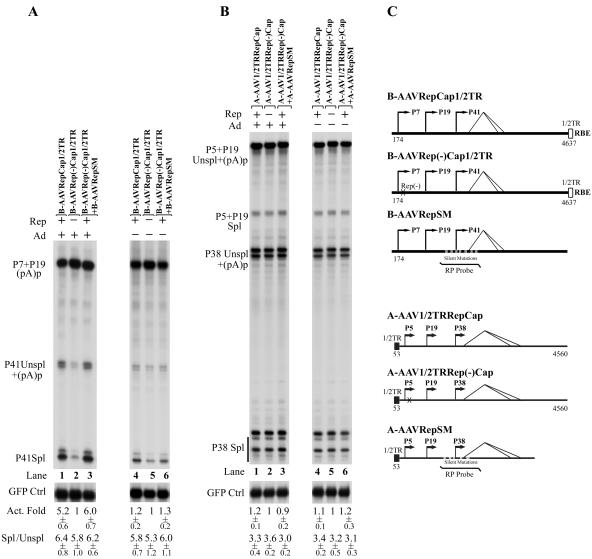
In 293 cells in the presence of helper Ad5 the P41 promoter of B-AAV was active within a RepCap plasmid that retains a portion of its right-hand ITR containing a consensus Rep-binding site described more fully below (Fig. 7A, lane 1). (Only a single RBE-containing [1/2]ITR sequence was included to preclude replication of the molecule.) This activation was dependent upon the B-AAV Rep protein: when a translation termination signal was introduced into the large Rep coding region at nt 541, P41 activity was reduced ∼5-fold (Fig. 7A, compare lane 2 to lane 1), and this activity was regained when the B-AAV Rep protein was supplemented in trans (Fig. 7A, compare lane 3 to lane 2). In the absence of Ad5, however, expression of the P41 promoter in E1A- and E1B-expressing 293 cells within the B-AAV RepCap plasmid was reduced compared to the levels of the transfected internal green fluorescent protein (GFP) control, and its activity was only modestly affected either by premature termination of the Rep ORF (Fig. 7A, compare lane 4 to lane 5) or by further addition in trans of Rep to the Rep(−) construct (Fig. 7A, compare lane 6 to lane 5). These results suggested that, similar to AAV2 rather than AAV5, efficient activity of the B-AAV P41 promoter required the B-AAV Rep protein together with the helper adenovirus.
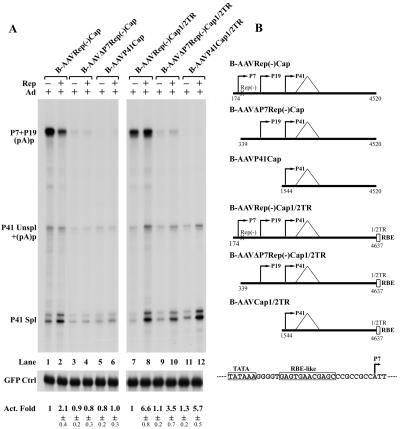
FIG. 7.
Transactivation, but not splicing, of P41-generated transcripts was dependent on B-AAV Rep and adenovirus. (A) B-AAV P41 transcripts. 293 cells were transfected, in the presence (+) or absence (−) of Ad5 (MOI of 5), with the following plasmids, which are diagrammed in Fig. 7C: B-AAVRepCap[1/2]TR, which contains half of the right-hand ITR which includes an RBE and the TRS (lanes 1 and 4); B-AAVRep(−)Cap[1/2]TR (in which Rep ORF was terminated, lanes 2 and 5); and B-AAVRep(−)Cap[1/2]TR, together with a Rep-supplying plasmid (B-AAVRepSM) (lanes 3 and 6). Total RNA (10 μg), isolated 36 to 40 h after transfection, was protected by the B-AAV RP probe (nt 1841 to 2039), as diagrammed. (B) A-AAV P38 transcripts. 293 cells were transfected, in the presence (+) or absence (−) of Ad5 (MOI of), with the following plasmids, which are diagrammed in panel C: A-AAV[1/2]TRRepCap (lane 1,4), which contains the right half of the A-AAV left-hand end ITR and the RepCap region; A-AAV[1/2]TRRep(−)Cap (lanes 2 and 5), in which the Rep-encoding ORF was terminated, and the plasmids A-AAV[1/2]TRRep(−)Cap and A-AAVRepSM (a A-AAV Rep supplying plasmid) (lanes 3 and 5). Total RNA (10 μg), isolated 36 to 40 h after transfection, was protected by the A-AAV RP probe (nt 1690 to 1885), as diagrammed. Plasmid C1GFP (Clontech) was cotransfected as an internal control, and RNA generated from this plasmid was protected by a probe to the GFP coding region (40). The levels of P41 (or P38) RNA (Unspl+Spl) was normalized to GFP RNA, and the activation fold (Act. Fold) is presented. The ratio of spliced P41 (or P38) transcripts to unspliced transcripts (Spl/Unspl) is also shown. Quantifications of the activation fold and the ratio of Spl/Unspl RNAs are shown with the standard deviations, and the values are averages of at least two independent experiments.
Similar experiments were performed with the A-AAV RepCap plasmids in 293 cells (Fig. 7B). (In the A-AAV constructs, ITR sequences were retained at the left-hand end rather than the right-hand end because of cloning constraints.) Surprisingly, the levels of P38-generated RNAs were high, regardless of the presence of Rep protein or Ad5 coinfection (Fig. 7B). Thus, the A-AAV capsid gene promoter displayed a high constitutive activity largely independent of additional adenovirus gene products or its Rep proteins, similar to the capsid-gene promoter of AAV5.
In addition, these experiments demonstrate that, in contrast to AAV2, the ratio of spliced versus unspliced B-AAV and A-AAV capsid-gene RNAs were not significantly affected by either Rep or adenovirus (Fig. 7A and B). [Although the bands detected by the RP probed that are designated as unspliced also included P41-generated products that are polyadenylated at (pA)p, these RNAs represent a negligible contribution to the amount of unspliced P41-generated RNAs for this determination.]
The B-AAVRepCap[1/2]ITR and B-AAVRep(−)Cap[1/2] ITR constructs contain both an AAV2 RBE-like element (GAGY) in the P7 region (diagrammed in Fig. 8B) and a consensus RBE (3×GAGT/C) within the B-AAV ITR sequences which are present in this construct (27). When the ITR sequences of B-AAVRep(−)Cap[1/2] ITR were removed, the low basal level of P41 could be enhanced approximately twofold by the addition of Rep in trans (Fig. 8A, compare lane 2 to lane 1). This activation was presumably dependent upon Rep-binding sequences within the P7 promoter, because further deletion, to either nt 339 [B-AAVΔP7Rep(−)Cap] or nt 1544 (B-AAVP41Cap), abrogated this response (Fig. 8A, compare lanes 3 to 6 to lanes 1 to 2). Reintroduction of the [1/2]ITR sequences to the clone with P7 deleted [B-AAVΔP7Rep(−)Cap[1/2]ITR, lanes 9 to 10] or the P41 minimal clone (B-AAVP41Cap[1/2]ITR, lanes 11 to 12) rescued the responsiveness of P41 to Rep. This was also seen for the full-length Rep(−)Cap construct (Fig. 7A, compare lanes 2 and lane 3, and Fig. 8A, lanes 7 and 8). Thus, efficient activation of B-AAV P41 by Rep in the presence of adenovirus depends on a Rep-binding site in cis and likely the targeting of Rep to the transcription template.
FIG. 8.
Rep transactivation of the B-AAV P41 promoter requires both adenovirus and the Rep-binding site. 293 cells were transfected, in the presence of Ad5 (MOI of 5), with the reporter plasmids diagrammed in panel B, with (+) or without (−) the Rep-supplying plasmid (B-AAVRepSM). Plasmid C1GFP (Clontech) was cotransfected as a control and was protected by a probe to the GFP coding region (40). The levels of P41 RNA (Unspl+Spl) were normalized to GFP RNA, and the activation fold (Act. Fold) is presented. The quantifications of the activation fold and the spl/unspl RNA ratio were averages with standard deviations of at least from two independent experiments. A partial sequence of the P7 promoter is diagrammed in panel B; the TATA box and the putative RBE-like sequence are indicated.
Comparison of transcription initiation from AAV ITR.
Although their RBEs are for the most part interchangeable, the AAV ITRs thus far characterized generally fall into three groups (the AAV2-like, the AAV5-like, and A-AAV) based on functional differences in their terminal resolution sites (TRS) (5, 8, 24, 39, 43). Significant initiation of transcription has been identified within the ITRs of both AAV2 (16, 19) and AAV5 (39). For AAV5, the start site for ITR-initiated transcription has been mapped to the AAV5 TRS. The analyses described above indicate that both B-AAV and A-AAV generate transcripts that initiate within their ITR region. Reporter constructs (diagrammed in Fig. 9) were constructed and used to evaluate the relative levels of transcription initiation within various AAV ITRs in HeLa cells. In the absence of helper Ad5, initiation from the AAV2 ITR, B-AAV ITR, and A-AAV ITR was similar and approximately 10-fold higher than initiation from the AAV5 ITR (Fig. 9). In the presence of Ad5, initiation from all of the ITRs was significantly stimulated. Transcription from the B-AAV ITR was stimulated to the greatest degree—at 10-fold it reached levels at least three times that of the AAV2 ITR and the A-AAV ITR (Fig. 9). Accumulated levels of luciferase activity derived from the AAV5 ITR construct was ∼10-fold less than that from B-AAV ITR construct in presence of Ad5, although these ITRs share 96% sequence identity.
DISCUSSION
A-AAV and B-AAV were first identified in the 1970s (11, 23, 30, 53) as contaminants of avian and bovine adenovirus stocks, respectively. Both viruses have recently received renewed interest, and their full nucleotide sequences have been recently reported (5, 14, 45). We present here the first detailed transcription profile of both viruses, which we obtained following the infection of cell lines derived from their native hosts. These represent the first reported expression profiles of nonprimate AAVs. Both viruses show similarities to the transcription profile of AAV5; however, the profile of A-AAV exhibited similarities to the prototype AAV2 strain as well. In addition, like AAV2, the B-AAV P41 capsid gene promoter required both Ad5 and its Rep protein targeted in cis for efficient activation; however, the P38 capsid gene promoter of A-AAV was constitutively active, similar to AAV5. The transcriptional activity of the B-AAV ITR was particularly strong.
A major feature of the transcription profiles of both B-AAV and A-AAV was that the RNAs generated from the viral upstream initiation sites (within the ITR, P7, and P19) were polyadenylated at the internal (pA)p site within the central intron, similar to AAV5 (39) and in contrast to AAV2 (29, 49, 50). B-AAV and A-AAV differed, however, in the extent to which this signal was used. For B-AAV, virtually all of the detectable RNAs generated from upstream promoters was polyadenylated at (pA)p. Thus, rather than existing in both a spliced and an unspliced form as found for RNAs generated by the AAV2 upstream promoters, the Rep-encoding RNAs generated by B-AAV, like those of AAV5, existed only as unspliced RNAs terminating within the intron. This RNA profile predicts that, like AAV5 (39), B-AAV encodes only Rep 78 and Rep 52, and we have shown this to be the case (data not shown).
In contrast, only approximately half of the RNAs generated by the A-AAV upstream promoters were polyadenylated internally at (pA)p. Thus, whereas the transcription profile of A-AAV resembled that of AAV5, a significant portion of the Rep-encoding RNAs of A-AAV extended to the right-hand end of the genome, as do the Rep-encoding RNAs generated by AAV2. However, in contrast to AAV2, only a small portion of the P5 and P19 RNAs polyadenylated at the distal polyadenylation site (pA)d were found to be spliced. This suggested that A-AAV, like AAV2, produced Rep 68 and Rep 40 in addition to Rep 78 and Rep 52; however, the relative levels of Rep 68 and Rep 40 would be predicted to be considerably less than what was observed for AAV2. Thus, at the protein expression level, A-AAV is most likely more similar to AAV5, encoding much greater relative levels of Rep 78 and Rep52, even though half of the A-AAV Rep-encoding RNAs extend to the right-hand end of the genome.
Why AAV5 and B-AAV can tolerate the absence of Rep 68 and Rep 40, whereas A-AAV can tolerate relative levels much lower than those seen for AAV2, is not clear. Rep 78 and Rep 68 are known to be redundant for many required activities during AAV infection (9, 13), although it has recently been shown that Rep 68 specifically interacts with 14-3-3 proteins, likely thus reducing Rep binding to AAV DNA, thereby modulating AAV genome replication (20). However, at least for B-AAV, the absence of Rep 68 and Rep 40 does not seem to suppress viral replication. Our source for B-AAV in these studies was an ATTC stock of bovine adenovirus and, upon passage, both the bovine adenovirus and the B-AAV remained at consistently high infectious titers (>107 PFU of BAd/ml and 1012 genome copies/ml of B-AAV). It has been previously reported that AAV1-, AAV2-, and AAV3-contaminated adenovirus stocks contain approximately 102 to 103 times as many AAV particles as adenovirus particles (21). In our B-AAV/BAd stocks, assuming 1 PFU of BAd/103 particles, the particle ratio of B-AAV/BAd was also maintained upon passage at approximately 102 to 103. Thus, it seems that lack of the Rep 68 and Rep 40 did not decrease B-AAV replication significantly in coinfection with bovine adenovirus.
We have previously found that in 293 cells the AAV5 P41 promoter within AAV5 RepCap constructs exhibits relatively high constitutive activity that is largely independent of either its Rep protein or additional Ad5 gene products (39; Ye et al., unpublished). Our data demonstrate that this is also the case for A-AAV P41, which displays similarly high constitutive levels of activity. In contrast, the constitutive activity of the B-AAV capsid gene promoter is quite low and yet, like P40 of AAV2, it can be significantly activated by the addition of Rep in the presence of Ad5. Thus, A-AAV, which is more divergent from AAV5 at the nucleotide level than is B-AAV, has a pattern of capsid gene promoter activity more similar to AAV5, while B-AAV, which is more similar at the nucleotide level to AAV5, shows a pattern of capsid gene expression more similar to AAV2. Why the capsid gene promoter of some AAVs and not others require activation by their Rep proteins in trans, and how this is governed, is currently under investigation. These results do, however, suggest that constitutively high activity of the capsid-gene promoter is not necessarily linked to a transcription profile featuring the absence of Rep 68 and Rep 40.
Interestingly, although the large Rep protein of B-AAV, like its AAV2 counterpart, functions as a transcriptional activator, it has no obvious role in the processing of B-AAV P41-generated RNA. This is in contrast to the AAV2 large Rep proteins, which enhance the splicing of AAV2 P40-generated RNAs (40). Further comparison of these two systems may help unravel the mechanism of this important function of AAV2 Rep.
It has been observed that the AAV ITRs exhibit endogenous transcriptional activity (16, 19, 39), and here we have compared the activity associated with a number of AAV ITRs. In the absence of Ad5, the transcriptional activity of the ITRs of AAV2, B-AAV, and A-AAV were quite similar, whereas that of AAV5 was somewhat less. Although the ITR structure of B-AAV shares 96% homology with AAV5 (7), transcription from the B-AAV ITR was, surprisingly, 10 times higher than that from the AAV5 ITR in the presence of Ad5. The B-AAV ITR contains an extra C that forms one more base-pair in the T stem, resulting in a more GC-rich region (CGGCCC), which might account for the higher levels of transcripts produced. The significance of ITR-initiated transcripts in the viral life cycle is unknown, but for B-AAV, levels of ITR-initiated RNAs in MDBK cells were approximately the same as for those generated from the P7 promoter.
The results presented here show that AAV5, B-AAV, and A-AAV display great similarity in their genetic profiles, which distinguishes them from the AAVs thus far examined. In addition, we have recently determined that the goat AAV, which is 95% identical to AAV5 at the nucleotide level, also has an expression profile similar to AAV5 (J. Qiu and D. Pintel, unpublished data). These results suggest that AAV5 and the animal A-AAV, B-AAV, and goat AAV may share a nonprimate origin, separating them from the human AAVs AAV1-4 and AAV6-9.
It is becoming clear that there is a much wider variation in the expression profiles of members of the Dependovirus genera of the parvovirus family of viruses than previously thought. Further detailed analysis of the transcription profile of the various types of AAVs will certainly lead to important information concerning the taxonomy, evolution, and molecular biology of this important virus family.
Acknowledgments
We thank John Chiorini at the National Institutes of Health for plasmids and valuable discussion. We thank Lisa Burger for excellent technical assistance.
This study was supported by PHS grants RO1 AI46458, RO1 AI56310, and RO1 AI21302 from NIAID to D.J.P.
REFERENCES
- 1.Arbetman, A. E., M. Lochrie, S. Zhou, J. Wellman, C. Scallan, M. M. Doroudchi, B. Randlev, S. Patarroyo-White, T. Liu, P. Smith, H. Lehmkuhl, L. A. Hobbs, G. F. Pierce, and P. Colosi. 2005. Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties. J. Virol. 79:15238-15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atchison, R. W., Casto, B. C., and Hammon, W. M. 1965. Adenovirus-associated defective virus particles. Science 149:754-756. [DOI] [PubMed] [Google Scholar]
- 3.Bantel-Schaal, U., and H. H. zur. 1984. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology 134:52-63. [DOI] [PubMed] [Google Scholar]
- 4.Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 218:1-23. [DOI] [PubMed] [Google Scholar]
- 5.Bossis, I., and J. A. Chiorini. 2003. Cloning of an avian adeno-associated virus (AAAV) and generation of recombinant AAAV particles. J. Virol. 77:6799-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C. L., R. L. Jensen, B. C. Schnepp, M. J. Connell, R. Shell, T. J. Sferra, J. S. Bartlett, K. R. Clark, and P. R. Johnson. 2005. Molecular characterization of adeno-associated viruses infecting children. J. Virol. 79:14781-14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiorini, J. A., F. Kim, L. Yang, and R. M. Kotin. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiorini, J. A., L. Yang, Y. Liu, B. Safer, and R. M. Kotin. 1997. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J. Virol. 71:6823-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiorini, J. A., B. Zimmermann, L. Yang, R. H. Smith, A. Ahearn, F. Herberg, and R. M. Kotin. 1998. Inhibition of PrKX, a novel protein kinase, and the cyclic AMP-dependent protein kinase PKA by the regulatory proteins of adeno-associated virus type 2. Mol. Cell. Biol. 18:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, J. K., J. B. McFerran, E. R. McKillop, and W. L. Curran. 1979. Isolation of an adeno associated virus from sheep. Arch. Virol. 60:171-176. [DOI] [PubMed] [Google Scholar]
- 11.Coria, M. F., and H. D. Lehmkuhl. 1978. Isolation and identification of a bovine adenovirus type 3 with an adenovirus-associated virus. Am. J. Vet. Res. 39:1904-1906. [PubMed] [Google Scholar]
- 12.Dawson, G. J., V. J. Yates, P. W. Chang, and J. J. Oprandy. 1982. Is avian adeno-associated virus an endogenous virus of chicken cells? Nature 298:580-582. [DOI] [PubMed] [Google Scholar]
- 13.Di, P. G., and J. A. Chiorini. 2003. PKA/PrKX activity is a modulator of AAV/adenovirus interaction. EMBO J. 22:1716-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estevez, C., and P. Villegas. 2004. Sequence analysis, viral rescue from infectious clones and generation of recombinant virions of the avian adeno-associated virus. Virus Res. 105:195-208. [DOI] [PubMed] [Google Scholar]
- 15.Farkas, S. L., Z. Zadori, M. Benko, S. Essbauer, B. Harrach, and P. Tijssen. 2004. A parvovirus isolated from royal python (Python regius) is a member of the genus Dependovirus. J. Gen. Virol. 85:555-561. [DOI] [PubMed] [Google Scholar]
- 16.Flotte, T. R., S. A. Afione, R. Solow, M. L. Drumm, D. Markakis, W. B. Guggino, P. L. Zeitlin, and B. J. Carter. 1993. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 268:3781-3790. [PubMed] [Google Scholar]
- 17.Gao, G., L. H. Vandenberghe, M. R. Alvira, Y. Lu, R. Calcedo, X. Zhou, and J. M. Wilson. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberman, R. P., T. J. McCown, and R. J. Samulski. 2000. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J. Virol. 74:8732-8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, S. I., M. A. Kawano, K. Ishizu, H. Watanabe, M. Hasegawa, S. N. Kanesashi, Y. S. Kim, A. Nakanishi, K. Kataoka, and H. Handa. 2004. Rep68 protein of adeno-associated virus type 2 interacts with 14-3-3 proteins depending on phosphorylation at serine 535. Virology 320:144-155. [DOI] [PubMed] [Google Scholar]
- 21.Hoggan, M. D., N. R. Blacklow, and W. P. Rowe. 1966. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. USA 55:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden, R. M., P. Ward, C. Giraud, E. Winocour, and K. I. Berns. 1996. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 93:11288-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luchsinger, E., R. Strobbe, G. Wellemans, D. Dekegel, and S. Sprecher-Goldberger. 1970. Haemagglutinating adeno-associated virus (AAV) in association with bovine adenovirus type 1. Arch. Gesamte Virusforsch. 31:390-392. [DOI] [PubMed] [Google Scholar]
- 24.Lusby, E., K. H. Fife, and K. I. Berns. 1980. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J. Virol. 34:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayor, H. D., and J. L. Melnick. 1966. Small deoxyribonucleic acid-containing viruses (picodnavirus group). Nature 210:331-332. [DOI] [PubMed] [Google Scholar]
- 26.McCarty, D. M., M. Christensen, and N. Muzyczka. 1991. Sequences required for coordinate induction of adeno-associated virus p19 and p40 promoters by Rep protein. J. Virol. 65:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty, D. M., D. J. Pereira, I. Zolotukhin, X. Zhou, J. H. Ryan, and N. Muzyczka. 1994. Identification of linear DNA sequences that specifically bind the adeno-associated virus Rep protein. J. Virol. 68:4988-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarty, D. M., S. M. Young, Jr., and R. J. Samulski. 2004. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 38:819-845.:819-845. [DOI] [PubMed] [Google Scholar]
- 29.Muzyczka, N., and K. I. Berns. 2001. Parvoviridae: the viruses and their replication, p. 2327-2359. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 30.Myrup, A. C., S. B. Mohanty, and F. M. Hetrick. 1976. Isolation and characterization of adeno-associated viruses from bovine adenovirus types 1 and 2. Am. J. Vet. Res. 37:907-910. [PubMed] [Google Scholar]
- 31.Naeger, L. K., J. Cater, and D. J. Pintel. 1990. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J. Virol. 64:6166-6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naeger, L. K., R. V. Schoborg, Q. Zhao, G. E. Tullis, and D. J. Pintel. 1992. Nonsense mutations inhibit splicing of MVM RNA in cis when they interrupt the reading frame of either exon of the final spliced product. Genes Dev. 6:1107-1119. [DOI] [PubMed] [Google Scholar]
- 33.Pereira, D. J., D. M. McCarty, and N. Muzyczka. 1997. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J. Virol. 71:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira, D. J., and N. Muzyczka. 1997. The adeno-associated virus type 2 p40 promoter requires a proximal Sp1 interaction and a p19 CArG-like element to facilitate Rep transactivation. J. Virol. 71:4300-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pintel, D., D. Dadachanji, C. R. Astell, and D. C. Ward. 1983. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 11:1019-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu, J., F. Cheng, L. R. Burger, and D. Pintel. 2006. The transcription profile of Aleutian mink disease virus in CRFK cells is generated by alternative processing of pre-mRNAs produced from a single promoter. J. Virol. 80:654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu, J., F. Cheng, Y. Yoto, Z. Zadori, and D. Pintel. 2005. The expression strategy of goose parvovirus exhibits features of both the Dependovirus and Parvovirus genera. J. Virol. 79:11035-11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu, J., R. Nayak, and D. J. Pintel. 2004. Alternative polyadenylation of adeno-associated virus type 5 RNA within an internal intron is governed by both a downstream element within the intron 3′ splice acceptor and an element upstream of the P41 initiation site. J. Virol. 78:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu, J., R. Nayak, G. E. Tullis, and D. J. Pintel. 2002. Characterization of the transcription profile of adeno-associated virus type 5 reveals a number of unique features compared to previously characterized adeno-associated viruses. J. Virol. 76:12435-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu, J., and D. J. Pintel. 2002. The adeno-associated virus type 2 Rep protein regulates RNA processing via interaction with the transcription template. Mol. Cell. Biol. 22:3639-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu, J., and D. J. Pintel. 2004. Alternative polyadenylation of adeno-associated virus type 5 RNA within an internal intron is governed by the distance between the promoter and the intron and is inhibited by U1 small nuclear RNP binding to the intervening donor. J. Biol. Chem. 279:14889-14898. [DOI] [PubMed] [Google Scholar]
- 42.Qiu, J., Y. Yoto, G. E. Tullis, and D. J. Pintel. 2006. Parvovirus RNA processing strategies, p. 253-273. In M. E. Bloom (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 43.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samulski, R. J., L. S. Chang, and T. Shenk. 1989. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J. Virol. 63:3822-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 78:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnepp, B. C., R. L. Jensen, C. L. Chen, P. R. Johnson, and K. R. Clark. 2005. Characterization of adeno-associated virus genomes isolated from human tissues. J. Virol. 79:14793-14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoborg, R. V., and D. J. Pintel. 1991. Accumulation of MVM gene products is differentially regulated by transcription initiation, RNA processing and protein stability. Virology 181:22-34. [DOI] [PubMed] [Google Scholar]
- 48.Siegl, G., R. C. Bates, K. I. Berns, B. J. Carter, D. C. Kelly, E. Kurstak, and P. Tattersall. 1985. Characteristics and taxonomy of Parvoviridae. Intervirology 23:61-73. [DOI] [PubMed] [Google Scholar]
- 49.Trempe, J. P., and B. J. Carter. 1988. Alternate mRNA splicing is required for synthesis of adeno-associated virus VP1 capsid protein. J. Virol. 62:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trempe, J. P., and B. J. Carter. 1988. Regulation of adeno-associated virus gene expression in 293 cells: control of mRNA abundance and translation. J. Virol. 62:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weitzman, M. D., S. R. Kyostio, R. M. Kotin, and R. A. Owens. 1994. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc. Natl. Acad. Sci. USA 91:5808-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wistuba, A., S. Weger, A. Kern, and J. A. Kleinschmidt. 1995. Intermediates of adeno-associated virus type 2 assembly: identification of soluble complexes containing Rep and Cap proteins. J. Virol. 69:5311-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yates, V. J., A. M. el-Mishad, K. J. McCormick, and J. J. Trentin. 1973. Isolation and characterization of an Avian adenovirus-associated virus. Infect. Immun. 7:973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]