Human noroviruses are the major cause of nonbacterial, epidemic gastroenteritis worldwide (19, 23, 33, 46) and cause significant numbers of endemic cases, as well. One study from 1999 estimated that, in the United States alone, human noroviruses cause 23 million cases of gastroenteritis and 50,000 hospitalizations per year (49). Norovirus outbreaks involve people of all ages and often occur in crowded locations, such as cruise ships, aircraft carriers, nursing homes, hospitals, schools, and restaurants (23). Noroviruses are classified as class B biological agents due to their high infectivity and stability and to the suddenness of outbreaks and the debilitating nature of the disease. Despite the significant economic impact and considerable morbidity caused by human noroviruses, no drug or vaccine is currently available to treat or prevent human norovirus disease. In addition, many aspects of norovirus biology are not well understood. This is due in large part to the absence of a cell culture system or small animal model for human noroviruses (16, 23).
Although most noroviruses have been associated with gastrointestinal disease in humans, noroviruses of cattle, swine and mice have also been identified (36, 44, 70). Of these potential experimental models, the murine norovirus (MNV) is the only norovirus that replicates in cell culture and in a small animal (36, 75). Moreover, laboratory mice are a versatile and relatively inexpensive model for the analysis of viral pathogenesis. A recent analysis of a large number of mouse serum samples from research colonies in the United States and Canada identified MNV-1 reactive antibodies in 22.1% of serum samples (30). In addition, over 35 new isolates of MNV have been found in research colonies (GenBank accession no. DQ223041 to DQ223043 and DQ269192 to DQ269205; unpublished observations). Therefore, MNV is one of the most prevalent pathogens in research mice today. Independent of its value as a potential model for norovirus infection, the impact of MNV infection on biomedical research, which is highly dependent on mice as experimental models, may be of great significance.
The MNV model system provides the first opportunity to understand the relationship between basic mechanisms of norovirus replication in tissue culture and pathogenesis in a natural host. The mouse model also provides an opportunity to use defined mutations in host genes to identify molecular components required for norovirus infection and for the host response to norovirus infection. To date, the MNV model system has revealed a tropism for cells of the hematopoietic lineage, specifically, macrophages and dendritic cells. In addition, in vivo studies using the MNV model system demonstrate a fundamental role for innate immune responses in the control of norovirus infection and an important role for adaptive immune responses in the clearance of norovirus infection from the intestine and other tissues.
This review summarizes what is currently known about MNV, highlights parallels that may exist between murine and human noroviruses, and concludes by comparing the strengths and weaknesses of several model systems for human norovirus infection. Although the MNV model may never recapitulate all aspects of human norovirus infection, we hope that this review will provide the impetus for others to use the MNV system to gain insight into many aspects of norovirus biology and pathogenesis that cannot be explored without a cell culture system or a genetically manipulable host. Over time, the relevance of such studies to understanding human norovirus infection will become apparent.
DISCOVERY OF MURINE NOROVIRUS 1
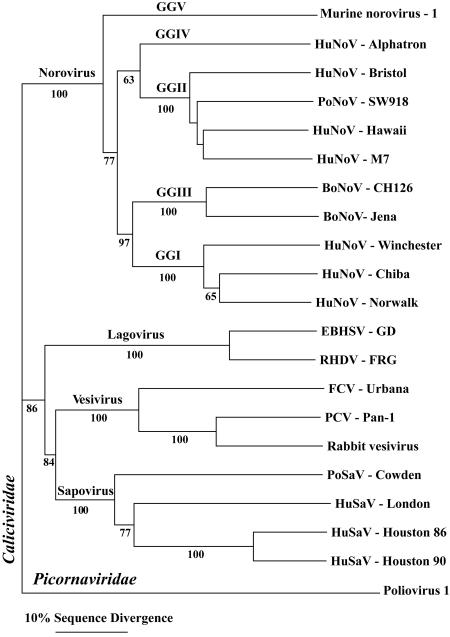
The first norovirus to infect mice, MNV-1, was described in 2003 (36). Severely immunocompromised mice lacking the recombination-activating gene 2 (RAG2) and signal transducer and activator of transcription 1 (STAT-1) (RAG2/STAT1−/− mice) sporadically succumbed to a systemic disease that could be serially passaged by intracerebral (i.c.) inoculation. Subsequently, this agent was shown to experimentally infect wild-type and immunocompromised mice after peroral (p.o.) and intranasal (i.n.) inoculation (see below) and to naturally infect a significant proportion of laboratory mice nationwide (30). Therefore, the initial isolation of MNV from brain tissue is not a reflection of its intrinsic biology. No known human or mouse pathogen could be isolated from the brain of i.c.-inoculated RAG2/STAT1−/− mice by standard diagnostic laboratory practices. Significantly, the unknown pathogen had features that are characteristic of most viruses: the pathogen could be filtered through a 0.22-μm filter and was interferon (IFN) sensitive. Mice lacking both the alpha/beta interferon (IFN-α/β) and the IFN-γ receptors (IFN-αβγR−/− mice), but not wild-type mice, were highly susceptible to the pathogen. To identify the unknown pathogen, representational difference analysis was utilized, and sequences that were similar but not identical to those of previously sequenced noroviruses were obtained. Since the cloned sequences obtained by representational difference analysis aligned along norovirus genomes, a combination of rapid amplification of cDNA ends and PCR was used to clone and sequence the entire 7,382-bp polyadenylated MNV-1 genome (36). The consensus sequence of the MNV-1 brain homogenate from IFN-αβγR−/− mice was recently confirmed and updated based on sequence analysis of PCR amplicons (GenBank accession no. AY228235). Phylogenetic analysis of the viral capsid protein (Fig. 1) and viral genome clearly demonstrates that MNV-1 is a previously unknown norovirus (36).
FIG. 1.
Phylogenic analysis of the Caliciviridae. Maximum parsimony analysis was performed using the PAUP* program on an alignment of the capsid protein sequence of MNV-1 with capsid protein sequences of representative members of the four genera of Caliciviridae. The numbers below the branches indicate bootstrap values exceeding 50%. Neighbor-joining analysis uncovered a topologically identical tree with very similar bootstrap values (data not shown). Capsid protein sequences were derived from murine norovirus 1 (GenBank accession no. AY228235), human norovirus (HuNoV) Alphatron (AF195847), HuNoV Bristol (X76716), porcine norovirus (PoNoV) SW918 (AB074893), HuNoV Hawaii (U07611), HuNoV M7 (AY130761), bovine norovirus (BoNoV) CH126 (AF320625), BoNoV Jena (AJ011099), HuNoV Winchester (AJ277609), HuNoV Chiba (AB042808), HuNoV Norwalk (M87661), European brown hare syndrome virus (EBHSV; Z69620), rabbit hemorrhagic disease virus (RHDV; M67473), feline calicivirus (FCV; L40021), primate calicivirus (PCV; AF091736), rabbit vesivirus (AJ866991), porcine sapovirus (PoSaV) Cowden (AF182760), human sapovirus (HuSaV) London (U95645), HuSaV Houston 86 (U95643), HuSaV Houston 90 (U95644), and VP1 of poliovirus 1 Mahoney (V01149).
CHARACTERISTICS OF MNV
The most important characteristics of MNV for the analysis of the biology and pathogenesis of noroviruses are the biological and molecular properties that MNV shares with other noroviruses and with caliciviruses in general. While MNV-1 was originally isolated by serial passage from the brain of severely immunocompromised mice after i.c. inoculation (36), it is now clear that this virus is an effective enteric pathogen. MNV-1 is virulent in immunocompromised mice after p.o. and i.n. inoculation (36) and infects wild-type mice after p.o. inoculation (see below). In addition, persistently infected RAG−/− mice (see below) shed infectious MNV-1 that can be transmitted from mouse to mouse in a cage or from contaminated bedding to uninfected mice (unpublished observations). Wild-type mice housed with persistently infected RAG−/− mice or on contaminated bedding from infected RAG−/− mice develop MNV-1-reactive antibodies within 3 to 4 weeks of exposure. Most significantly, many new MNV strains have been isolated from the feces or diarrhea of laboratory mice (GenBank accession no. DQ269192 to DQ269205; unpublished observations). The relationship between gastrointestinal disease in mice and infection with these new MNV isolates is unknown. These studies indicate that MNV shares with its human counterparts the capacity to spread via the fecal-oral and, possibly, respiratory routes.
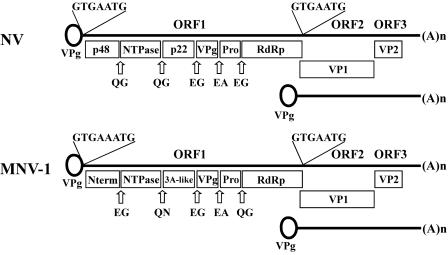
From a molecular point of view, MNV-1 shares many biochemical and genetic features with human noroviruses. MNV-1 has the size (28 to 35 nm in diameter), shape (icosahedral), and buoyant density (1.36 ± 0.04 g/cm3) characteristic of human noroviruses (23, 36). In addition, analysis of the MNV-1 genome identifies the three open reading frames (ORF) characteristic of noroviruses and vesiviruses, two genera within the Caliciviridae (Fig. 2). ORF1 of MNV-1 encodes a predicted 187.5-kDa polyprotein containing the 2C helicase, 3C protease, and 3D polymerase motifs found in other caliciviruses and picornaviruses. ORF2 of MNV-1 encodes a 58.9-kDa capsid protein that can self-assemble into virus-like particles when expressed in a baculovirus expression system, similar to other caliciviruses (36). ORF3 of MNV-1 encodes a putative 22.1-kDa basic protein.
FIG. 2.
Genome organization of NV and MNV-1. Cleavage sites in NV and MNV-1 are indicated by open arrows, and the amino acids surrounding the cleavage site are also shown (4, 43, 45; Sosnovtsev et al., unpublished). The subgenomic RNA is shown below the genomic RNA. The presence of a viral protein linked to genomic and subgenomic RNA (VPg) is predicted, but not proven, for noroviruses.
Proteolytic processing of the ORF1 polyprotein into some of the MNV-1 nonstructural proteins can be visualized in MNV-1-infected RAW 264.7 cells (75). Although proteolytic processing sites have been mapped in the ORF1 polyprotein of several human noroviruses using in vitro expression systems (3, 43, 45, 65, 67), it has not been possible to verify the utilization of these cleavage sites during authentic virus infection. A recent study of MNV-1 demonstrated that proteolytic processing of the ORF1 polyprotein (Fig. 2) in a cell-free translation system correlated closely with that observed for virus-infected cells (S. V. Sosnovtsev, G. Belliot, K. O. Chang, V. G. Prikhodko, L. B. Thackray, C. E. Wobus, S. M. Karst, H. W. Virgin, and K. Y. Green, unpublished data). Additional studies using MNV will permit further elucidation of the role of viral and host proteins during authentic norovirus replication.
The analysis of norovirus replication is in its infancy. However, the conserved molecular features of murine and human norovirus genomes suggest that the study of MNV may define mechanisms of norovirus replication. Two aspects of norovirus replication that have been examined thus far using MNV-1 are the expression of a subgenomic RNA and the rearrangement of intracellular membranes during viral replication. Until recently, a subgenomic RNA expressed during viral replication had been demonstrated only for animal caliciviruses that can be propagated in tissue culture (6, 17, 18, 28, 50, 62). For these viruses, synthesis of the capsid protein is initiated from the subgenomic RNA and involves sequences conserved at the 5′ end of the genomic and subgenomic RNA. For noroviruses, the conservation of nucleotides at the 5′ end of the genome and a region just upstream of ORF2 suggested that the capsid (ORF2) and basic protein (ORF3) might also be expressed from a subgenomic RNA (Fig. 2). Recently, three studies have confirmed that a subgenomic RNA is expressed during norovirus replication. A Northern blot analysis of RNA isolated from MNV-1-infected cells shows increasing amounts of a subgenomic RNA over time (75). The same subgenomic RNA species are also observed with MNV-1-infected wild-type and STAT1−/− macrophages (unpublished observations). Furthermore, a subgenomic-length RNA is detected after transfection of cells with a cDNA clone of Norwalk virus (NV) or U201, both human noroviruses (2, 37). The presence of a subgenomic RNA species during murine and human norovirus replication suggests that noroviruses, like some other RNA viruses, regulate synthesis of structural proteins by using an abundant subgenomic message.
The second aspect of norovirus replication examined using MNV-1 implicates intracellular membranes in norovirus replication. A dramatic reorganization of intracellular membranes has been observed during infection with feline calicivirus (FCV), a vesivirus (47, 69). However, it was not known whether a similar rearrangement of intracellular membranes occurs during norovirus infection. Analysis of MNV-1-infected cells by electron microscopy shows striking changes in morphology, including an extensive reorganization of intracellular membranes and the loss of an intact Golgi apparatus (75). Increasing amounts of viral particles are observed near rearranged individual or confronting intracellular membranes that ultimately occupy most of the cytoplasm. These observations are consistent with the idea that noroviruses, like other positive-strand RNA viruses, replicate in association with intracellular membranes. However, additional studies using MNV are needed to conclusively demonstrate a requirement for these membranes during norovirus replication.
It is likely that many fundamental mechanisms of replication will be conserved between murine and human noroviruses. Importantly, the recent development of a replication system for human noroviruses (2, 37) will permit comparisons of authentic murine and human norovirus replication and should allow the identification of conserved mechanisms. The physiological relevance of norovirus-specific replication mechanisms may then be determined in vivo using the MNV system.
MNV INFECTION OF WILD-TYPE MICE
Although MNV-1 was initially isolated from severely immunocompromised mice, subsequent studies demonstrate that this virus also infects wild-type mice. A robust seroconversion to the capsid protein is seen in inbred 129 and outbred CD1 mice inoculated p.o. or i.n. with MNV-1 (30, 36). More importantly, adult (8-week-old) 129 mice have detectable levels of viral RNA in the liver, spleen, and proximal intestine (but not in the lung, brain, blood, or feces) 1 day after p.o. inoculation with 3 × 104 PFU of MNV-1 brain homogenate (36). While MNV-1 RNA is not detected in the liver, spleen, or proximal intestine of adult 129 mice 3 days after inoculation, viral RNA is detected in the feces at 7 days and in the mesenteric lymph node, spleen, and jejunum at 5 weeks in a subset of juvenile (4-week-old) CD1 mice after inoculation with 1 × 107 PFU of plaque-purified MNV-1 (30, 36). Even more striking, MNV RNA is detected in the mesenteric lymph node, spleen, jejunum, and feces of juvenile CD1 mice for at least 8 weeks after inoculation with 1 × 106 PFU of three new murine norovirus isolates, MNV2, MNV3, and MNV4 (C. C. Hsu, L. K. Riley, H. M. Wills, and R. S. Livingston, submitted for publication). It is an intriguing possibility that MNV and other noroviruses may continually replicate in a subset of apparently wild-type hosts, potentially providing a reservoir for norovirus epidemics. Although continuous replication has not been documented for human noroviruses, long-term replication is commonly observed for FCV (56, 72). The possibility that MNV persistently infects the lymphoid system is intriguing, given the capacity of this virus to productively infect macrophages and dendritic cells (see below).
The characteristic clinical manifestations of human norovirus infection are projectile vomiting and/or explosive, watery diarrhea, as well as low-grade fever, malaise, nausea, and abdominal cramping or pain (23). In addition, a certain proportion of human norovirus infections are asymptomatic (22, 60). A murine model cannot recapitulate norovirus-induced vomiting, since mice lack an emetic reflex. However, other symptoms of human norovirus infection can be measured. To date, adult 129 and juvenile CD1 mice do not exhibit any clinical symptoms when inoculated with MNV-1 (30, 36). In addition, juvenile CD1 mice do not develop any clinical symptoms when inoculated with three new strains of MNV, namely, MNV2, MNV3, and MNV4 (Hsu et al., submitted). However, these studies have examined only a few murine norovirus isolates and have focused on a limited number of mouse strains. Given the diversity of host responses observed for different mouse strains and the ongoing characterization of new MNV isolates, we have only begun to understand the pathogenic potential of MNV in laboratory mice. Interestingly, only six amino acids differ between wild-type porcine enteric calicivirus-Cowden (PEC-Cowden), a sapovirus, and tissue culture-adapted PEC-Cowden (24). However, wild-type PEC-Cowden causes diarrhea in gnotobiotic pigs, while tissue culture-adapted PEC-Cowden does not (25). In light of these data, a more thorough investigation using different mouse and virus strains, infection routes, and virus doses is clearly needed to determine the presence or absence of MNV-induced disease in wild-type hosts.
INNATE IMMUNITY IS REQUIRED TO CONTROL NOROVIRUS INFECTION
The discovery of a norovirus that infects a genetically defined host provides a unique opportunity to identify molecular components required for resistance to norovirus infection. MNV-1 causes systemic and lethal disease in STAT1−/− mice, unlike wild-type mice, by all routes analyzed (p.o., i.n., i.c., intraperitoneal, and footpad) (36; unpublished observations). Susceptible STAT1−/− mice have high levels of MNV-1 RNA in all organs examined (lung, liver, spleen, proximal intestine, and brain) and in blood and feces 1, 3, and 7 days after p.o. inoculation. Although the exact cause of death of MNV-1-infected STAT1−/− mice is not known, these mice have histopathological signs of pneumonia and loss of splenic architecture 3 days after p.o. inoculation and severe liver inflammation by day 7 (36). Of note, histopathological signs of encephalitis have only been seen in the brain of RAG/STAT−/− mice after direct i.c. inoculation with MNV-1 (36). The importance of STAT-1 in controlling MNV-1 infection in vivo is mirrored in tissue culture, where MNV-1 replicates to higher levels in STAT1−/− macrophages and dendritic cells than their wild-type counterparts (75). These studies demonstrate that STAT1-dependent responses limit norovirus replication in the cell. This cell-intrinsic antiviral function of STAT-1 likely plays a significant role in the susceptibility of STAT1−/− mice to MNV infection and disease.
STAT-1 is one of the primary mediators of both type I and type II IFN responses (55, 58). In vitro, MNV-1 replication in IFN-αβR−/− macrophages is comparable to the high-level replication present in STAT1−/− macrophages, while MNV-1 replication in IFN-γR−/− macrophages is similar to replication in wild-type counterparts (75). The latter is not surprising, since IFN-γ is unlikely to be generated in cell cultures that lack T and NK cells. These data demonstrate a role for STAT-1 and type I IFN in limiting MNV-1 replication in vitro. Initial studies in vivo demonstrate that, like STAT1−/− mice, IFN-αβγR−/− mice are highly susceptible to lethal MNV-1 infection (36). Further studies are needed to define the individual roles of IFN-αβ and IFN-γ during MNV-1 infection in vivo. This is especially true in light of the fact that the initial animal studies analyzed only lethality (36) and, therefore, more subtle contributions of IFN-αβ and IFN-γ in controlling MNV infection remain undefined.
The most interesting future direction for these studies will be to identify the host factors responsible for the impressive effects of STAT-1 and IFN on MNV-1 replication and pathogenesis. Two well-known mediators of IFN-inducible antiviral pathways are protein kinase RNA activated (PKR) and inducible nitric oxide synthase (iNOS). Significant mortality is not seen in PKR−/− or iNOS−/− mice after inoculation with MNV-1 (36). In addition, MNV-1 replication in PKR−/− and iNOS−/− macrophages is similar to replication in wild-type macrophages (75). While more subtle contributions of PKR and iNOS to MNV-1 resistance were not addressed in these initial studies, the combination of in vitro and in vivo data suggest that other mediators of IFN- and STAT1-dependent antiviral activity are required to control MNV-1 replication and disease.
The role of innate immunity during MNV infection suggests an explanation for the short clinical course of human norovirus disease. The characteristic rapid onset (∼1 day) and resolution (∼2 days) of human norovirus disease (23) occurs before one might plausibly invoke a role for adaptive immunity in control of norovirus-induced disease. Studies of MNV suggest that innate immunity, specifically IFN- or STAT1-dependent immune responses, may be responsible for the rapid control of human norovirus disease. Furthermore, the induction of a systemic disease in immunocompromised mice after mucosal inoculation of MNV-1 suggests that humans with deficient innate immune responses may experience a more severe and disseminated form of norovirus disease with unknown clinical consequences. Interestingly, the susceptibility of humans to virus infections can differ based on allelic variations in genetic composition (8, 9). One striking example is how susceptibility to human norovirus infection is determined by allelic variation in human histo-blood group antigens (HBGA) and secretor status (31, 32, 34, 41, 42, 61). In light of the role of innate immunity during murine norovirus infection, it would be interesting to examine whether the penetrance, severity, and persistence of human norovirus infection are influenced by allelic variations in innate immunity.
ADAPTIVE IMMUNITY IS REQUIRED TO CLEAR NOROVIRUS INFECTION
Studies of MNV have led to several important findings regarding the role of the adaptive immune response in viral shedding and clearance. Like wild-type mice, RAG1−/− and RAG2−/− mice are resistant to MNV-1-induced lethality (36). These studies show that the adaptive immune response, in striking contrast with the STAT1-dependent innate immune response, is not required for protection against lethal MNV infection. However, unlike wild-type mice, RAG−/− mice have high levels of viral RNA in all organs analyzed (lung, liver, spleen, proximal intestine, brain) and in blood and feces 90 days after p.o. inoculation (36). In unpublished studies, we have found that the high levels of viral RNA correlate with the presence of infectious MNV-1 in multiple tissues of RAG−/− mice. The continuous replication of MNV-1 in RAG−/− mice, in contrast to the rapid clearance of MNV-1 from wild-type mice, demonstrates that components of the adaptive immune response, specifically B and/or T cells, are required to contain and clear MNV-1 infection. These studies also show that MNV-1 is capable of continuously replicating in tissues for long periods of time without inducing overt disease or lethality, a property that might contribute to persistent norovirus infection in wild-type hosts.
The role of adaptive immunity in preventing the dissemination and continuous replication of MNV may have important implications for human norovirus infection. Although human norovirus disease has a rapid onset and resolution, human noroviruses can be shed for up to 3 weeks after infection (23, 60). Studies of MNV suggest that adaptive immunity, mediated by B and/or T cells, may be responsible for clearance of human norovirus infection. In addition, studies using MNV suggest that humans with compromised adaptive immune responses may experience disseminated, long-term norovirus infection. While human norovirus infection outside the gastrointestinal tract has not been documented, several cases of prolonged norovirus shedding (4 months to >2 years) have been described in transplant patients with compromised adaptive immune systems (21, 38, 39, 51, 53).
While studies of MNV-1 infection in RAG−/− mice identified a role for B and/or T cells in viral control and clearance, future studies are needed to address the relative importance of B and T cells and their mechanisms of action. Initial studies indicate that one mechanism may be through the generation of a neutralizing antibody response, which is at least partially protective in vivo. Wild-type mice infected with MNV-1 generate antibodies that neutralize MNV-1 (75; unpublished observations). Polyclonal serum from MNV-1-inoculated wild-type mice and monoclonal antibodies derived from the spleen of an MNV-1-inoculated mouse inhibit MNV-1 replication and plaque formation (75). In addition, the MNV-1-reactive polyclonal serum is able to delay MNV-1-induced lethality in RAG2/STAT1−/− mice after intraperitoneal transfer (unpublished observations). While a neutralizing antibody response to human norovirus infection cannot be directly measured in the absence of a tissue culture system, blockade of human norovirus binding to their respective HBGA has been developed as a surrogate assay to detect potential neutralizing antibodies (27, 59). These data suggest an important role for antibody in prevention of norovirus disease, but more detailed studies are clearly needed to determine immune mechanisms. This area of research promises to be one for which the MNV system is particularly well suited, given the wealth of immunologic reagents and mice with defined mutations in immune system components that are currently available.
MNV HAS A TROPISM FOR MACROPHAGES AND DENDRITIC CELLS
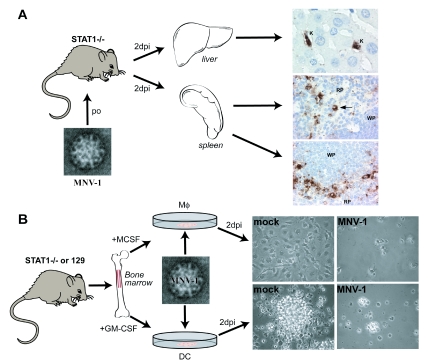
While the tropism of several animal caliciviruses has been investigated in vitro and in vivo (13, 20, 40, 48, 54), studies to define the tropism of noroviruses during natural infection are just beginning. Immunohistochemical staining of tissue sections from STAT1−/− mice 2 days after p.o. inoculation with MNV-1 brain homogenate shows virus-specific staining in cells of the liver and spleen (Fig. 3A). In the liver, resident macrophages or Kupffer cells are stained with anti-MNV-1 polyclonal antiserum; in the spleen, cells with macrophage-like morphology are stained in the red pulp and marginal zone, and cells of dendritic cell-like morphology are stained in the white pulp (75). These data suggest that MNV-1 infects macrophages and dendritic cells in vivo. Additional studies using cell lineage-specific markers and a more comprehensive survey of host tissues in both wild-type and immunocompromised mice are needed to confirm the site of MNV replication in vivo.
FIG. 3.
Identification of MNV cellular and tissue tropism. (A) MNV has a tropism for cells of macrophage and dendritic cell-like morphology in vivo. Immunohistochemistry was performed on sections from liver and spleen 2 days postinoculation (2dpi) with a peroral (po) dose of MNV-1 (75). Resident macrophages of the liver or Kupffer cells (K) and cells in the spleen consistent with macrophage (indicated by an arrow) and dendritic cellmorphology and localization stain with an anti-MNV-1 antibody. RP, red pulp; WP, white pulp. (B) MNV has a tropism for macrophages and dendritic cells in vitro. Bone marrow precursors were cultured in macrophage colony stimulating factor (+MCSF) or granulocyte macrophage colony stimulating factor (+GM-CSF) to generate macrophages (Mφ) and dendritic cells (DC), respectively (75). Monolayers of STAT1−/− macrophages or dendritic cells show cytopathic effects 2 days postinoculation (2dpi) with MNV-1.
In vitro, MNV-1 replicates efficiently in cultured bone marrow-derived murine macrophages and dendritic cells (Fig. 3B) but not in murine embryonic fibroblasts, murine hepatocyte, or neuroblastoma cell lines, mouse embryonic stem (ES) cells, or ES cell-derived embryoid bodies (75; unpublished observations). MNV-1 also replicates robustly in the murine macrophage cell line RAW 264.7, leading to the development of a plaque assay and the first tissue culture system for a norovirus (75). Furthermore, all murine macrophage and dendritic cell lines tested (RAW 264.7, IC21, J774A.1, P388D1, WBC264-9C, and JAWSII) support MNV-1 replication (75; unpublished observations). These studies clearly demonstrate that MNV-1 has a tropism for cells of the hematopoietic lineage, including macrophages and dendritic cells. These findings clearly warrant more detailed studies to examine the role of macrophages and dendritic cells in MNV infection in vivo.
The unexpected tropism of MNV-1 for macrophages and dendritic cells may have important implications for understanding norovirus infection and disease. Human noroviruses are thought to have an enteric tropism limited to the upper intestinal tract, since intestinal biopsies from volunteers challenged with NV or Hawaii virus show villous broadening and blunting in the jejunum and an infiltration of mononuclear cells (1, 14, 63, 64). Similar pathology is seen in biopsies from pediatric transplant patients with high-volume diarrhea associated with human norovirus infection (38, 51). However, the site of human norovirus replication in vivo has not been demonstrated. Interestingly, recent data demonstrated that a subset of dendritic cells can form transepithelial dendrites capable of directly sampling the intestinal lumen (52). Infection of transepithelial dendritic cells in the lumen of the intestine may provide a potential route of infection for noroviruses. Therefore, the tropism of human noroviruses in vivo should be reconsidered in light of the data from MNV-1.
While the capacity to culture MNV provides an important tool, it would be valuable to have a human cell line or primary cell that is permissive for human norovirus replication. While recombinant NV virus-like particles are able to specifically bind to multiple cell lines, including human intestinal cells (Caco-2 cells), only a small proportion of particles are internalized into the cell (74). However, Caco-2 cells, as well as many other epithelial and fibroblast cell lines, are not susceptible to human norovirus infection (16). Data from the MNV system indicate that efforts to develop a culture system for human noroviruses should be revisited, with an emphasis on attempting to grow the virus in macrophages and dendritic cells and in cells lacking IFN responses or STAT-1. Although the role of STAT-1 in human norovirus infection has not been investigated, the down-regulation of IFN-αβ- and IFN-γ-mediated STAT-1 activation correlates with the susceptibility of cultured cells to infection with PEC (12).
CLOSING COMMENTS
Until the discovery of MNV, in 2003, understanding of norovirus biology and pathogenesis was hampered by the lack of a cell culture system and a small animal model (16, 23). Despite this lack, significant advances have been made in understanding basic mechanisms of norovirus infection and pathogenesis, including the determination of the first sequence of a norovirus, the crystal structure of a norovirus capsid, and the role of HBGA and secretor status in susceptibility to human norovirus infection (34, 35, 42, 57). In addition, many advances in understanding mechanisms of calicivirus replication have come from studies using related animal caliciviruses, such as FCV (23). The recently developed replication system for human noroviruses (2, 37) may now be used to directly compare norovirus and calicivirus replication at the cellular level.
Analysis of mechanisms of pathogenesis requires the integrated use of multiple techniques, including molecular virology, tissue culture, and in vivo studies. The well-established tissue culture and reverse genetics systems for FCV has yielded a wealth of information about calicivirus replication in vitro (23), while the PEC model system of diarrhea has permitted detailed histological analysis of the small intestine during calicivirus infection in vivo (Table 1). However, neither FCV nor PEC is a norovirus (Fig. 1). Models for human norovirus infection using bovine or porcine noroviruses (Fig. 1) are currently in development; however, studies of these viruses are limited by the absence of a tissue culture system (Table 1). In addition, most animal models for human norovirus infection are unable to identify molecular components required for norovirus infection or the host response to norovirus infection, due to the difficulty in genetically manipulating bovine, porcine, and feline hosts (Table 1).
TABLE 1.
Comparison of several model systems for human norovirus infection
| Parameter | Characteristics of indicated model system
|
|||||
|---|---|---|---|---|---|---|
| Human norovirus | Murine norovirus | Porcine norovirus | Bovine norovirus | Feline calicivirus | Porcine enteric calicivirus | |
| Genus | Norovirus | Norovirus | Norovirus | Norovirus | Vesivirus | Sapovirus |
| Genomic organizationa | 3 ORF (35) | 3 ORF (36) | Unknown | 3 ORF (44) | 3 ORF (7) | 2 ORF (24) |
| Growth in tissue culture | No (16) | Yes (75) | No | No | Yes (15) | Yes (10, 12) |
| Reverse genetics | Yes (2, 37) | No | No | No | Yes (68) | Yes (11) |
| Symptoms in wild-type host | Diarrhea, vomiting, nausea, asymptomatic (22, 23, 60) | Asymptomatic (36) | Asymptomatic (71) | Diarrhea (5) | Upper respiratory disease, systemic disease (29, 54) | Diarrhea (25) |
| Histological changes in intestine | Villous blunting (1, 14, 63, 64) | Unknown | Unknown | Villous blunting (26) | None | Villous blunting (25) |
| Detection of viral antigen in lesions | No | Yes, in macrophages and dendritic cells of STAT1−/− mice (75) | Unknown | Yes, in villous enterocytes (26) | Yes, in alveolar macrophages, epithelial cells of oral ulcers, tonsils (29, 54) | Yes, in villous enterocytes (25) |
| Infectious route | Fecal-oral, respiratory (22) | Fecal-oral, respiratory (36, 75) | Unknown | Oral (5, 26) | Respiratory (29) | Oral (25) |
| Virus shed in feces | Yes (22) | Yes (36) | Detected in cecum contents (71) | Yes (5) | No | Yes (25) |
| Cost per unit | NAb | Low | High | High | Intermediate | High |
| Genetic manipulation of host | NA | Inbred strains, knockout mice, transgenic mice | Limited (73) | Limited (66) | Not available | Limited (73) |
Number of ORFs.
NA, not applicable.
Therefore, we believe that, while advances in many of these model systems are expected in the future, the identification of the first norovirus that infects a genetically manipulable host and the development of the first tissue culture system for a norovirus (Table 1) provide a major opportunity to uncover new aspects of norovirus biology and pathogenesis in vitro and in vivo. As discussed in this review, the MNV model system has already provided new insight into how noroviruses grow and interact with their host and has generated questions regarding the relationship between noroviruses and cells of the hematopoietic lineage and the role of innate immunity in the control of norovirus infection. It is our hope that many investigators will begin to utilize the MNV model system, giving rise to an interactive and fast-moving field, to provide a better understanding of the biology and pathogenesis of an important cause of human disease.
Acknowledgments
C.E.W. was supported by NIH grant U54 AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research. L.B.T. was supported by NIH training grant AI007163. Work on MNV in the laboratory of H.W.V. was funded by grants RO1 AI054483 and RO1 AI065982.
Technical assistance was received from the histopathology research core and genome center at Washington University. Phylogenetic analysis was performed by Scott Kelley at San Diego State University.
We thank Kim Green and Stanislav Sosnovtsev at the NIH and members of the Virgin laboratory for critical readings of the manuscript and helpful discussions.
C.E.W. and L.B.T. contributed equally to this work.
REFERENCES
- 1.Agus, S. G., R. Dolin, R. G. Wyatt, A. J. Tousimis, and R. S. Northrup. 1973. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann. Intern. Med. 79:18-25. [DOI] [PubMed] [Google Scholar]
- 2.Asanaka, M., R. L. Atmar, V. Ruvolo, S. E. Crawford, F. H. Neill, and M. K. Estes. 2005. Replication and packaging of Norwalk virus RNA in cultured mammalian cells. Proc. Natl. Acad. Sci. USA 102:10327-10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belliot, G., S. V. Sosnovtsev, T. Mitra, C. Hammer, M. Garfield, and K. Y. Green. 2003. In vitro proteolytic processing of the MD145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J. Virol. 77:10957-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blakeney, S. J., A. Cahill, and P. A. Reilly. 2003. Processing of Norwalk virus nonstructural proteins by a 3C-like cysteine proteinase. Virology 308:216-224. [DOI] [PubMed] [Google Scholar]
- 5.Bridger, J. C., G. A. Hall, and J. F. Brown. 1984. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 43:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burroughs, J. N., and F. Brown. 1978. Presence of a covalently linked protein on calicivirus RNA. J. Gen. Virol. 41:443-446. [DOI] [PubMed] [Google Scholar]
- 7.Carter, M. J., I. D. Milton, J. Meanger, M. Bennett, R. M. Gaskell, and P. C. Turner. 1992. The complete nucleotide-sequence of a feline calicivirus. Virology 190:443-448. [DOI] [PubMed] [Google Scholar]
- 8.Casanova, J. L., and L. Abel. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 4:55-66. [DOI] [PubMed] [Google Scholar]
- 9.Casanova, J. L., and L. Abel. 2005. Inborn errors of immunity to infection: the rule rather than the exception. J. Exp. Med. 202:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, K. O., Y. Kim, K. Y. Green, and L. J. Saif. 2002. Cell-culture propagation of porcine enteric calicivirus mediated by intestinal contents is dependent on the cyclic AMP signaling pathway. Virology 304:302-310. [DOI] [PubMed] [Google Scholar]
- 11.Chang, K. O., S. S. Sosnovtsev, G. Belliot, Q. H. Wang, L. J. Saif, and K. Y. Green. 2005. Reverse genetics system for porcine enteric calicivirus, a prototype Sapovirus in the Caliciviridae. J. Virol. 79:1409-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, K. O., S. V. Sosnovtsev, G. Belliot, Y. Kim, L. J. Saif, and K. Y. Green. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 101:8733-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke, I. N., and P. R. Lambden. 1997. Viral zoonoses and food of animal origin: caliciviruses and human disease. Arch. Virol. Suppl. 13:141-152. [DOI] [PubMed] [Google Scholar]
- 14.Dolin, R., A. G. Levy, R. G. Wyatt, T. S. Thornhill, and J. D. Gardner. 1975. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am. J. Med. 59:761-768. [DOI] [PubMed] [Google Scholar]
- 15.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 16.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85:79-87. [DOI] [PubMed] [Google Scholar]
- 17.Dunham, D. M., X. Jiang, T. Berke, A. W. Smith, and D. O. Matson. 1998. Genomic mapping of a calicivirus VPg. Arch. Virol. 143:2421-2430. [DOI] [PubMed] [Google Scholar]
- 18.Ehresmann, D. W., and F. L. Schaffer. 1979. Calicivirus intracellular RNA: fractionation of 18-22 s RNA and lack of typical 5′-methylated caps on 36 S and 22 S San Miguel sea lion virus RNAs. Virology 95:251-255. [DOI] [PubMed] [Google Scholar]
- 19.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 20.Flynn, W. T., L. J. Saif, and P. D. Moorhead. 1988. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am. J. Vet. Res. 49:819-825. [PubMed] [Google Scholar]
- 21.Gallimore, C. I., D. Lewis, C. Taylor, A. Cant, A. Gennery, and J. J. Gray. 2004. Chronic excretion of a norovirus in a child with cartilage hair hypoplasia (CHH). J. Clin. Virol. 30:196-204. [DOI] [PubMed] [Google Scholar]
- 22.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34-43. [DOI] [PubMed] [Google Scholar]
- 23.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human Caliciviruses, p. 841-874. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 24.Guo, M., K. O. Chang, M. E. Hardy, Q. Zhang, A. V. Parwani, and L. J. Saif. 1999. Molecular characterization of a porcine enteric calicivirus genetically related to Sapporo-like human caliciviruses. J. Virol. 73:9625-9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo, M., J. Hayes, K. O. Cho, A. V. Parwani, L. M. Lucas, and L. J. Saif. 2001. Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC. J. Virol. 75:9239-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall, G. A., J. C. Bridger, B. E. Brooker, K. R. Parsons, and E. Ormerod. 1984. Lesions of gnotobiotic calves experimentally infected with a calicivirus-like (Newbury) agent. Vet. Pathol. 21:208-215. [DOI] [PubMed] [Google Scholar]
- 27.Harrington, P. R., L. Lindesmith, B. Yount, C. L. Moe, and R. S. Baric. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 76:12335-12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbert, T. P., I. Brierley, and T. D. Brown. 1997. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J. Gen. Virol. 78:1033-1040. [DOI] [PubMed] [Google Scholar]
- 29.Hoover, E. A., and D. E. Kahn. 1975. Experimentally induced feline calicivirus infection: clinical signs and lesions. J. Am. Vet. Med. Assoc. 166:463-468. [PubMed] [Google Scholar]
- 30.Hsu, C. C., C. E. Wobus, E. K. Steffen, L. K. Riley, and R. S. Livingston. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 12:1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, P. W., T. Farkas, W. M. Zhong, S. Thornton, A. L. Morrow, and J. Xi. 2005. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutson, A. M., F. Airaud, J. LePendu, M. K. Estes, and R. L. Atmar. 2005. Norwalk virus infection associates with secretor status genotyped from sera. J. Med. Virol. 77:116-120. [DOI] [PubMed] [Google Scholar]
- 33.Hutson, A. M., R. L. Atmar, and M. K. Estes. 2004. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol. 12:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 35.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 36.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 37.Katayama, K., G. S. Hansman, T. Oka, S. Ogawa, and N. Takeda. 26. February 2006. Investigation of norovirus replication in a human cell line. Arch. Virol. [Epub ahead of print.] [DOI] [PubMed]
- 38.Kaufman, S. S., N. K. Chatterjee, M. E. Fuschino, M. S. Magid, R. E. Gordon, D. L. Morse, B. C. Herold, N. S. LeLeiko, A. Tschernia, S. S. Florman, G. E. Gondolesi, and T. M. Fishbein. 2003. Calicivirus enteritis in an intestinal transplant recipient. Am. J. Transplant. 3:764-768. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman, S. S., T. K. Chatterjee, T. E. Fuschino, D. L. Morse, R. A. Morotti, M. S. Magid, G. E. Gondolesi, S. S. Florman, and T. M. Fishbein. 2005. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J. Pediatr. Gastroenterol. Nutr. 40:328-333. [DOI] [PubMed] [Google Scholar]
- 40.Kimura, T., I. Mitsui, Y. Okada, T. Furuya, K. Ochiai, T. Umemura, and C. Itakura. 2001. Distribution of rabbit haemorrhagic disease virus RNA in experimentally infected rabbits. J. Comp. Pathol. 124:134-141. [DOI] [PubMed] [Google Scholar]
- 41.Lindesmith, L., C. Moe, J. LePendu, J. A. Frelinger, J. Treanor, and R. S. Baric. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 79:2900-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 43.Liu, B., I. N. Clarke, and P. R. Lambden. 1996. Polyprotein processing in Southampton virus: identification of 3C-like protease cleavage sites by in vitro mutagenesis. J. Virol. 70:2605-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, B. L., P. R. Lambden, H. Gunther, P. Otto, M. Elschner, and I. N. Clarke. 1999. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 73:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, B. L., G. J. Viljoen, I. N. Clarke, and P. R. Lambden. 1999. Identification of further proteolytic cleavage sites in the Southampton calicivirus polyprotein by expression of the viral protease in E. coli. J. Gen. Virol. 80:291-296. [DOI] [PubMed] [Google Scholar]
- 46.Lopman, B. A., D. W. Brown, and M. Koopmans. 2002. Human caliciviruses in Europe. J. Clin. Virol. 24:137-160. [DOI] [PubMed] [Google Scholar]
- 47.Love, D. N., and M. Sabine. 1975. Electron microscopic observation of feline kidney cells infected with a feline calicivirus. Arch. Virol. 48:213-228. [DOI] [PubMed] [Google Scholar]
- 48.Maeda, Y., Y. Tohya, Y. Matsuura, M. Mochizuki, and T. Sugimura. 2002. Early interaction of canine calicivirus with cells is the major determinant for its cell tropism in vitro. Vet. Microbiol. 87:291-300. [DOI] [PubMed] [Google Scholar]
- 49.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyers, G., C. Wirblich, and H. J. Thiel. 1991. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology 184:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morotti, R. A., S. S. Kaufman, T. M. Fishbein, N. K. Chatterjee, M. E. Fuschino, D. L. Morse, and M. S. Magid. 2004. Calicivirus infection in pediatric small intestine transplant recipients: Pathological considerations. Hum. Pathol. 35:1236-1240. [DOI] [PubMed] [Google Scholar]
- 52.Niess, J. H., S. Brand, X. Gu, L. Landsman, S. Jung, B. A. McCormick, J. M. Vyas, M. Boes, H. L. Ploegh, J. G. Fox, D. R. Littman, and H. C. Reinecker. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254-258. [DOI] [PubMed] [Google Scholar]
- 53.Nilsson, M., K. O. Hedlund, M. Thorhagen, G. Larson, K. Johansen, A. Ekspong, and L. Svensson. 2003. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 77:13117-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pesavento, P. A., N. J. MacLachlan, L. Dillard-Telm, C. K. Grant, and K. F. Hurley. 2004. Pathologic, immunohistochemical, and electron microscopic findings in naturally occurring virulent systemic feline calicivirus infection in cats. Vet. Pathol. 41:257-263. [DOI] [PubMed] [Google Scholar]
- 55.Platanias, L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375-386. [DOI] [PubMed] [Google Scholar]
- 56.Povey, R. C., R. C. Wardley, and H. Jessen. 1973. Feline picornavirus infection: the in vivo carrier state. Vet. Rec. 92:224-229. [DOI] [PubMed] [Google Scholar]
- 57.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 58.Ramana, C. V., M. P. Gil, R. D. Schreiber, and G. R. Stark. 2002. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 23:96-101. [DOI] [PubMed] [Google Scholar]
- 59.Rockx, B., R. S. Baric, G. de, E. Duizer, and M. P. Koopmans. 2005. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J. Med. Virol. 77:439-446. [DOI] [PubMed] [Google Scholar]
- 60.Rockx, B., M. de Wit, H. Vennema, J. Vinje, E. De Bruin, Y. van Duynhoven, and M. Koopmans. 2002. Natural history of human calicivirus infection: a prospective cohort study. Clin. Infect. Dis. 35:246-253. [DOI] [PubMed] [Google Scholar]
- 61.Rockx, B. H., H. Vennema, C. J. Hoebe, E. Duizer, and M. P. Koopmans. 2005. Association of histo-blood group antigens and susceptibility to norovirus infections. J. Infect. Dis. 191:749-754. [DOI] [PubMed] [Google Scholar]
- 62.Schaffer, F. L., D. W. Ehresmann, M. K. Fretz, and M. I. Soergel. 1980. A protein, VPg, covalently linked to 36S calicivirus RNA. J. Gen. Virol. 47:215-220. [DOI] [PubMed] [Google Scholar]
- 63.Schreiber, D. S., N. R. Blacklow, and J. S. Trier. 1973. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N. Engl. J. Med. 288:1318-1323. [DOI] [PubMed] [Google Scholar]
- 64.Schreiber, D. S., N. R. Blacklow, and J. S. Trier. 1974. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J. Infect. Dis. 129:705-708. [DOI] [PubMed] [Google Scholar]
- 65.Seah, E. L., J. A. Marshall, and P. J. Wright. 1999. Open reading frame 1 of the Norwalk-like virus Camberwell: completion of sequence and expression in mammalian cells. J. Virol. 73:10531-10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shemesh, M., M. Gurevich, E. Harel-Markowitz, L. Benvenisti, L. S. Shore, and Y. Stram. 2000. Gene integration into bovine sperm genome and its expression in transgenic offspring. Mol. Reprod. Dev. 56:306-308. [DOI] [PubMed] [Google Scholar]
- 67.Someya, Y., N. Takeda, and T. Miyamura. 2000. Complete nucleotide sequence of the chiba virus genome and functional expression of the 3C-like protease in Escherichia coli. Virology 278:490-500. [DOI] [PubMed] [Google Scholar]
- 68.Sosnovtsev, S., and K. Y. Green. 1995. RNA transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require VpG for infectivity. Virology 210:383-390. [DOI] [PubMed] [Google Scholar]
- 69.Studdert, M. J., and J. D. O'Shea. 1975. Ultrastructural studies of the development of feline calicivirus in a feline embryo cell line. Arch. Virol. 48:317-325. [DOI] [PubMed] [Google Scholar]
- 70.Sugieda, M., H. Nagaoka, Y. Kakishima, T. Ohshita, S. Nakamura, and S. Nakajima. 1998. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch. Virol. 143:1215-1221. [DOI] [PubMed] [Google Scholar]
- 71.Sugieda, M., and S. Nakajima. 2002. Viruses detected in the caecum contents of healthy pigs representing a new genetic cluster in genogroup II of the genus “Norwalk-like viruses.” Virus Res. 87:165-172. [DOI] [PubMed] [Google Scholar]
- 72.Wardley, R. C., and R. C. Povey. 1977. The clinical disease and patterns of excretion associated with three different strains of feline caliciviruses. Res. Vet. Sci. 23:7-14. [PubMed] [Google Scholar]
- 73.Webster, N. L., M. Forni, M. L. Bacci, R. Giovannoni, R. Razzini, P. Fantinati, A. Zannoni, L. Fusetti, L. Dalpra, M. R. Bianco, M. Papa, E. Seren, M. S. Sandrin, I. F. C. Mc Kenzie, and M. Lavitrano. 2005. Multi-transgenic pigs expressing three fluorescent proteins produced with high efficiency by sperm mediated gene transfer. Mol. Reprod. Dev. 72:68-76. [DOI] [PubMed] [Google Scholar]
- 74.White, L. J., J. M. Ball, M. E. Hardy, T. N. Tanaka, N. Kitamoto, and M. K. Estes. 1996. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J. Virol. 70:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of a Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]