Abstract
Despite the utility of recombinant adenoviral vectors in basic research, their therapeutic promise remains unfulfilled. Most engineered adenoviral vectors use a heterologous promoter to transcribe a foreign gene. We show that adenoviruses containing the cytomegalovirus immediate-early promoter induce the expression of the proapoptotic cellular protein TAp73 via the cyclin-dependent kinase-retinoblastoma protein-E2F pathway in murine embryonic fibroblasts. Cells transduced with these vectors also expressed high levels of the adenoviral E4-orf6/7 and E2A proteins. By contrast, adenoviruses containing the ubiquitin C promoter failed to elicit these effects. E4-orf6/7 is necessary and sufficient for increased TAp73 expression, as shown by using retrovirus-mediated E4-orf6/7 expression and adenovirus with the E4-orf6/7 gene deleted. Activation of TAp73 likely occurs via E4-orf6/7-induced dimerization of E2F and subsequent binding to the inverted E2F-responsive elements within the TAp73 promoter. In addition, adenoviral vectors containing the cytomegalovirus immediate-early promoter, but not the ubiquitin C promoter, cooperated with chemotherapeutic agents to decrease cellularity in vitro. In contrast to murine embryonic fibroblasts, adenoviruses containing the ubiquitin C promoter, but not the cytomegalovirus immediate-early promoter, induced both E4-orf6/7 and TAp73 in human foreskin fibroblasts, emphasizing the importance of cellular context for promoter-dependent effects. Because TAp73 is important for the efficacy of chemotherapy, adenoviruses that increase TAp73 expression may enhance cancer therapies by promoting apoptosis. However, such adenoviruses may impair the long-term survival of transduced cells during gene replacement therapies. Our findings reveal previously unknown effects of foreign promoters in recombinant adenoviral vectors and suggest means to improve the utility of engineered adenoviruses by better controlling their impact on viral and cellular gene expression.
Adenoviral (Ad) vectors are valuable tools for the study of many fundamental biological processes because of their ability to efficiently transduce quiescent cells. In addition, many clinical gene transfer protocols use adenoviral vectors for gene replacement to treat genetic disorders, such as cystic fibrosis; for cancer therapy to preferentially kill tumor cells; or for immunization (24). A large number of adenoviral vectors used in basic research and in clinical trials drive transcription of the inserted gene with the immediate-early promoter of cytomegalovirus (CMV).
The adenoviral genome is a 36-kb double-stranded DNA chromosome that exists as an episome in the infected cell. Adenoviral genes are arranged bidirectionally, and alternative splicing creates many distinct gene products. The study of adenovirus has dramatically enhanced our understanding of fundamental cellular processes. For example, investigations seeking to understand the control of the adenoviral E2A promoter led to the discovery of the E2F transcription factors and the pivotal role that the retinoblastoma protein (pRb) plays in controlling E2F transactivation and entry into the cell cycle (2). Studies also revealed that the adenoviral E1A protein regulates E2F activity by inhibiting pRb's sequestration of E2F factors and that E4-orf6/7 aids in the dimerization and subsequent binding of E2F to a subset of E2F-responsive viral and cellular promoters (2).
Human adenoviral infections typically result in minimal inflammation due to a complex interaction of viral gene products that modulate the host's immune response and may create conditions that favor a persistent infection (27, 38). Replication-defective first-generation adenoviral vectors, which have the E1A, E1B, and usually the E3 gene deleted, induce very strong innate and adaptive immune responses that limit the utility of the vector. Second-generation adenoviral vectors, which have one or more of the E2 genes essential for viral DNA replication deleted and are therefore replication incompetent or have the E4 gene deleted and are replication defective, exhibit improved performance as gene therapy vectors but still generate strong innate and adaptive immune responses (10). These findings highlight the possibility that expression of viral genes retained in first- and second-generation vectors reduces therapeutic utility via negative effects on the host cell and/or the promotion of immune responses.
The p73 transcription factor is a member of the p53 family that is upregulated and posttranslationally activated following DNA damage (34). Together with a third member of the p53 family, p63, the proapoptotic isoform TAp73 contributes to apoptosis. In fact, p53-dependent apoptosis and transcription are dependent on p63/p73, and vice versa (11). The TAp73 promoter is directly regulated by E2F, with preferential regulation by the E2F1 family member following DNA damage (34). A second p73 isoform, ΔNp73, possesses an alternative 5′ exon transcribed from an internal promoter. ΔNp73 is thought to oligomerize with TAp73 and TAp63 and directly compete with p53 for binding to promoters, thus antagonizing their proapoptotic functions (20, 35, 51). Adding to this complexity, the p73 gene encodes at least six C-terminal splice variants (20).
Chemotherapeutic agents that induce apoptosis depend in part upon the induction and transactivation potential of TAp73. However, some tumor-associated variants of p53 bind and inactivate p73, thus lowering the rate of clinical response to certain proapoptotic therapies (3, 21, 25, 32). Therefore, treatments that elevate expression of TAp73 and enable it to overcome dominant-negative mutant p53 may prove efficacious for cancer therapy.
While using recombinant adenoviruses to study the transcriptional regulation of TAp73, we consistently found that adenoviral vectors using particular foreign promoters increased TAp73 transcript levels in fibroblasts. Because of the proapoptotic role of TAp73 and the current difficulties of attaining long-term expression in clinical trials that use adenoviral vectors, we investigated the link between adenoviral transduction and TAp73 expression.
MATERIALS AND METHODS
Cell culture.
Mice were housed in the University of Colorado at Denver and Health Sciences Center animal resource center, and all mouse experiments were approved by the University of Colorado at Denver and Health Sciences Center Animal Care and Use Committee. BALB/c mice were purchased from Jackson Laboratory, and mouse embryo fibroblasts (MEFs) were prepared as described earlier (1). Adenoviral stocks were created as described previously (38, 48). For MEF transductions, adenoviral vectors were titered based on green fluorescent protein (GFP) expression in coxsackievirus/adenovirus receptor-expressing EL4 thymoma (26) cells transduced at multiplicities of infection (MOIs) of less than 1, by indirect immunofluorescence specific for the viral E2A-encoded DNA binding protein (DBP) (Ad CMV-p16) (8), or by particles [Ad E1A with or without poly(A)] (38). Due to differing susceptibilities to adenoviral transduction, adenoviral vectors were independently titered on human foreskin fibroblasts (HFFs) based on GFP expression.
Adenoviral and retroviral constructions.
The construction of Ad CMV-GFP (26), Ad ubiquitin C (UbC)-GFP (49), Ad CMV-nil (formerly named MbAd2) (39), and Ad CMV-p16 (22) was described previously. Ad CMV-GFP-dpTP was constructed by electroporation of Escherichia coli strain BJ5183 carrying the plasmid pAdEasy-1 modified to contain the majority of the chromosome of Ad5dl300ΔpTP (38) with PmeI-digested pAdTrack (18). A recombinant plasmid containing the CMV-GFP cassette from pAdTrack was grown and digested with PacI to release the viral chromosome. The DNA was used to transfect HEK293-pTP cells to generate virus. The virus was plaque purified prior to large-scale growth. Ad CMV-GFP-dE4 was constructed by electroporation of E. coli strain BJ5183 carrying the plasmid pAdEasy-2 with PmeI-digested pAdTrack (18). A recombinant plasmid containing the CMV-GFP cassette from pAdTrack was grown and digested with PacI to release the viral chromosome. The DNA was used to cotransfect HEK293T cells with pCMV-E4-orf6 to generate virus. After incubation for 7 days, the cells were frozen and thawed to release the virus and used to infect HEK293 cells that had been transfected with pCMV-E4-orf6 to increase the virus titer. After incubation for 7 days, the cells were frozen and thawed to release the virus, and a large volume of cell lysate was used to infect a small number of HEK293 cells to promote virus growth in the absence of complementation for E4 (52). The virus was grown on a large scale by high-multiplicity infection of HEK293 cells.
To construct Ad CMV-GFP-dE4-orf6/7, Ad5dl356 (15) DNA (which does not encode E4-orf6/7) prepared from purified virions was digested with ClaI, which cleaves uniquely within the E1A gene. The large DNA fragment was purified by centrifugation on a 10 to 40% sucrose gradient and used along with PmeI-digested pAdTrack (22) to cotransfect HEK293 cells. The virus was plaque purified, and a GFP-positive plaque was selected, grown on a large scale, and purified.
The protein IX (pIX) coding region was amplified from pAdEasy-1 (18) with Pfu Turbo (Stratagene, Inc.) and the primers GCGTAGATCTATGAGCACCAACTCGTTTGATGG and ATCTAAGCTTTTAAACCGCATTGGGAGGGGAGG and ligated into MSCV2.2+IRESGFP (47) using BglII and PmeI. E4-orf6/7 cDNA was inserted as a BamHI/SalI fragment into the BglII/ClaI sites of MSCViresGFP. Sequencing confirmed the nucleotide sequences of all amplified products.
Viral transduction of cells.
MEFs or HFFs suspended at 10 million per ml of serum-free Dulbecco's modified Eagle's medium (DMEM) were transduced with adenovirus for 60 min with frequent agitation and then plated in DMEM supplemented with 10% fetal bovine serum (FBS). Following overnight culture, the cells were washed once with phosphate-buffered saline and incubated for 2 days in DMEM plus 0.2% FBS. The cells were harvested 22 h after the medium was replaced with DMEM plus 10% FBS. For retroviral transductions, the ecotropic Phoenix packaging line (45) was transiently transfected with the mouse stem cell virus (MSCV) vector, along with pCLEco (31), using Fermentas ExGen500; 24 h after the medium was replaced, the retroviral supernatant was filtered using a 0.2-μm syringe filter, diluted 1:3 with fresh DMEM plus 10% FBS plus 4 μg/ml Polybrene, and added to adherent MEFs. Fresh retroviral supernatant was added three times over a 12-hour period, and the MEFs were transduced with adenovirus the following day.
Reverse transcription (RT)-PCR.
RNA was isolated using TRIzol (Invitrogen Corp.), denatured (95°C for 3 min), DNase treated (Ambion; 37°C for 30 min), and further purified using the RNeasy Mini Kit (QIAGEN Inc.). TaqMan Universal PCR Master Mix, No AmpErase UNG, and 6-6-carboxyfluorescein/6-carboxytetramethylrhodamine-labeled probes (Applied Biosystems) were used for the detection of 18S rRNA (48), cyclin A2, DHFR, cdc6, and Ets2 (9). SYBR Green Master Mix (Applied Biosystems) was used for the detection of p21 (48) and pIX.
The primers and probes utilized were as follows: cycA2 (TCAAGACTCGACGGGTTGC, TCCATGAAGGACCAGCAGTG, and probe CCTCTTAAGGACCTTCCTATAAACGATGAGCATG), DHFR (CGGAGGCAGTTCTGTTTACCA, CCTGCATGATCCTTGTCACAA, and probe AAGCCATGAATCAACCAGGCCACCTTAG), cdc6 (GTTCTGTGCCCGCAAAGTG, AGCTCGCCTGCAAACATCC, and probe CTGCTGTTTCAGGAGACATCCGTAAAGCG), and pIX (GCGTAGATCTATGAGCACCAACTCGTTTGATGG and AGGAAGCCTTCAGGGCAGAAACC). Real-time RT-PCR was performed with Moloney murine leukemia virus-RT (Invitrogen Corp.) in a model 7700 thermocycler (Perkin-Elmer) as described earlier (9): 48°C for 30 min (1 cycle), 95°C for 10 min (1 cycle), 95°C for 15 s, and 60°C for 1 min (40 cycles). A 1-min 72°C extension step was added for PCR for pIX RNA. Reverse transcriptions of murine TAp73 and ΔNp73 were done independently from the real-time PCR using GGCACTGCTGAGCAAATTGAACTG with incubation for 50 min at 45°C, followed by 5 min at 85°C. Nineteen microliters of the RT reaction mixture was added to a 6-μl mixture containing AmpliTaq GOLD (Applied Biosystems), the reverse primer GGCACTGCTGAGCAAATTGA, and a gene-specific forward primer and probe (TA, GCGAGGAGTCCAACATGGAT and probe CTTCCACCTGCAAGGCATGGCC; ΔNp73, CCACGAGCCTACCATGCTTTAC and probe CGGTGACCCCATGAGACACCTCG). Oligo(dT) was used for the reverse transcription of human TAp73, followed by the TAp73-specific primer and probe set from Applied Biosystems (assay identifier, Hs00232088_m1).
Western blotting.
Protein was prepared from cells using RIPA buffer. The antibodies used were α-p73 (NeoMarkers; GC15), α-E4-orf6/7 (which recognizes both E4-orf6 and E4-orf6/7) (28), α-DNA binding protein (36) (a gift from A. J. Levine), and α-β-actin (sc-1616). Secondary antibodies used were α-mouse horseradish peroxidase (HRP), α-rabbit HRP, and α-goat HRP (Bio-Rad), and the blots were developed using Pierce SuperSignal Pico or Femto chemiluminescence detection. Western blotting was performed with 0.2% Tween 20 in the antibody solutions and washes.
Flow cytometry and chemotherapeutics.
Flow cytometry and GFP quantification were performed on single-cell suspensions with an XL cytometer (Coulter). For relative cell counts (chemotherapeutic drug assays), all wells were harvested in the same manner, and immediately following mixing, were analyzed for 30 seconds using the cytometer's automatic collection system. Etoposide (Sigma) (in DMSO) and 5-fluorouracil (5-FU) (Sigma) (in phosphate-buffered saline) were added to MEFs after the 2-day incubation in DMEM plus 0.2% FBS and incubated for 4 days prior to analysis. DMSO served as the carrier control.
RESULTS
Adenoviral vectors containing the CMV promoter induce p73 expression.
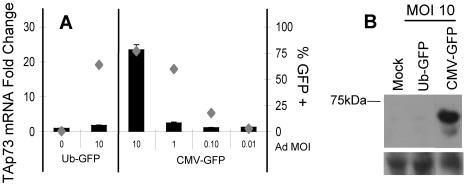
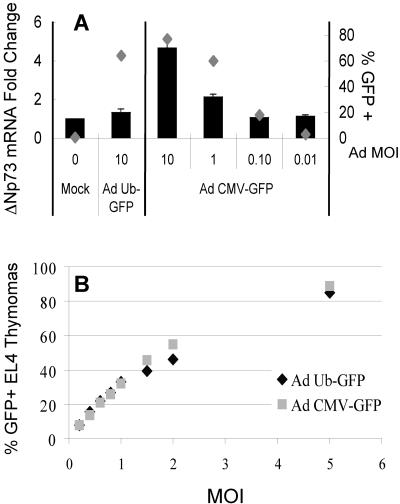
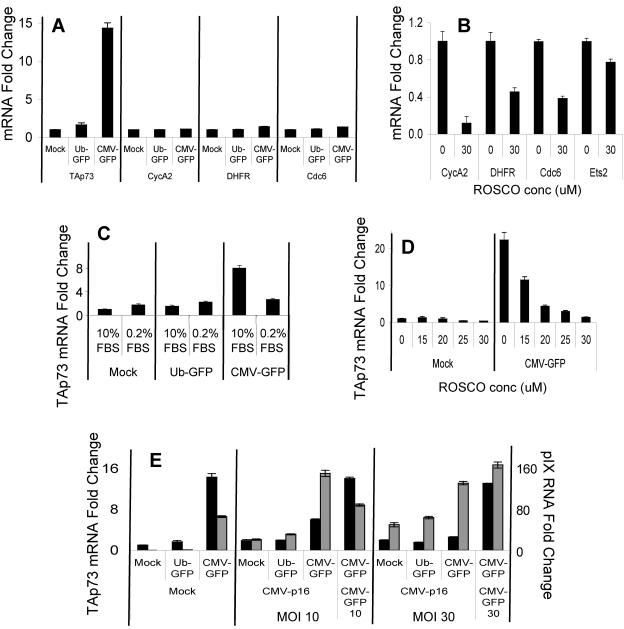
While investigating the regulation of p73 transcription, we consistently found that transcript levels of TAp73 varied among MEFs transduced with adenoviral vectors that differed only in the promoter used to drive expression of GFP (Fig. 1 shows schemata of recombinant adenoviruses used in this study). Adenoviral vectors that used the CMV, but not the human UbC promoter induced high levels of TAp73 mRNA (Fig. 2A) and TAp73α protein (Fig. 2B) and, to a lesser extent, ΔNp73 mRNA levels (Fig. 3A). To establish that the effective viral titers of the two adenoviruses were comparable, mouse EL4 thymoma cells expressing the coxsackievirus and adenovirus receptors were transduced over a wide range of MOIs. Very similar percentages of GFP-expressing cells were measured at all MOIs, confirming consistent viral titers (Fig. 3B). Additionally, the mean fluorescence intensities of EL4 cells transduced by the two viruses were equivalent, and thus, differences in promoter strength did not affect the titer calculations.
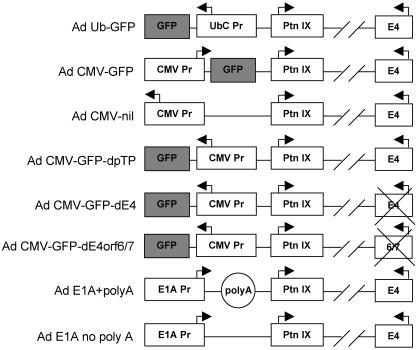
FIG. 1.
Adenoviral vectors used in these studies are presented schematically, with modifications of the E1 and E4 regions indicated, along with the location of the pIX gene (Ptn IX). Note that all vectors have the E1A and E1B genes deleted. Not drawn to scale.
FIG. 2.
Adenoviral vectors expressing GFP from the CMV promoter, but not the UbC promoter, induce TAp73 expression. MEFs suspended in serum-free medium were transduced with adenovirus at the indicated MOI, plated at subconfluency for 16 h in complete medium, changed to starvation medium (0.2% serum) for 2 days to synchronize their cell cycles, and then fed for 22 h in complete medium prior to harvest. (A) The transcript levels of TAp73 relative to 18S rRNA were determined by real-time RT-PCR. Indistinguishable results were obtained by normalization to β-actin. Parallel samples were used for GFP analysis (gray diamonds). The standard error of the mean is included (obscured when the error is very small). (B) TAp73 protein was detected by immunoblotting. A nonspecific band served as the loading control (lower blots).
FIG. 3.
Adenoviral vectors expressing GFP from the CMV promoter, but not the UbC promoter, induce ΔNp73 expression. (A) MEFs were transduced with adenovirus at the indicated MOI, serum starved, and then restimulated with serum-containing medium as described in the legend to Fig. 2. The transcript levels of ΔNp73 relative to 18S rRNA were determined by real-time RT-PCR. Parallel samples were used for GFP analysis (gray diamonds). The standard error of the mean is included (obscured when the error is very small). (B) EL4 thymoma cells expressing the coxsackievirus/adenovirus receptor required for adenoviral entry (26) were transduced over a wide range of MOIs, and the percentage that expressed GFP was quantified via flow cytometry 16 h later.
Because MEFs transduced with the adenoviral vector using the CMV promoter produced a ninefold-greater intensity of GFP fluorescence than the vector using the UbC promoter (data not shown), we tested the possibility that the elevated GFP levels following Ad CMV-GFP transduction caused the induction of TAp73. Surprisingly, an adenoviral vector that possessed the CMV promoter but lacked any foreign coding sequence, Ad CMV-nil, increased TAp73 transcript levels (Fig. 4A). Therefore, GFP expression is not responsible for TAp73 induction by Ad CMV-GFP.
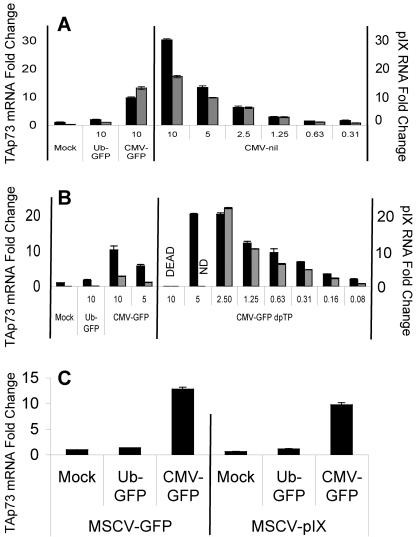
FIG. 4.
Neither GFP expression nor adenoviral DNA replication is responsible for induction of TAp73 and adenoviral pIX RNA. MEFs were transduced as before with adenoviral vectors that (A) possess the CMV promoter but do not encode GFP (or any foreign gene) or (B) express GFP from the CMV promoter but cannot replicate due to deletion of the pTP gene. TAp73 mRNA (black bars, left y axis) and adenoviral pIX RNA (gray bars, right y axis) relative to 18S rRNA were quantified by real-time RT-PCR (ND, not determined; “DEAD” indicates that cell killing at these MOIs prevented mRNA analyses). (C) Adenoviral pIX is not responsible for increased transcript levels of TAp73. Prior to adenoviral transductions (MOI, 10), MEFs were transduced with an MSCV-based retrovirus expressing GFP alone or in conjunction with adenoviral pIX. TAp73 mRNA relative to 18S rRNA was quantified by real-time RT-PCR. The expression of pIX mRNA was confirmed by RT-PCR and found to be substantially higher than levels observed in cells transduced with Ad CMV-GFP (data not shown). The standard error of the mean is included (obscured when the error is very small).
Although the adenoviruses used in this study are considered replication defective due to deletion of the E1 genes, it is possible that limited replication (13, 41) could have increased p73 expression. To determine if eliminating the potential for viral DNA replication affects TAp73 expression, MEFs were transduced with an adenoviral vector with the preterminal protein (pTP) gene deleted and that directs expression of GFP from the CMV promoter (Ad CMV-GFP-dpTP) (38). This adenovirus induced TAp73 transcript levels even more efficiently than Ad CMV-GFP (Fig. 4B). This result suggests that adenoviral DNA replication does not contribute to TAp73 induction by CMV promoter-containing adenoviruses.
Adenoviral vectors containing the CMV promoter induce adenoviral protein IX.
Ad CMV-GFP induces TAp73 less efficiently than Ad CMV-GFP-dpTP. The CMV promoter in CMV-GFP-dpTP directs transcription toward the left end of the viral chromosome. This orientation differs from that found in Ad CMV-GFP (Fig. 1) and could place enhancer elements closer to downstream adenoviral promoters. The possibility that enhancer elements within the CMV promoter affected the transcription of downstream adenoviral genes was tested by measuring the transcript levels of the most proximal adenoviral gene, encoding pIX. Although all adenovirus-transduced MEFs expressed RNAs that included the pIX sequence, Ad CMV-GFP and Ad CMV-nil induced more pIX-bearing RNAs than Ad UbC-GFP (Fig. 4A). Moreover, Ad CMV-GFP-dpTP induced pIX RNA more efficiently than Ad CMV-GFP (Fig. 4B). Levels of TAp73 and pIX transcripts exhibited a strong linear correlation over various amounts of infecting virus (correlation coefficient, 0.98). We have confirmed that transcription of the coding DNA strand of the pIX gene is induced by performing reverse transcription in the presence of a single oligonucleotide that primes the sense mRNA (data not shown).
Because of the strong correlation between TAp73 and pIX-related RNAs, we tested the possibility that pIX is sufficient to alter the expression of TAp73. MEFs were transduced with an MSCV-based retrovirus expressing GFP alone or in conjunction with pIX. After transducing over 90% of the control and experimental MEFs with retrovirus and confirming pIX mRNA expression, we transduced them with adenovirus as described above. As shown in Fig. 4C, retrovirus-expressed pIX alone, or in conjunction with Ad UbC-GFP or Ad CMV-GFP, had no effect on TAp73 expression. Therefore, pIX is not sufficient to alter TAp73 transcript levels.
TAp73 transcripts are induced via the cyclin-dependent kinase (CDK)-pRb-E2F pathway.
Three transcription factors have been shown to increase TAp73 transcription: TAp73, p53, and E2F1 (6, 19, 34, 43). We transduced p73−/−, p53−/−, and E2F1−/− MEFs with adenovirus, and in each case, Ad CMV-GFP induced TAp73 transcripts equally well in the presence or absence of p73, p53, or E2F1 protein, thus indicating that these transcription factors are not individually required for TAp73 induction by Ad CMV-GFP (data not shown). To further assess the role of p53, we measured the induction of one of its well-established targets, p21, and found that Ad CMV-GFP transduction did not alter p21 transcript levels in wild-type MEFs (data not shown).
TAp73 transcription is thought to be controlled primarily by E2F via well-established sites in the TAp73 promoter (20). Since the adenoviral protein E1A inhibits the ability of the pocket proteins pRb, p130, and p107 to sequester E2F transcription factors, contamination of our adenoviral stocks with E1A-expressing viruses could result in TAp73 induction. However, despite Ad CMV-GFP inducing the transcript levels of TAp73, the levels of three other well-established E2F targets, DHFR, Cdc6, and CycA2, remained unchanged (Fig. 5A). We confirmed our ability to quantify regulated mRNA expression of these E2F targets by using roscovitine, a pharmacological agent that preferentially inhibits CDK2 and CDK1 activity (30), to inhibit CDK activity and, thus, E2F-driven transcription (Fig. 5B). These results demonstrate that the upregulation of TAp73 does not reflect a general enhancement of the expression of all E2F target genes and is not a result of contaminating E1A protein.
FIG. 5.
CDK-pRb-E2F pathway is required for adenoviral induction of TAp73. (A) MEFs were transduced as before (MOI, 10), and TAp73, CycA2, DHFR, and Cdc6 RNAs relative to 18S rRNA were quantified by real-time RT-PCR. (B) MEFs were incubated in 30 μM roscovitine or dimethyl sulfoxide (DMSO) control, and CycA2, DHFR, and Cdc6 RNAs relative to 18S rRNA were quantified by real-time RT-PCR. (C) MEFs were transduced as before (MOI, 10), except the 0.2% starvation medium was not replaced or (D) roscovitine or DMSO control was added when the starvation medium was replaced with 10% FBS-DMEM. (E) MEFs were transduced with the indicated adenoviral vectors at the indicated MOIs. Additional Ad CMV-GFP was added to equalize the MOIs when Ad CMV-p16 was used. TAp73 mRNA (black bars, left y axis) and adenoviral pIX RNA (gray bars, right y axis) relative to 18S rRNA were quantified by real-time RT-PCR. The standard error of the mean is included (obscured when the error is very small).
While E2F1 is not required for TAp73 induction by Ad CMV-GFP, it is possible that the other transactivating E2Fs, E2F2 and E2F3, compensate for E2F1 loss (7). Hyperphosphorylation of pRb family members by CDKs causes the release of E2F transcription factors (42). We therefore used three independent means to assess the role of the CDK-pRb-E2F pathway in the induction of TAp73 following adenoviral transduction: serum starvation, a pharmacological CDK inhibitor, and a genetically encoded CDK inhibitor. First, MEFs were transduced with adenovirus and then maintained in low-serum medium (0.2% FBS) for 2 days to elicit quiescence. In contrast to MEFs maintained in normal growth medium, the quiescent MEFs failed to induce TAp73 following Ad CMV-GFP transduction (Fig. 5C). Among many possibilities, this result could reflect a requirement for transactivating E2F, which is absent in growth factor-deprived cells. To test this possibility, adenovirus-transduced MEFs were incubated in the presence of roscovitine. As shown in Fig. 5D, roscovitine inhibited the Ad CMV-GFP induction of TAp73 in a dose-dependent manner. Importantly, mRNA levels of Ets2, a non-E2F target, were not significantly affected by 30 μM roscovitine, suggesting an absence of nonspecific effects at this concentration (data not shown).
Finally, the role of the CDK-pRb-E2F pathway in TAp73 induction by Ad CMV-GFP was tested by overexpressing p16Ink4a (14), a CDK4 and CDK6 inhibitor that prevents pRb family protein phosphorylation and thus E2F activation. MEFs were transduced as before in the presence or absence of Ad CMV-p16 at various MOIs. The quantity of CMV promoter-containing adenoviral vector was kept constant by adjusting the Ad CMV-GFP MOI. As shown in Fig. 5E, Ad CMV-p16 inhibited TAp73 induction in a dose-dependent fashion. Ad CMV-p16 did not alter the mRNA of a non-E2F target (Ets2) (data not shown). In addition, Ad CMV-p16 transduction increased pIX transcript levels while decreasing TAp73 induction (Fig. 5E). In total, these experiments strongly suggest that CDK activation, and thus presumably E2F, is required for the TAp73 induction observed after Ad CMV-GFP transduction.
Adenoviral E4-orf6/7 induces TAp73.
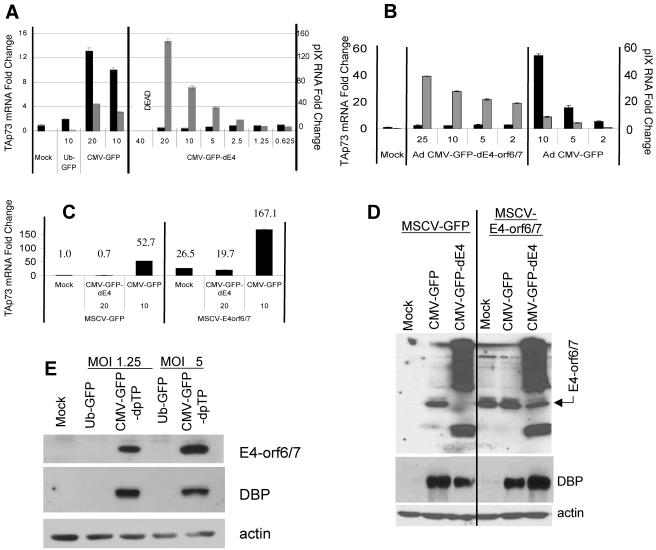
These findings led us to hypothesize that enhancer elements within the CMV promoter stimulate the expression of downstream adenoviral proteins. These adenoviral products could then directly or indirectly increase TAp73 transcript levels via the CDK-pRb-E2F pathway. To identify candidate adenoviral proteins that upregulate TAp73 transcript levels, we assessed whether an adenoviral vector possessing the CMV promoter but lacking the E4 gene induces TAp73. Remarkably, the adenovirus with E4 deleted failed to induce TAp73 transcripts but did increase the quantities of adenoviral pIX RNA and caused death at higher MOIs (Fig. 6A).
FIG. 6.
Adenoviral protein E4-orf6/7 is necessary and sufficient to induce p73. (A and B) MEFs were transduced with the indicated viruses or (C) prior to adenoviral transductions, MEFs were transduced with an MSCV-based retrovirus expressing GFP alone or in conjunction with E4-orf6/7. TAp73 RNA (black bars, left y axis) and adenoviral pIX RNA (gray bars, right y axis) relative to 18S rRNA were quantified by real-time RT-PCR. The standard error of the mean is included (obscured when the error is very small). “DEAD” indicates that cell killing at these MOIs prevented mRNA analyses. (D) Adenoviral DNA DBP and E4 proteins were detected by immunoblotting. β-Actin detection served as the loading control. (E) Adenoviral DBP and E4-orf6/7 protein were detected by immunoblotting. β-Actin served as the loading control.
Among the six known E4 proteins, E4-orf6/7 has been shown to promote dimerization and subsequent binding of E2F to a subset of viral and cellular E2F-responsive promoters (46). To determine if E4-orf6/7 is required for upregulating TAp73 transcript levels, we assessed whether an adenoviral vector possessing the CMV promoter but lacking the E4-orf6/7 gene (Ad CMV-GFPdE4orf6/7) induces TAp73. As shown in Fig. 6B, the adenovirus lacking the E4-orf6/7 coding region failed to induce TAp73 transcripts while retaining the ability to induce pIX message, thus demonstrating that E4-orf6/7 is necessary for TAp73 induction following transduction with CMV-possessing adenoviral vectors.
In order to test whether expression of E4-orf6/7 is sufficient to induce TAp73 transcript levels, MEFs were transduced with an MSCV-based retrovirus expressing GFP alone or in conjunction with E4-orf6/7. Retrovirus-expressed E4-orf6/7 was sufficient to induce TAp73 transcript levels, and transduction with Ad CMV-GFP, but not Ad CMV-GFP-dE4, further increased TAp73 mRNA (Fig. 6C). Anti-E4 Western blots from MEFs transduced with Ad CMV-GFP, but not Ad CMV-GFP-dE4, revealed a unique protein species corresponding to the appropriate size of E4-orf6/7 (Fig. 6D). The retrovirus engineered to expresses E4-orf6/7 produced a similar-size protein at levels comparable to cells transduced by Ad CMV-GFP. For unknown reasons, the anti-E4 antibody revealed both higher- and lower-molecular-weight species following transduction with Ad CMV-GFP-dE4. Note that retroviral expression of E4-orf6/7 in MEFs induced TAp73 to a greater extent in the presence of Ad CMV-GFP, though the amount of E4-orf6/7 was not noticeably altered (Fig. 6C and D). This result suggests that Ad CMV-GFP transduction may promote TAp73 expression through additional viral gene products in addition to E4-orf6/7, although these mechanisms must also be dependent on E4-orf6/7. A second adenoviral product, the DBP encoded by the E2A gene, was induced by both Ad CMV-GFP and Ad CMV-GFP-dE4 (Fig. 6D). By contrast, transduction with Ad UbC-GFP did not lead to detectable expression of either the DBP or the E4 protein in MEFs (Fig. 6E). In total, these experiments demonstrate that the expression of E4-orf6/7 following adenoviral transduction is necessary and sufficient to induce the expression of TAp73.
Adenoviral vectors lacking exogenous promoters induce TAp73.
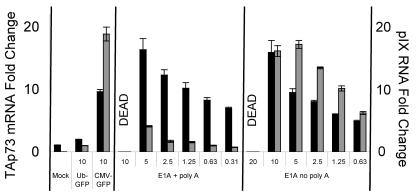
We have demonstrated that adenoviral vectors containing a CMV promoter induce adenoviral pIX, DBP, and E4-orf6/7, as well as cellular TAp73. We next explored whether an adenovirus that contains no foreign promoter or foreign enhancer elements would do the same. Ad E1A+polyA possesses the E1A promoter in its endogenous orientation but with the intervening sequence between the E1A and pIX promoters replaced with the poly(A) signal of simian virus 40 (SV40) (and with the E1 coding regions deleted [Fig. 1 shows schema]). Our previous studies using a similar adenovirus expressing GFP from the E1A promoter indicated that the strength of this promoter is at least 10-fold weaker than the CMV promoter in fibroblasts (26). Although this virus induces pIX less efficiently than Ad CMV-GFP, it induces TAp73 much more efficiently (Fig. 7). In addition, an adenovirus identical to Ad E1A+polyA, except lacking the SV40 poly(A) signal just prior to the pIX promoter, induces TAp73 with similar efficiency but increases the levels of pIX-containing RNAs to a much greater extent (Fig. 7). Thus, neither the strength of the promoter at the 5′ end of the adenoviral genome nor the extent of pIX induction can predict the magnitude of TAp73 expression. Furthermore, an adenoviral vector lacking exogenous promoter sequences can induce the expression of TAp73.
FIG. 7.
Adenoviral vectors possessing only endogenous elements induce adenoviral pIX and TAp73 expression. MEFs were transduced as before with adenoviral vectors that maintain the natural E1A promoter but lack foreign promoter sequences. As indicated in Fig. 1, the two adenoviral vectors used differed in that only the first possessed an SV40 polyadenylation sequence between the E1A promoter and the pIX coding sequence. TAp73 mRNA (black bars, left y axis) and adenoviral pIX RNA (gray bars, right y axis) relative to 18S rRNA were quantified by real-time RT-PCR. “DEAD” indicates that cell killing at these MOIs prevented mRNA analyses. For both viruses, a linear correlation (correlation coefficient, 0.99) exists between pIX RNA and TAp73 induction as a function of the MOI used for transduction being decreased. The standard error of the mean is included (obscured when the error is very small).
Adenoviral vectors containing the CMV promoter augment chemotherapeutics.
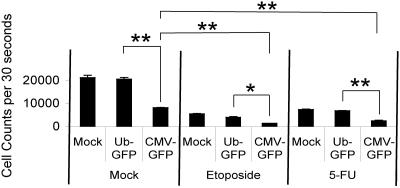
As discussed above, p73 is involved in the apoptosis associated with chemotherapeutic drugs, such as 5-FU and etoposide (20). We therefore tested whether the chemotherapeutic effects would be augmented by Ad CMV-GFP transduction. As shown in Fig. 8, treatment with either 5-FU or etoposide decreased the numbers of live cells (2.9- and 3.9-fold, respectively). Transduction with Ad CMV-GFP, but not Ad UbC-GFP, also decreased cell numbers substantially (2.6-fold). Importantly, the combination of the chemotherapeutic 5-FU or etoposide with Ad CMV-GFP transduction had a cooperative effect and decreased cell numbers more than either alone (8.4- and 15-fold, respectively). A significant portion of this effect was due to death, since the final number of cells was less than that originally plated. Thus, adenoviral vectors containing the CMV promoter cooperate with chemotherapeutics to mediate decreased cellularity.
FIG. 8.
Chemotherapeutics and Ad CMV-GFP cooperatively decrease cellularity. MEFs were transduced with adenovirus (MOI, 30) as before, except 0.2% FBS starvation medium was replaced with 10% FBS-DMEM containing DMSO, etoposide (10 μM), or 5-FU (100 μM). Four days following the addition of chemotherapeutics, triplicate wells were harvested and the quantity of live cells was assessed by forward and side scatter as described in Materials and Methods. The standard error of the mean is included. *, P < 0.005; **, P < 0.0005 using Student's two-sample homoscedastic t test.
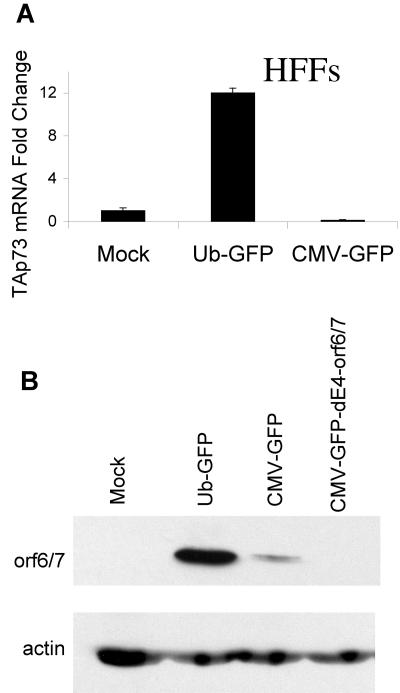
Adenoviral vectors expressing GFP from the UbC promoter, but not the CMV promoter, induce E4-orf6/7 and TAp73 expression in HFFs.
We have shown that MEFs transduced with adenoviral vectors containing the CMV promoter, but not the UbC promoter, induce cellular TAp73 via adenoviral E4-orf6/7 and the CDK-pRb-E2F pathway. We next investigated the generality of our findings by transducing HFFs with adenovirus. In contrast to our results with MEFs, Ad UbC-GFP, but not Ad CMV-GFP, strongly induced high levels of cellular TAp73 mRNA (Fig. 9A) and adenoviral E4-orf6/7 protein (Fig. 9B). All HFFs expressed GFP following adenoviral transduction, with the CMV-containing adenoviral vectors causing about threefold-greater fluorescence (mean fluorescence intensity) than Ad UbC-GFP (data not shown), further emphasizing that the induction of viral and cellular genes does not correlate with the strength of the promoter driving GFP. These results are consistent with our previous experiments demonstrating TAp73 induction via E4-orf6/7 and suggest that exogenous promoters within recombinant adenoviral vectors upregulate adenoviral, and thus cellular, proteins in a species-specific or cell-type-specific fashion.
FIG. 9.
Adenoviral vectors expressing GFP from the UbC promoter, but not the CMV promoter, induce E4-orf6/7 and TAp73 expression in human foreskin fibroblasts. HFFs were transduced with adenovirus (MOI, 10) as previously described. (A) The transcript levels of TAp73 relative to 18S rRNA were determined by real-time RT-PCR. The standard error of the mean is included (obscured when the error is very small). To rule out a “virus-switching” mistake, these experiments were repeated several times, the virus stocks used were the same as those used for the experiments above with MEFs, and PCR amplification of the promoters followed by sequencing was used to confirm the identities of the viral stocks. (B) E4-orf6/7 protein was detected by immunoblotting. β-Actin served as the loading control (lower blots).
DISCUSSION
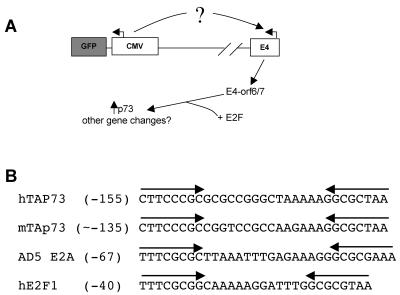
Although recombinant adenoviruses have proven useful in the laboratory, their use in therapeutic applications has faced difficulties. Our data demonstrate that adenoviral vectors possessing the E4 gene and either the CMV promoter in MEFs or the UbC promoter in HFFs upregulate expression of the adenoviral protein E4-orf6/7 that subsequently leads to the induction of TAp73 (Fig. 10A shows a model). The adenoviral protein E4-orf6/7 dimerizes E2F factors and increases the transactivation of the inverted E2F-responsive sites in the E2F1 and adenoviral E2 promoters (46). Both the murine and human TAp73 promoters have two inverted putative E2F sites separated by a similar number of base pairs (Fig. 10B), fitting with our finding that E4-orf6/7 induces TAp73 while other E2F targets remain unchanged (40). Promoters of some cellular apoptotic genes may have acquired inverted E2F sites to aid in the prevention of adenoviral spread, or E4-orf6/7 may have evolved to induce apoptotic E2F targets to prevent the inflammation necessary for a productive immune response. Most likely, both possibilities have influenced the coevolution of the cellular and adenoviral genomes.
FIG. 10.
Alignment of E2F-responsive sites in the TAp73, Ad5 E2A, and E2F1 promoters. (A) Model for adenoviral-vector-mediated deregulation of cellular gene expression dependent on both the choice of promoter driving ectopic gene expression and E4-orf6/7. The CMV promoter is used in this example, but the extent of E4-orf6/7-dependent deregulation of cellular gene expression mediated by a particular ectopic promoter depends on the cellular context. (B) Alignment of human and mouse TAp73, Ad5 E2A, and human E2F1 promoter sequences with predicted (for p73) or demonstrated (E2A and E2F1) E2F binding sequences (arrows).
A surprising result was that, while Ad CMV vectors but not Ad UbC vectors caused increased expression of E4-orf6/7 and p73 in MEFs, the exact opposite was true in HFFs, with only Ad UbC vectors upregulating these viral and cellular genes. Given the limited number of cell types examined, it would be premature to conclude that species differences account for the opposing results, although we can conclude that adenoviral-vector-mediated upregulation of these genes is an important issue for both murine and human models. While the CMV promoter has defined enhancer activity (29), how this enhancer activity (or that of the UbC promoter) influences expression of viral genes at the opposite end of the viral chromosome is a complete mystery, as is why such enhancer activity would exhibit dramatic cell type specificity (not correlated with promoter activity, as measured by GFP expression). These results also indicate the requirement for cell-type- or species-specific host factors for the upregulation of E4-orf6/7 expression mediated by either the CMV or UbC promoters/enhancers. Clearly, the choice of promoter for ectopic gene expression in a particular cell type can dramatically determine impacts on viral and cellular gene expression.
High-efficiency transduction of a wide range of cell types has allowed many investigators, including ourselves, to use adenovirus vectors to deliver and express nonviral genes. In light of our findings, results that solely depend on the use of an adenovirus vector should be interpreted cautiously. Although such experiments can be judiciously controlled by transduction with appropriate control viruses, the possibility that a particular effect is due to synergy between the gene being investigated and adenoviral products that are being expressed due to the presence of the exogenous promoter must always be considered.
There have been previous indications that recombinant adenoviruses can induce unintended consequences via E4 that are independent of the foreign gene expressed. An adenovirus with E1 deleted modulates endothelial cell survival and angiogenic potential in a manner that correlates with E4-mediated induction of connexin 40 and inhibition of connexin 43 expression through PKA (55). The vectors used contain the CMV promoter driving expression of LacZ or no foreign gene, although a possible role for the CMV promoter and contributions of specific E4 proteins in altering connexin expression were not tested. Furthermore, prolongation of adenoviral survival and reduced adaptive immune response occurred after deletion of the E4 gene from an adenovirus with E1 deleted using a hybrid promoter containing the CMV immediate-early enhancer and chicken β-actin promoter to direct LacZ expression, suggesting that E4 gene expression in the absence of the E1 and E3 genes is deleterious in certain cell types (12). Finally, E1B 55K and E4 orf 3 and 6 proteins have been shown to inhibit cellular pathways that recognize and repair double-stranded DNA breaks (4, 5, 44, 50). While these pathways prevent recognition of adenoviral genome ends as DNA breaks, thus preventing concatemerization of viral genomes, they can also sensitize cells to agents that cause DNA damage, such as radiation therapies (16).
Adenoviruses have had a checkered history of clinical success in gene therapy trials. Despite continued complications, adenoviral strategies for gene addition, vaccination, and cancer treatment continue to hold promise. A series of well-publicized gene therapy trials utilized adenoviral vectors to delivery the cystic fibrosis transmembrane conductance regulator (CFTR) gene to the lung epithelia of cystic fibrosis patients. Although such treatments have sometimes resulted in clinically relevant improvements, they have often been inconsistent and transient (17, 23, 53, 54). A lack of long-term CFTR expression due to inflammation and loss of transduced cells was a primary obstacle. The choice of promoter used and the presence or absence of adenoviral sequences, such as E4, clearly could affect the success of future gene replacement studies using adenoviral vectors, in part by influencing viral effects on the expression of cellular genes, such as TAp73 (although effects on p73 are probably less relevant when the target cells are quiescent).
Genetically modified adenoviral vectors have shown promise in the treatment of head and neck squamous cell carcinomas, as well as other tumors. In fact, chemotherapy has increased efficacy when used in conjunction with Onyx-015, an E1B 55K mutant adenovirus that preferentially replicates in tumor cells with disrupted p53 pathways (37). Furthermore, the only currently approved gene therapy protocol effectively utilizes Ad CMV-p53 in combination with radiotherapy for the treatment of head and neck squamous cell carcinoma (33). These findings parallel our results, which demonstrate decreased numbers of Ad CMV-GFP-transduced MEFs grown in the presence of chemotherapeutics and suggest that human clinical-oncology studies may benefit by utilizing adenoviral vectors containing promoters shown to enhance TAp73 expression in the target cell type. In fact, given the dependence of recombinant adenovirus-mediated upregulation of p73 on CDK activity, ectopic promoter- and E4-orf6/7-mediated effects on cellular gene expression may be most relevant for gene therapy protocols for cancers, which inevitably exhibit deregulation of the CDK pathway. Perhaps the insights gained in our work can be used to optimize TAp73 induction and thus increase the therapeutic efficacy of these approaches.
In conclusion, our results demonstrate that adenoviral vectors containing the CMV promoter in MEFs or the UbC promoter in HFFs efficiently induce TAp73 expression via Ad E4-orf6/7. Based in part on our results presented here, manipulation of therapeutic adenoviral vectors to either limit or enhance their cytopathic effects should impact their clinical utility.
Acknowledgments
J.D. is supported by grants from the National Institutes of Health (RO1 CA77314) and by a Scholar Award from the Leukemia and Lymphoma Society. G.S.S. is supported by a Fellow Award from the Leukemia and Lymphoma Society. We thank K. Helm and M. Ashton of the Cancer Center Flow Cytometry Core (supported by grant 2 P30 CA 46934-09 and PO1HD38129). This work has been facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research (grant P30 AI054907).
REFERENCES
- 1.Attardi, L. D., S. W. Lowe, J. Brugarolas, and T. Jacks. 1996. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 15:3693-3701. [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Israel, H., and T. Kleinberger. 2002. Adenovirus and cell cycle control. Front. Biosci. 7:d1369-d1395. [DOI] [PubMed] [Google Scholar]
- 3.Bergamaschi, D., M. Gasco, L. Hiller, A. Sullivan, N. Syed, G. Trigiante, I. Yulug, M. Merlano, G. Numico, A. Comino, M. Attard, O. Reelfs, B. Gusterson, A. K. Bell, V. Heath, M. Tavassoli, P. J. Farrell, P. Smith, X. Lu, and T. Crook. 2003. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3:387-402. [DOI] [PubMed] [Google Scholar]
- 4.Boyer, J., K. Rohleder, and G. Ketner. 1999. Adenovirus E4 34k and E4 11k inhibit double strand break repair and are physically associated with the cellular DNA-dependent protein kinase. Virology 263:307-312. [DOI] [PubMed] [Google Scholar]
- 5.Carson, C. T., R. A. Schwartz, T. H. Stracker, C. E. Lilley, D. V. Lee, and M. D. Weitzman. 2003. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 22:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X., Y. Zheng, J. Zhu, J. Jiang, and J. Wang. 2001. p73 is transcriptionally regulated by DNA damage, p53, and p73. Oncogene 20:769-774. [DOI] [PubMed] [Google Scholar]
- 7.DeGregori, J. 2002. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim. Biophys. Acta 1602:131-150. [DOI] [PubMed] [Google Scholar]
- 8.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeRyckere, D., D. L. Mann, and J. DeGregori. 2003. Characterization of transcriptional regulation during negative selection in vivo. J. Immunol. 171:802-811. [DOI] [PubMed] [Google Scholar]
- 10.Everett, R. S., B. L. Hodges, E. Y. Ding, F. Xu, D. Serra, and A. Amalfitano. 2003. Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum. Gene Ther. 14:1715-1726. [DOI] [PubMed] [Google Scholar]
- 11.Flores, E. R., K. Y. Tsai, D. Crowley, S. Sengupta, A. Yang, F. McKeon, and T. Jacks. 2002. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416:560-564. [DOI] [PubMed] [Google Scholar]
- 12.Gao, G. P., Y. Yang, and J. M. Wilson. 1996. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J. Virol. 70:8934-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorziglia, M. I., M. J. Kadan, S. Yei, J. Lim, G. M. Lee, R. Luthra, and B. C. Trapnell. 1996. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J. Virol. 70:4173-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimison, B., T. A. Langan, and R. A. Sclafani. 2000. P16Ink4a tumor suppressor function in lung cancer cells involves cyclin-dependent kinase 2 inhibition by Cip/Kip protein redistribution. Cell Growth Differ. 11:507-515. [PubMed] [Google Scholar]
- 15.Halbert, D. N., J. R. Cutt, and T. Shenk. 1985. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J. Virol. 56:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart, L. S., S. M. Yannone, C. Naczki, J. S. Orlando, S. B. Waters, S. A. Akman, D. J. Chen, D. Ornelles, and C. Koumenis. 2005. The adenovirus E4orf6 protein inhibits DNA double strand break repair and radiosensitizes human tumor cells in an E1B-55K-independent manner. J. Biol. Chem. 280:1474-1481. [DOI] [PubMed] [Google Scholar]
- 17.Hay, J. G., N. G. McElvaney, J. Herena, and R. G. Crystal. 1995. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum. Gene Ther. 6:1487-1496. [DOI] [PubMed] [Google Scholar]
- 18.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin, M., M. C. Marin, A. C. Phillips, R. S. Seelan, D. I. Smith, W. Liu, E. R. Flores, K. Y. Tsai, T. Jacks, K. H. Vousden, and W. G. Kaelin, Jr. 2000. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407:645-648. [DOI] [PubMed] [Google Scholar]
- 20.Irwin, M. S., and W. G. Kaelin. 2001. p53 family update: p73 and p63 develop their own identities. Cell Growth Differ. 12:337-349. [PubMed] [Google Scholar]
- 21.Irwin, M. S., K. Kondo, M. C. Marin, L. S. Cheng, W. C. Hahn, and W. G. Kaelin. 2003. Chemosensitivity linked to p73 function. Cancer Cell 3:403-410. [DOI] [PubMed] [Google Scholar]
- 22.Jin, X., D. Nguyen, W. W. Zhang, A. P. Kyritsis, and J. A. Roth. 1995. Cell cycle arrest and inhibition of tumor cell proliferation by the p16INK4 gene mediated by an adenovirus vector. Cancer Res. 55:3250-3253. [PubMed] [Google Scholar]
- 23.Knowles, M. R., K. W. Hohneker, Z. Zhou, J. C. Olsen, T. L. Noah, P. C. Hu, M. W. Leigh, J. F. Engelhardt, L. J. Edwards, K. R. Jones, et al. 1995. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 333:823-831. [DOI] [PubMed] [Google Scholar]
- 24.Lai, C. M., Y. K. Lai, and P. E. Rakoczy. 2002. Adenovirus and adeno-associated virus vectors. DNA Cell Biol. 21:895-913. [DOI] [PubMed] [Google Scholar]
- 25.Lang, G. A., T. Iwakuma, Y. A. Suh, G. Liu, V. A. Rao, J. M. Parant, Y. A. Valentin-Vega, T. Terzian, L. C. Caldwell, L. C. Strong, A. K. El-Naggar, and G. Lozano. 2004. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119:861-872. [DOI] [PubMed] [Google Scholar]
- 26.Leon, R. P., T. Hedlund, S. J. Meech, S. Li, J. Schaack, S. P. Hunger, R. C. Duke, and J. DeGregori. 1998. Adenoviral-mediated gene transfer in lymphocytes. Proc. Natl. Acad. Sci. USA 95:13159-13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Q., and D. A. Muruve. 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10:935-940. [DOI] [PubMed] [Google Scholar]
- 28.Marton, M. J., S. B. Baim, D. A. Ornelles, and T. Shenk. 1990. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J. Virol. 64:2345-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier, J. L., and M. F. Stinski. 1996. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology 39:331-342. [DOI] [PubMed] [Google Scholar]
- 30.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 31.Naviaux, R. K., E. Costanzi, M. Haas, and I. M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olive, K. P., D. A. Tuveson, Z. C. Ruhe, B. Yin, N. A. Willis, R. T. Bronson, D. Crowley, and T. Jacks. 2004. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119:847-860. [DOI] [PubMed] [Google Scholar]
- 33.Pearson, S., H. Jia, and K. Kandachi. 2004. China approves first gene therapy. Nat. Biotechnol. 22:3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pediconi, N., A. Ianari, A. Costanzo, L. Belloni, R. Gallo, L. Cimino, A. Porcellini, I. Screpanti, C. Balsano, E. Alesse, A. Gulino, and M. Levrero. 2003. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat. Cell Biol. 5:552-558. [DOI] [PubMed] [Google Scholar]
- 35.Pozniak, C. D., S. Radinovic, A. Yang, F. McKeon, D. R. Kaplan, and F. D. Miller. 2000. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289:304-306. [DOI] [PubMed] [Google Scholar]
- 36.Reich, N. C., P. Sarnow, E. Duprey, and A. J. Levine. 1983. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology 128:480-484. [DOI] [PubMed] [Google Scholar]
- 37.Ries, S., and W. M. Korn. 2002. ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br. J. Cancer 86:5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaack, J., M. L. Bennett, J. D. Colbert, A. V. Torres, G. H. Clayton, D. Ornelles, and J. Moorhead. 2004. E1A and E1B proteins inhibit inflammation induced by adenovirus. Proc. Natl. Acad. Sci. USA 101:3124-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz, J. K., C. H. Bassing, I. Kovesdi, M. B. Datto, M. Blazing, S. George, X. F. Wang, and J. R. Nevins. 1995. Expression of the E2F1 transcription factor overcomes type beta transforming growth factor-mediated growth suppression. Proc. Natl. Acad. Sci. USA 92:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seelan, R. S., M. Irwin, P. van der Stoop, C. Qian, W. G. Kaelin, Jr., and W. Liu. 2002. The human p73 promoter: characterization and identification of functional E2F binding sites. Neoplasia 4:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenk, T., N. Jones, W. Colby, and D. Fowlkes. 1980. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harbor Symp. Quant. Biol. 44:367-375. [DOI] [PubMed] [Google Scholar]
- 42.Stevaux, O., and N. J. Dyson. 2002. A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol. 14:684-691. [DOI] [PubMed] [Google Scholar]
- 43.Stiewe, T., and B. M. Putzer. 2000. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat. Genet. 26:464-469. [DOI] [PubMed] [Google Scholar]
- 44.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418:348-352. [DOI] [PubMed] [Google Scholar]
- 45.Swift, S., J. Lorens, P. Achacoso, and G. P. Nolan. 1999. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Current protocols in immunology, unit 10.28, suppl. 31. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 46.Tauber, B., and T. Dobner. 2001. Adenovirus early E4 genes in viral oncogenesis. Oncogene 20:7847-7854. [DOI] [PubMed] [Google Scholar]
- 47.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11:281-288. [DOI] [PubMed] [Google Scholar]
- 48.Wan, Y. Y., and J. DeGregori. 2003. The survival of antigen-stimulated T cells requires NFκB-mediated inhibition of p73 expression. Immunity 18:331-342. [DOI] [PubMed] [Google Scholar]
- 49.Wan, Y. Y., R. P. Leon, R. Marks, C. M. Cham, J. Schaack, T. F. Gajewski, and J. DeGregori. 2000. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA 97:13784-13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weitzman, M. D., and D. A. Ornelles. 2005. Inactivating intracellular antiviral responses during adenovirus infection. Oncogene 24:7686-7696. [DOI] [PubMed] [Google Scholar]
- 51.Yang, A., N. Walker, R. Bronson, M. Kaghad, M. Oosterwegel, J. Bonnin, C. Vagner, H. Bonnet, P. Dikkes, A. Sharpe, F. McKeon, and D. Caput. 2000. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404:99-103. [DOI] [PubMed] [Google Scholar]
- 52.Yoder, S. S., and S. M. Berget. 1986. Role of adenovirus type 2 early region 4 in the early-to-late switch during productive infection. J. Virol. 60:779-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabner, J., L. A. Couture, R. J. Gregory, S. M. Graham, A. E. Smith, and M. J. Welsh. 1993. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75:207-216. [DOI] [PubMed] [Google Scholar]
- 54.Zabner, J., B. W. Ramsey, D. P. Meeker, M. L. Aitken, R. P. Balfour, R. L. Gibson, J. Launspach, R. A. Moscicki, S. M. Richards, T. A. Standaert, et al. 1996. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 97:1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, F., J. Cheng, G. Lam, D. K. Jin, L. Vincent, N. R. Hackett, S. Wang, L. M. Young, B. Hempstead, R. G. Crystal, and S. Rafii. 2005. Adenovirus vector E4 gene regulates connexin 40 and 43 expression in endothelial cells via PKA and PI3K signal pathways. Circ. Res. 96:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]