Abstract
In an accompanying report (Y. Eda, M. Takizawa, T. Murakami, H. Maeda, K. Kimachi, H. Yonemura, S. Koyanagi, K. Shiosaki, H. Higuchi, K. Makizumi, T. Nakashima, K. Osatomi, S. Tokiyoshi, S. Matsushita, N. Yamamoto, and M. Honda, J. Virol. 80:5552-5562, 2006), we discuss our production of a high-affinity humanized monoclonal antibody, KD-247, by sequential immunization with V3 peptides derived from human immunodeficiency virus type 1 (HIV-1) clade B primary isolates. Epitope mapping revealed that KD-247 recognized the Pro-Gly-Arg V3 tip sequence conserved in HIV-1 clade B isolates. In this study, we further demonstrate that in vitro, KD-247 efficiently neutralizes CXCR4- and CCR5-tropic primary HIV-1 clade B and clade B′ with matching neutralization sequence motifs but does not neutralize sequence-mismatched clade B and clade E isolates. Monkeys were provided sterile protection against heterologous simian/human immunodeficiency virus challenge by the passive transfer of a single high dose (45 mg per kg of body weight) of KD-247 and afforded partial protection by lower antibody doses (30 and 15 mg per kg). Protective neutralization endpoint titers in plasma at the time of virus challenge were 1:160 in animals passively transferred with a high dose of the antibody. The antiviral efficacy of the antibody was further confirmed by its suppression of the ex vivo generation of primary HIV-1 quasispecies in peripheral blood mononuclear cell cultures from HIV-infected individuals. Therefore, KD-247 promises to be a valuable tool not only as a passive immunization antibody for the prevention of HIV infection but also as an immunotherapy for the suppression of HIV in phenotype-matched HIV-infected individuals.
Because most primary strains of human immunodeficiency virus type 1 (HIV-1) are relatively resistant to neutralization, the specificities of antibodies that confer protective immunity against it are still not understood (22). Previously, we and others (9, 31) have reported that chimpanzees can be protected against infection with the T-cell-line-adapted strain HIV-1IIIB by passive transfer of either HIV immunoglobulin (Ig) (HIVIG) or anti-HIV-1IIIB V3 monoclonal antibodies (MAbs). Passive administration of the anti-HIV-1 gp41 human MAb 2F5 (24) to two chimpanzees prior to challenge with primary HIV-15016 resulted in a delay in plasma viremia and reduced viral load. Since the chimpanzee model is limited by the failure of HIV-1 to induce disease in these animals, a pathogenic model was developed in monkeys using a simian/human immunodeficiency virus (SHIV) strain that is capable of inducing high plasma viremia, CD4+-T-cell loss, and simian AIDS (11, 14, 15, 37). Following pathogenic SHIV 89.6P challenge, Mascola and colleagues (20) previously noted a synergistic effect with the passively transferred antibody HIVIG, a MAb against membrane-proximal external region 2F5 (27), and 2G12, a glycan-dependent MAb (41). Monkeys were afforded protective immunity against pathogenic SHIV DH12 by chimpanzee HIVIG and provided sterile protection against the challenge virus when given high-dose inoculations (27, 36). However, sterile protection was strain specific, and the antiserum did not bind a V3 loop peptide or block the interaction of gp120 with CD4. In several passive immunization studies using MAbs, the antibodies 2G12 and 2F5 as well as 4410, a MAb against membrane-proximal external region 4E10 (4), have been shown to inhibit SHIV in monkeys (2, 20, 21). Furthermore, human MAb b12, targeting the CD4-binding domain of gp120, has been reported to elicit complete protection against viral challenge (29) and partial protection against MAb 2G12 (22) in monkeys. Recently, passively transferred antibodies with 2G12, 2F5, and 4E10 were shown to delay the rebound of HIV-1 after the cessation of antiretroviral therapy, with that delay especially pronounced in acutely infected individuals. The in vivo effect of the neutralizing antibody cocktail was found to depend on 2G12 activity by escape mutant analysis (42).
It has been established that anti-V3 antibodies, induced by brief immunization protocols in animals, are capable of neutralizing HIV-1 in cell cultures and in animal challenge studies (13, 16, 27, 28). However, that capability has not been fully exploited because the V3 sequence is extremely diverse, and so the anti-V3 antibodies are extremely type specific and displayed little cross-reactivity. In the accompanying paper (8a), we describe how we sequentially immunized mice with V3 peptides derived from several different HIV-1 clade B field isolates. The antibody response could be traced to a tip sequence of the HIV-1 gp120 V3 domain, a relatively conserved motif (11, 18, 45). We reshaped anti-V3 MAb C25 into KD-247, a humanized MAb directed against the V3 tip motif Pro-Gly-Arg of the V3 domain. KD-247 cross-neutralized primary isolates with a matching neutralization sequence motif, suggesting that it could be used to overcome the previous limitations surrounding anti-V3 neutralizing antibody production by active immunization strategies.
In this study, we show that the humanized MAb KD-247 is suitable not only for use as a passive immunization antibody for the prevention of immunodeficiency virus infection but also to passively transfer antibodies for immunotherapy. Using 18 primary HIV-1 isolates, we evaluate the neutralizing capacity of KD-247. We also assess its efficacy against ex vivo generation of HIV from the peripheral blood mononuclear cells (PBMCs) of four HIV-infected individuals. Finally, we examine whether KD-247 can suppress HIV-1 replication in monkeys.
MATERIALS AND METHODS
Passive transfer of KD-247 to monkeys followed by pathogenic virus challenge.
All animals used in this study were mature, cycling, male cynomolgus monkeys (Macaca fascicularis) from the Tsukuba Primate Center, National Institute of Infectious Diseases (NIID), Japan. They were free of known simian retroviruses, herpesviruses, bacteria, and parasites. They were housed in accordance with the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science under the Japanese Law Concerning the Protection and Management of Animals (1, 38) and were maintained in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of NIID, Japan. Once approved by an institutional committee for biosafety level 3 experiments, these studies were conducted at the Tsukuba Primate Center, NIID, Japan, in accordance with the requirements specifically stated in the laboratory biosafety manual of the World Health Organization (44a).
The pathogenic SHIV strain C2/1 is an SHIV strain 89.6 variant isolated by in vivo passage in cynomolgus monkeys (37). The original SHIV 89.6 strain was kindly provided by Y. Lu at the Harvard AIDS Institute (Boston, MA) (19, 32). Virus stocks of SHIV C2/1 were stored at −125°C and thawed just prior to use. The challenge stock was provided by K. Shinohara of the National Institute of Infectious Diseases, Tokyo, Japan. Cynomolgus monkeys injected intravenously with SHIV C2/1 showed high levels of viremia and marked CD4+-T-cell depletion within 2 weeks after inoculation (1, 34, 35, 37). Naïve monkeys were intravenously administered 0, 15, 30, or 45 mg/kg of KD-247 along with either 45 mg/kg of purified normal human immunoglobulin (Nihon Pharmaceutical Co., Tokyo, Japan) or saline. Twenty-four hours after antibody transfer, the animals were intravenously challenged with 20 50% tissue culture infective doses (TCID50s) of SHIV C2/1.
In vitro virus neutralization assays.
The primary clinical isolate HIV-1MNp was kindly provided by J. Sullivan of the University of Massachusetts Medical School, Worcester, MA. The virus was confirmed to be neutralization resistant (5). Laboratory-adapted HIV-189.6 and HIV-1MN were obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health, Rockville, MD. GHOST cell neutralization assays were performed as described previously (5, 38). Briefly, GHOST cells expressing either CXCR4 or CCR5 coreceptors were used as targets of HIV-1 infection. The cells were then analyzed by FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA). The same concentration of either purified normal human immunoglobulin consisting primarily of the IgG1 subclass (Nihon Pharmaceutical Co.) or saline was used as control.
Neutralization activities in monkey plasma were assayed by detecting the neutralizing titers in the assay measuring 100% neutralization against the challenge virus as described previously by Nishimura et al. (26). In brief, plasma samples were serially diluted and incubated with 100 TCID50s of challenge virus, and M8166 cells were then incubated as previously described (26). The neutralization was expressed as the percent inhibition of simian immunodeficiency virus p27 antigen production in the culture supernatants (38, 39). Normal monkey plasma was used as a control.
PBMC-based virus neutralization assay.
HIV-1MN (H9/HTLV-III MN) was kindly provided by the AIDS Research and Reference Reagent Program, National Institutes of Health, Rockville, MD (45). The WHO primary isolates 92TH002, 92TH022, 92TH023 (all clade E), and 92TH014 (clade B′) were used as virus stocks (12). The primary isolates HIV-1JR-CSF and the CS and JCI series of HIV-1 isolates were provided by Y. Koyanagi (40) and Y. Okamoto (27). In vitro virus neutralization assays were performed as previously described (7, 12). Neutralization titers are expressed as either the concentration of serum IgG antibody or the reciprocal of the serum dilution that yielded a 50% (50% inhibitory concentration [IC50]) or 90% (IC90) reduction in HIV-1 p24 production over that seen in controls using purified serum IgG from healthy individuals or preimmune mouse sera.
Ex vivo virus neutralization assays.
The PBMCs of patients infected with HIV-1 were depleted of CD8+ cells by magnetic separation using polystyrene beads coated with anti-CD8 MAb (Dynabeads M-450 CD8; Dynal, Oslo, Norway). The negatively selected cells were stimulated with OKT3 antibody (1 μg/ml; Jannsen-Kyowa, Tokyo, Japan) and subsequently cultured in the presence of interleukin-2 (20 U/ml; Boehringer, Mannheim, Germany) together with KD-247 (60 and 240 μg/ml). The amount of HIV-1 p24 antigen in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA) (Dainabot, Tokyo, Japan). Approval by the ethical committee and written informed consent from all the human subjects were obtained according to the guidelines of the Ministry of Health, Labor, and Welfare, Japan, and to those of the Kumamoto University Medical School, Kumamoto, Japan.
Competitive PCR quantitation of SHIV RNA in plasma.
Quantitative competitive reverse transcription-PCR was performed as described previously by Piatak et al. (30), with both the substitution of a different competitor RNA and a different DNA template (35). The detection limit of this assay was 500 RNA copies/ml in monkey plasma.
Flow cytometric evaluation of cell surface antigen expression and absolute cell count.
Mouse MAbs conjugated with either fluorescein isothiocyanate, phycoerythrin (PE), PE-Cy5, or peridinin chlorophyll protein were used in flow cytometric analyses to detect cellular expression of monkey CD3 (NF-18; BioSource International Inc., Camarillo, CA), human CD4 (Nu-TH/I; Nichirei Co., Tokyo, Japan), CD8 (SK-1; Becton Dickinson & Co., San Jose, CA), and CD95 (CH11 and 7C11; Becton Dickinson) (30). To determine absolute cell counts, samples of whole blood were analyzed following the addition of fluorescein isothiocyanate-conjugated anti-CD3 (BioSource), PE-conjugated anti-CD4 (Becton Dickinson), and peridinin chlorophyll protein-conjugated anti-CD8 (Becton Dickinson) MAbs as previously described (35).
Plasma concentration of KD-247.
HIV-1 V3 peptide-based ELISA was used for quantification of KD-247 antibody. In brief, 96-well ELISA plates (Maxisorp; Nunc A/S, Roskilde, Denmark) were coated with 100 μl of a KD-247 antigen peptide (SP1 [YNKRKRIHIGPGRAFYTTKNC]) per well in 50 mM carbonate buffer (pH 9.3) at 1 μg/ml overnight at 4°C. KD-247 was diluted to concentrations ranging from 2.5 to 40 ng/ml as a reference. Bound KD-247 was detected with a peroxidase-conjugated anti-human IgG MAb (in-house preparation; The Chemo-Sero-Therapeutic Research Institute). The concentrations of KD-247 in the plasma of monkeys were determined using a calibration curve (SOFTmax; Molecular Devices Co., Menlo Park, CA).
Statistical analysis.
The plasma concentrations at various data points postdose were applied to a two-compartment model using an automatic pharmacokinetic analysis program (nonlinear least-squares method), and pharmacokinetic parameters were calculated.
RESULTS
Neutralization ability of the humanized antibody KD-247 against a panel of primary isolates as determined by a PBMC-based study.
In the initial series of the study, we showed that sequential immunization with synthetic V3 peptides from representatives of primary HIV-1 clade B isolates generated cross-reactive antisera and produced a high-affinity humanized MAb, KD-247, directed against the tip of the HIV-1 V3 domain, PGR. Furthermore, the humanized antibody more effectively neutralized several primary isolates of HIV-1 clade B than did previously reported neutralization antibodies (8a, 10, 23, 27). To further analyze the divergence of the cross-neutralization ability of the antibody by a PBMC-based HIV-1 neutralization assay, we used a panel of a total of 23 immunodeficiency viruses: 18 primary isolates of HIV-1 clade B, clade B′, and clade E viruses; 3 laboratory HIV-1 clade B viruses; and 2 highly pathogenic SHIVs (Table 1). The KD-247 antibody effectively neutralized HIV-1MN, HIV-1SF2, and HIV-189.6, containing the consensus V3 sequence of HIV-1 clade B, IGPGRAFY, with an IC90 and IC50 from 1 to 5 and from 0.1 to 1.0 μg/ml, respectively (Table 1, laboratory isolates, clade B). We next sought to assess whether the neutralization of primary isolates by KD-247 required a matching neutralization sequence motif. As expected, KD-247 effectively neutralized primary CCR5-tropic clade B and B′ isolates (IC90 and IC50 from 5 to 34 and from 0.4 to 3.2 μg/ml, respectively) and all four of the CXCR4-tropic clade B isolates (IC90 and IC50 from 4 to 6 and from 0.2 to 0.7 μg/ml, respectively) with matching IGPGR or V3 tip sequences. Thus, CCR5-tropic isolates with an IC90 of a mean concentration of neutralization antibody of 13.5 μg/ml were more than 2.8 times less sensitive to the neutralization by KD-247 than primary CXCR4-tropic isolates with a mean IC90 of 4.8 μg/ml. In contrast, the neutralization-resistant virus CS2-2 did not match the neutralization sequence motif, and the CS6-6 virus showed a QR insertion in the V3 tip sequence. The HIV-1 isolates containing a glutamine (Q) residue at position 20 in the V3 region, such as those of subtype E, were also resistant to neutralization by KD-247. Therefore, KD-247 effectively neutralizes both the CCR5- and CXCR4-tropic primary isolates with matching neutralization motifs.
TABLE 1.
PBMC-based neutralization of primary and laboratory isolates by KD-247a
| Isolate | Env V3 sequenceb | GHOST cell | KD-247
|
447-52D IC50c | |
|---|---|---|---|---|---|
| IC90 | IC50 | ||||
| Laboratory isolates, clade B | |||||
| HIV-1MN | CTRPNYNKRKRIHIGPGRAFYTTKNIIGTIRQAHC | X4 | 1 | 0.1 | 0.1 |
| HIV-1SF2 | -----N-T--G----------A-EK-V-D------ | X4 | 5 | 1.0 | 1.0 |
| HIV-189.6 | -----N-T-R-LS--------ARR----D------ | R5/X4 | 2.5 | 0.2 | >10 |
| Primary isolates, clade B | |||||
| HIV-1JR-CSF | ----SN-K--S------------GE---D------ | R5 | 5 | 0.4 | >10 |
| HIV-1CS2-2 | -----N-T--S--M---K-----GD---N----Y- | R5 | >50 | >50 | ND |
| HIV-1CS3-5 | ---I-N-T--S----------A-GE---N-K---- | R5 | 10 | 1.4 | ND |
| HIV-1CS4-4 | -I---N-T--G----L--WK--A-G--N------ | R5/X4 | >50 | >50 | ND |
| HIV-1CS6-6 | --G--N-T--S-R-QR------V-IGK--NM----- | R5 | >50 | >50 | ND |
| HIV-1CS6-8 | -I---N-T--G----------A-D----N------ | R5 | 8 | 1.2 | ND |
| HIV-1JCI-1 | ----HKTI----------------Q-E-N----- | X4 | 5 | 0.4 | ND |
| HIV-1JCI-2 | ----SN-T-R-------------RQ-R-D----- | X4 | 4 | 0.2 | ND |
| HIV-1JCI-3 | -----N-I--H------------RG-RD--K--- | R5 | 10 | 0.6 | ND |
| HIV-1JCI-5 | -------T--G---------V---G-RD--K--- | X4 | 4 | 0.2 | ND |
| HIV-1JCI-6 | ----SN-T-R---------S--A-Q-RGD------ | X4 | 6 | 0.7 | ND |
| HIV-1JCI-9 | -------T--G---------V---G-RD--K--- | R5 | 21 | 1.6 | ND |
| HIV-1JCI-11 | -------TS-G-R--------ASER-RD--K--- | R5 | 34 | 3.2 | ND |
| HIV-1JCI-22 | -----N-I--H------------RG-RD--K--- | R5 | 12 | 1.2 | ND |
| Primary isolates, clade B′ | |||||
| HIV-192TH014 | -----N-T--S-PL-----W---GQ---D------ | R5 | 8 | 0.9 | >1.5 |
| Primary isolates, clade E | |||||
| HIV-192TH002 | ----SN-T-TS-T----QV--R-GD---D--K-Y- | R5 | >50 | >50 | ND |
| HIV-192TH022 | ----SN-T-TS-T----QV--R-GD---D--K-Y- | R5 | >50 | >50 | >10 |
| HIV-192TH023 | ----SN-T-TS-N----QV--R-GD---D--K-Y- | R5 | >50 | >50 | ND |
| SHIV-B | |||||
| SHIV 89.6PD | -----N-T-R-LS--------ARR----D------ | R5/X4 | 5 | 0.5 | ND |
| SHIV C2/1 | -----N-T-E-LS--------ARR----D------ | R5/X4 | 5 | 0.5 | ND |
The HIV-1 sequences were confirmed by proviral DNA sequencing of virus-infected cells.
Dashes indicate sequence homology to HIV-1MN, and spaces represent the presence of a deletion.
ND, not done.
Ex vivo suppressive effects of KD-247 on the generation of HIV-1 quasispecies from PBMCs of HIV-infected individuals.
To fully assess the antiviral efficacy of KD-247, we next sought to determine whether it would suppress the generation of HIV-1 from PBMCs of HIV-infected individuals and whether it would do so as efficiently as an established anti-V3 humanized antibody, Cβ1 (23). As shown in Table 2, we investigated the effect of KD-247 at concentrations of 60 and 240 μg/ml on the ex vivo generation of HIV-1 using CD8+-T-cell-depleted PBMC cultures from four Japanese individuals infected with HIV-1 clade B (Env V3 sequence in Table 2). In the presence of KD-247 at concentrations of 60 and 240 μg/ml, the generation of viruses from PBMCs of KU008 was reduced in a dose-dependent manner, with 3.56- and 3.85-log reductions in the culture supernatants, respectively; reductions of 2.82 and 3.14 logs of virus generation from PBMCs of KU045 were also detected in the presence of 60 and 240 μg/ml of KD-247, respectively, KU037 showed a reduction of 3.56 logs at only 240 μg/ml. However, KU040 showed no dose-dependent suppressive effects of virus generation by KD-247. When the irrelevant antibodies of Cβ1 and normal serum IgG were added to cell cultures, they showed no suppressive effects on virus generation (data not shown). These results demonstrate that KD-247 effectively neutralizes nonpassage viruses generated in the primary culture of PBMCs from individuals infected with HIV-1 clade B with neutralization sequence motifs matching that of the quasispecies, IGPGR.
TABLE 2.
Ex vivo neutralizing activity of KD-247 against HIV-1 present in PBMC cultures established using cells from HIV-infected individualsa
| Patient | HIV-1 Env V3 sequence (no. of clones) | PBMCs, (no. of cells/well) | KD-247 (μg/ml) | p24 (log10 pg/ml) |
|---|---|---|---|---|
| KU008 | CTRPHNNTRKSIHIGPGRAFYATGDIIGNIRQAHC (3) | 6.5 × 105 | 0 | 3.93 |
| ------------------------E---D--R-- (2) | 60 | 0.37 | ||
| ------------------------E---D----- (1) | 240 | 0.08 | ||
| ----------------------------D----- (1) | ||||
| KU045 | CTRPNNNTRKGIHIGPGRAFYGTDIVGDIRQAHC (5) | 7.3 × 105 | 0 | 3.70 |
| -----------------------E-T-N----Y- (2) | 60 | 0.88 | ||
| ---------------------------N------ (1) | 240 | 0.56 | ||
| KU037 | CTRPNNNTRKSIPIGPGRAFYATGDIIGDIRKAHC (3) | 1.3 × 106 | 0 | 3.81 |
| -------I-------------------------- (1) | 60 | 3.86 | ||
| -I------G------------------------- (1) | 240 | 0.25 | ||
| KU040 | CTRPNNNTRKSVHIGPGRAWYATGEIIGNIRQAHC (2) | 8.0 × 105 | 0 | 4.12 |
| --------------A----F-------------- (1) | 60 | 2.34 | ||
| -----------I--------H------------- (1) | 240 | 2.62 | ||
| ---H-------I-L---G--H---D--------- (1) |
Ex vivo neutralization activity was directly detected by using CD8+ cell-depleted PBMCs from HIV-infected individuals as described in Materials and Methods.
The number of analyzed DNA clones from each patient is indicated in parentheses. Dashes indicate sequences identical to those of the upper major clone from each patient.
Induction of complete protection of monkeys against a highly pathogenic SHIV strain by a single passive transfer of a high dose of KD-247.
PBMCs from 12 juvenile male cynomolgus monkeys were first evaluated in vitro to establish their susceptibility to infection with the SHIV C2/1 challenge stock in standard viral infectivity assays (35, 37) (data not shown). Challenge virus SHIV C2/1 originated from SHIV 89.6 but did share an identical envelope sequence with the parental strain, HIV-189.6, and showed 17 nucleotide mutations with amino acid changes (1, 34). The neutralization sensitivity of SHIV C2/1 to KD-247 was found to be similar to that of HIV-189.6, with an IC90 and IC50 of 5 and 0.5 μg/ml in human PBMC-based neutralization assays, respectively (Table 1, laboratory isolates, clade B and SHIV-B), suggesting that the neutralization potency of KD-247 in vitro might be sufficient to warrant passive transfer experiments.
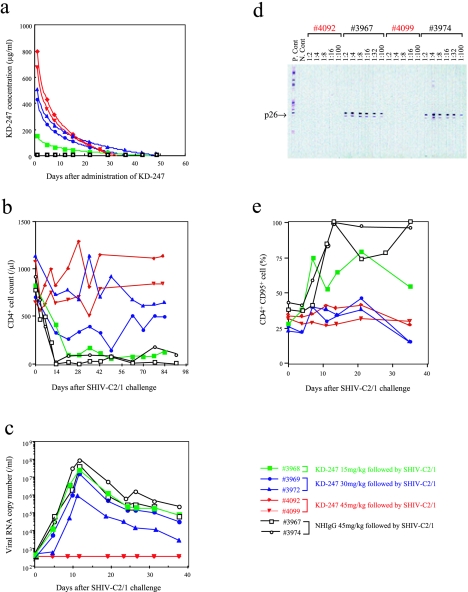
Of the 12 monkeys, 5 were inoculated with KD-247, 2 were inoculated with control normal human IgG (NHIgG) (45 mg/kg), and the remaining 5 were given saline alone. Of the five animals receiving KD-247, two were given a dose of 45 mg/kg, two received 30 mg/kg, and one received 15 mg/kg. Twenty-four hours after antibody transfer, all 12 monkeys were given an intravenous challenge of 20 TCID50s/ml SHIV (Fig. 1). At the time of viral challenge, the plasma concentrations of KD-247 were 151, 443, 496, 866, and 678 μg/ml of the antibody in immune sera from monkeys 3968, 3969, 3972, 4092, and 4099, respectively (Fig. 1a). The area under the plasma concentration time curve (AUC) values for monkeys 3968, 3969, 3972, 4092, and 4099 were calculated from the antibody concentration data to be 1.8, 3.5, 5.0, 6.5, and 5.6 mg · day/ml, respectively.
FIG. 1.
KD-247 efficiently protects monkeys from pathogenic virus challenge. A total of 12 cynomolgus monkeys were used for virus challenge studies with SHIV C2/1. In the first group, five monkeys were intravenously inoculated with various doses of KD-247, followed by 20 TCID50s of SHIV C2/1 challenge 24 h after antibody transfer. Monkeys in the second and third groups were injected prior to virus challenge with either 45 mg/kg of normal human immunoglobulin (two monkeys) or saline alone (five monkeys). The following parameters were measured in monkeys given KD-247: (a) concentration of KD-247 in plasma following passive transfer, (b) CD4+-T-cell counts, (c) plasma viremia, (d) Western blot analysis using an HIV-2 Western blot kit (Diagnostics Pasteur, Marnes-La-Coquette, France) (6) of serum samples obtained at autopsy from monkeys given a single high dose (45 mg/kg) of KD-247 (monkeys 4092 and 4099) or NHIgG controls (monkeys 3967 and 3974), and (e) CD95 antigen expression on PBMCs from monkeys challenged with SHIV.
The percentage of CD4+ T cells and the levels of plasma viremia were also monitored after SHIV challenge (Fig. 1b and c). All monkeys that were intravenously inoculated with normal human IgG or saline showed a loss of CD4+ T cells within 7 days of viral challenge, accompanied by plasma viremia reaching 107 to 108 viral RNA copies/ml (data from the five control monkeys that received saline alone are not shown). Of the two control monkeys that received 45 mg/kg of NHIgG, both seroconverted against SHIV p27 antigen (monkeys 3967 and 3974) (Fig. 1d). At autopsy, all control monkeys showed CD4+-T-cell depletion in lymphoid organs, a finding consistent with our previous observations using this model (35, 37).
Both monkeys that received a single high dose of 45 mg of KD-247 per kg of body weight prior to SHIV challenge were completely protected from viral challenge, maintaining stable CD4+-T-cell counts and not seroconverting or exhibiting plasma viremia (Fig. 1b to e, monkeys 4092 and 4099, indicated by red lines and red characters). When evaluated at autopsy using PCR for SHIV gag proviral DNA, their tissues showed no sign of infection (data not shown). The titers in plasma resulting from 100% in vitro neutralization against 100 TCID50s of the challenge virus at the time of virus challenge were 1:160 in both monkeys 4092 and 4099. The titers in partially protected monkeys 3969 and 3972 were 1:40 and 1:80, respectively. No neutralization activity of less than 1:10 was measured in the animals receiving 45 mg/kg of NHIgG (monkeys 3967 and 3974). Thus, although the highest titers of neutralization activities were detected in plasma from protected animals, the neutralization activity was high even in animals with only partial protection.
Administration of lower doses of KD-247, 30 mg/kg to two monkeys (monkeys 3969 and 3972, indicated by blue lines and blue characters in Fig. 1) and 15 mg/kg to one monkey (monkey 3968, indicated by green lines and green characters in Fig. 1), afforded partial protection from SHIV infection. Monkey 3972 (Fig. 1, closed triangle with blue line) showed better partial protection than monkey 3969, which received 30 mg/kg of antibody. That superior degree of partial protection may be related to better blood concentration of the antibody and to better AUC values. All three monkeys described above seroconverted against SHIV p27 antigen (data not shown), but their loss of CD4+ T cells seemed to be inversely proportional to the plasma concentration of KD-247 (Fig. 1a and b). Although the CD4+-T-cell decline indicated minimal protection in the monkey given 15 mg/kg of KD-247 (monkey 3968) (Fig. 1b), CD95 antigen expression, a marker for cell stimulation, was significantly lowered in this animal and completely inhibited in the other four monkeys receiving KD-247 (Fig. 1e), suggesting that KD-247 significantly suppressed PBMC stimulation by the virus challenge in these animals (monkeys 3969, 3972, 4092, and 4099).
These results therefore demonstrate that KD-247 efficiently neutralizes primary HIV isolates regardless of cell tropism. Furthermore, passive immunization with a single dose of 45 mg of antibodies per kg of body weight 24 h prior to viral challenge completely protected animals from viral challenge, showing that at high concentrations, KD-247 lowers the viral load and induces sterilizing immunity in the monkey model.
DISCUSSION
In this study, KD-247 proved an effective antiviral agent for the targeting of phenotype-matched viruses, one capable of both in vitro neutralization of primary isolates and in vivo passive transfer of the antibody as well as of suppressive effects against ex vivo generation of HIV from HIV-infected individuals. Although it has already been established that brief immunizations with a V3 peptide can elicit neutralizing antibodies to homologues of the CXCR4-tropic virus, the limitations of anti-V3 antibodies have been known for over a decade (8, 13, 16, 28). Also, at reasonable IC50s, the anti-V3 antibodies did not neutralize CCR5-tropic strains. In the accompanying paper (8a), we described the derivation of a humanized MAb, KD-247, that was produced by sequential immunization using six different HIV-1 Env V3 peptides derived from HIV-1 clade B field isolates. We suggested that KD-247 could potentially overcome the previous limitations to immunologically exploiting the anti-V3 antibody induced by brief immunization protocols, i.e., its extraordinary sequence variability and the associated isolate specificity of anti-V3 antibodies (27, 38). The findings of our current study suggest that KD-247 may curb the spread of viral infection and reduce viral loads in HIV-infected individuals who have been determined to share the V3 tip sequence of the virus by virus neutralization phenotype-matching analysis.
In vitro, KD-247 has potent neutralizing activity against a variety of primary HIV-1 clade B isolates, including CCR5-tropic viruses, at low concentrations. We found that KD-247 neutralized a variety of clade B primary viruses containing IGPGR V3 sequences, although its neutralization ability was affected by some of the surrounding amino acids of the V3 tip region, as discussed in the accompanying paper (8a). Based upon these results, we should be able to predict the neutralization ability of KD-247 by prior sequencing of the HIV-1 Env V3 region of the target virus. Using the previously published sequences found in the Los Alamos HIV-1 sequence database, we determined that the IGPGRA sequence is present in the majority of HIV-1 clade B isolates (45) to which KD-247 would be expected to have cross-neutralization activity. Moreover, KD-247 significantly curbed the generation of primary HIV-1 quasispecies in ex vivo cultures of CD8+-T-cell-depleted PBMCs from seropositive individuals. However, as described above, the major limitation of KD-247 as an antiviral agent is its inability to neutralize variants expressing amino acid alterations in the binding site PGR motif and additional amino acids.
What are the properties that make KD-247 an effective neutralizer of CCR5-tropic viruses? First, the site-specific binding of KD-247 to epitopes on the virus envelope glycoprotein seems to be key to its virus neutralization ability. Indeed, the results of the Pepscan analysis reported in the accompanying paper suggest that KD-247 can react with core V3 sequences from various HIV-1 clade B isolates (8a). The shortest peptide that was reactive with KD-247 was IGPGR, but that epitope was stabilized by the addition of one or more amino acids. Furthermore, IGPGRA and GPGRAF sequences occur in the majority of HIV-1 isolates from donors in the United States (17). The results of Pepscan with replacement peptides also suggest that KD-247 has broad binding activity to HIV-1. While the number of amino acid substitutions tolerated in the central PGR sequence of the V3 tip peptide was small, replacement of amino acids in the flanking region was relatively permissible. Second, ex vivo neutralization assays using patient-derived isolates containing APGR and GPGG sequences in the V3 tip showed incomplete neutralization (Table 2, KU040). Thus, KD-247 would be expected to bind with HIV-1 quasispecies having a recognition sequence similar to the neutralization phenotype. Third, as the accompanying paper demonstrates, high-affinity antibody binding is apparently required for neutralization, because the kinetic parameters of KD-247 were identified to be fast on and slow off rates, similar to those of a type-specific MAb, Rμ5.5, although the equilibrium dissociation constant value of KD-247 for binding to a control SP1 peptide was higher than that of Rμ5.5 (8a). This is a reasonable assumption, since the epitope of KD-247 (IGPGR) is shorter than that of Rμ5.5 (IHIGPGRAFYT). The high association rate of KD-247 might be responsible for exerting the observed cross-neutralization activity against various primary isolates. These results are consistent with the hypothesis that virus neutralization can be explained by the kinetic parameters of antibody binding.
Most recent passive transfer studies with monoclonal antibodies used common combinations of broadly cross-reactive human MAbs capable of neutralizing primary HIV-1 isolates. In monkeys, human MAbs b12 (29) and 2G12 (20) were shown to induce complete and partial protection, respectively, against viral challenges. In contrast, the MAb chosen for this study, KD-247, is a humanized antibody induced by sequential immunization with a set of V3 peptides from primary isolates. Because the KD-247 IC90 value from an in vitro neutralization assay in our study, 5.0 μg/ml of the antibody, approximates that obtained by a single antibody, b12 (3), and a combination of the two MAbs 2F5 and 2G12 or a triple combination of HIVIG, 2F5, and 2G12, as previously reported (41, 43), we postulated that KD-247 was sufficiently potent to achieve protection of monkeys against a pathogenic SHIV challenge. Since our previous experience (9) has taught us to expect approximately 500 to 1,000 μg/ml in sera from monkeys passively immunized with 30 to 45 mg of antibody per kg of body weight, the potency of KD-247 should prove sufficient for passive transfer experiments of effective antibodies in animals in vivo. We also expected that a single passive transfer of KD-247 via inoculation with 15 and 30 mg of antibody would result in approximately 150 to 500 μg/ml of plasma concentration at the time of viral challenge. As expected, we found an AUC value of 1.8 to 5.0 mg · day/ml. Consequently, we found that animals passively immunized with 45 mg/kg of KD-247 showed 678 and 866 μg/ml of KD-247 in plasma at the time of viral challenge and an AUC value of 5.6 and 6.5 mg · day/ml. Those animals were provided sterile protection against intravenous challenge with the pathogenic virus SHIV C2/1. The protective endpoint titers of neutralization antibodies in plasma at the time of virus inoculation were 1:160 in both animals that elicited sterile immunity, and a high titer of neutralization activity in plasma was similarly detected in completely protected monkeys, as described previously by Nishimura et al. (26) and Parren et al. (29). Thus, the high titers of neutralization activity in plasma confer sterile protection against viral challenge in the passively immunized animals with neutralizing antibodies. Furthermore, the pharmacokinetic information consisting of the plasma concentration of the neutralizing antibodies at the time of viral challenge and the AUC value may be closely related to the ability of the antibody to provide sterile protection against viral challenge. Since those protected macaques demonstrated the inhibition of CD4+ cell loss, the pharmacokinetic properties of KD-247 may also be closely associated with the inhibition of CD4+ cell decline in the peripheral circulation of the challenged monkeys.
In this study, we also detected lower viremia with lesser CD4+ cell decline in animals that were inoculated with intermediate doses of antibody. However, we noted that the lesser doses of the antibody provided complete protection against enhanced rates of the CD4+ CD95+ cell subpopulation in the peripheral circulation of the challenged animals, suggesting that the reshaping MAb might be able to control the activation of peripheral CD4+ T cells in animals by its passive transfer. Although the number of monkeys enrolled in this study was limited, it remains noteworthy that a single inoculation with KD-247, even at a suboptimal dose for viral protection, appeared to be effective for maintaining CD4+ T cells in monkeys inoculated with virus. Since it has been previously reported that the limited effect of neutralizing antibody may be related to the rapid appearance of an escape mutant in infected individuals, high titers of neutralization activity should be generated in the passively immunized animals (25, 33, 44). In our preliminary study, we isolated the escape mutant from the neutralization resistance virus HIV-1JR-FL in the presence of KD-247: at passage 8 of the culture in the presence of 1,000 μg/ml KD-247, one amino acid substitution, GPGR to GPER, was identified in the V3 tip (K. Yoshimura et al., unpublished results). Collectively, these results suggest that KD-247 shows clinical promise both for passive immunization and as a strategy for preventing viral spread in phenotype-matched HIV-infected individuals.
Acknowledgments
We thank Richard M. Krause and Malcolm Martin, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD; Susan Zolla-Pazner, New York University School of Medicine, New York, NY; and Ruth Connor, Aaron Diamond AIDS Research Center, New York, NY, for their helpful discussions and revision of the manuscript.
This work was supported by the Panel on AIDS of the US-Japan Cooperative Medical Science Program and the Health Science Foundation, Japan.
REFERENCES
- 1.Ami, Y., Y. Izumi, K. Matsuo, K. Someya, M. Kanekiyo, S. Horibata, N. Yoshino, K. Sakai, K. Shinohara, S. Matsumoto, T. Yamada, S. Yamazaki, N. Yamamoto, and M. Honda. 2005. Priming-boosting vaccination with recombinant Mycobacterium bovis bacillus Calmette-Guérin and a nonreplicating vaccinia virus recombinant leads to long-lasting and effective immunity. J. Virol. 79:12871-12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso, R. M., M. B. Zwick, R. L. Stanfield, R. Kunert, J. M. Binley, H. Katinger, D. R. Burton, and I. A. Wilson. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163-173. [DOI] [PubMed] [Google Scholar]
- 5.Cecilia, D., V. N. Kewalramani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, S. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z., A. Luckay, D. L. Sodora, P. Telfer, P. Reed, A. Gettie, J. M. Kanu, and R. F. Sadek. 1997. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 71:3953-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chujoh, Y., K. Matsuo, H. Yoshizaki, T. Nakasatomi, K. Someya, Y. Okamoto, S. Naganawa, S. Haga, H. Yoshikura, A. Yamazaki, S. Yamazaki, and M. Honda. 2002. Cross-clade neutralizing antibody production against human immunodeficiency virus type 1 clade E and B′ strains by recombinant Mycobacterium bovis BCG-based candidate vaccine. Vaccine 20:797-804. [DOI] [PubMed] [Google Scholar]
- 8.Durda, P. J., L. Bacheler, P. Clapham, A. M. Jenoski, B. Leece, T. J. Matthews, A. McKnight, R. Pomerantz, M. Rayner, and K. J. Weinhold. 1990. HIV-1 neutralizing monoclonal antibodies induced by a synthetic peptide. AIDS Res. Hum. Retrovir. 6:1115-1123. [DOI] [PubMed] [Google Scholar]
- 8a.Eda, Y., M. Takizawa, T. Murakami, H. Maeda, K. Kimachi, H. Yonemura, S. Koyanagi, K. Shiosaki, H. Higuchi, K. Makizumi, T. Nakashima, K. Osatomi, S. Tokiyoshi, S. Matsushita, N. Yamamoto, and M. Honda. 2006. Sequential immunization with V3 peptides from primary human immunodeficiency virus type 1 produces cross-neutralizing antibodies against primary isolates with a matching narrow-neutralization sequence motif. J. Virol. 80:5552-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emini, E. A., W. A. Schleif, J. H. Nunberg, A. J. Conley, Y. Eda, S. Tokiyoshi, S. D. Putney, S. Matsushita, K. E. Cobb, C. M. Jett, J. W. Eichberg, and K. K. Murthy. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728-730. [DOI] [PubMed] [Google Scholar]
- 10.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 11.Hattori, T., K. Shiozaki, Y. Eda, S. Tokiyoshi, S. Matsushita, H. Inaba, M. Fujimaki, T. Meguro, K. Yamada, M. Honda, K. Nishikawa, and K. Takatsuki. 1991. Characteristics of the principal neutralizing determinant of HIV-1 prevalent in Japan. AIDS Res. Hum. Retrovir. 7:825-830. [DOI] [PubMed] [Google Scholar]
- 12.Honda, M., K. Matsuo, T. Nakasone, Y. Okamoto, H. Yoshizaki, K. Watanabe, Y. Fukushima, W. Sugiura, S. Haga, Y. Katsura, K. Kitamura, H. Tasaka, K. Komuro, T. Yamada, T. Asano, A. Yamazaki, and S. Yamazaki. 1995. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guérin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc. Natl. Acad. Sci. USA 92:10693-10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javaherian, K., A. J. Langlois, C. McDanal, K. L. Ross, L. I. Eckler, C. L. Jellis, A. T. Profy, J. R. Rusche, D. P. Bolognesi, S. D. Putney, and T. J. Matthews. 1989. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc. Natl. Acad. Sci. USA 86:6768-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L. J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed monkeys. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, M. K. Reimann, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laman, J. D., M. M. Schellekens, Y. H. Abacioglu, G. K. Lewis, M. Tersmette, R. A. Fouchier, J. P. Langedijk, E. Claasen, and W. J. Boersma. 1992. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J. Virol. 66:5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRosa, G. J., J. P. Davide, K. Weinhold, J. A. Waterbury, A. T. Profy, J. A. Lewis, A. J. Langlois, G. R. Dreemsman, R. N. Boswell, P. Shadduck, L. H. Holley, M. Karplus, D. P. Bolognesi, T. J. Matthews, E. A. Emini, and S. D. Putney. 1990. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science 249:932-935. [DOI] [PubMed] [Google Scholar]
- 18.Los Alamos Database and Analysis Staff. 2003. Part II. HIV-1/SIVepz complete genome alignments, p. 123-317. In T. Leitner, B. Foley, B. Hahn, P. A. Marx, F. McCutchan, J. W. Mellors, S. Wolinksy, and B. Korber (ed.), HIV Sequence Compendium 2003. Publication LA-UR 04-7420. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 19.Lu, Y., M. S. Salvato, C. D. Pauza, J. Li, J. Sodroski, K. Manson, M. Wyand, N. Letvin, S. Jenkins, N. Touzjian, C. Chutkowski, N. Kushner, M. LeFaile, L. G. Payne, and B. Roberts. 1996. Utility of SHIV for testing HIV-1 vaccine candidates in monkeys. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:99-106. [DOI] [PubMed] [Google Scholar]
- 20.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankle, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 21.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathews, T. J. 1994. Dilemma of neutralizing resistance of HIV-1 field isolates and vaccine development. AIDS Res. Hum. Retrovir. 10:633-636. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita, S., H. Maeda, K. Kimachi, Y. Eda, Y. Maeda, T. Murakami, S. Tokiyoshi, and K. Takatsuki. 1992. Characterization of a mouse/human chimeric monoclonal antibody (Cβ1) to a principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. AIDS Res. Hum. Retrovir. 8:1107-1115. [DOI] [PubMed] [Google Scholar]
- 24.Muster, T., F. Steindl, M. Purtscher, A. Trkola, G. Himmler, F. Rükler, and H. Katinger. 1993. Conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 256:54-63. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto, Y., Y. Eda, A. Ogura, S. Shibata, T. Amagai, Y. Katsura, T. Asano, K. Kimachi, K. Makizumi, and M. Honda. 1998. In SCID-hu mice, passive transfer of a reshaping antibody prevents infection and atrophic change of medulla in human thymic implant due to intravenous inoculation of primary HIV-1 isolate. J. Immunol. 160:69-76. [PubMed] [Google Scholar]
- 28.Palker, T. J., M. E. Clark, A. J. Langlois, T. J. Matthews, K. J. Weinhold, R. R. Randall, D. P. Bolognesi, and B. F. Haynes. 1988. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc. Natl. Acad. Sci. USA 85:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piatak, M., Jr., K. C. Luk, B. Williams, and J. D. Lifson. 1993. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques 14:70-81. [PubMed] [Google Scholar]
- 31.Prince, A. M., H. Reesink, D. Pascual, B. Horowitz, I. Hewlett, K. K. Murthy, K. E. Cobb, and J. W. Eichberg. 1991. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retrovir. 7:971-973. [DOI] [PubMed] [Google Scholar]
- 32.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai, K., K. Shinohara, E. Takahashi, Y. Izumi, Y. Ami, Y. Sasaki, Y. Suzaki, S. Ando, T. Nakasone, and M. Honda. 2001. Molecular cloning of a pathogenic simian-human immunodeficiency virus for HIV/AIDS monkey model, p. 84. Proceedings of the Sixth International Congress on AIDS in Asia and the Pacific. Melbourne, Australia.
- 35.Sasaki, Y., Y. Ami, K. Shinohara, E. Takahashi, S. Ando, K. Someya, Y. Suzaki, T. Nakasone, and M. Honda. 2000. Induction of CD95 ligand expression on CD8+ T-lymphocyte correlates with HLA-DR expression and contributes to apoptosis of CD95-upregulated CD4+ T-cells in monkeys by infection with a pathogenic simian/human immunodeficiency virus. Clin. Exp. Immunol. 121:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Wiley, M. W. Cho, and M. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara, K., K. Sakai, S. Ando, Y. Ami, N. Yoshino, E. Takahashi, K. Someya, Y. Suzaki, T. Nakasone, Y. Sasaki, M. Kaizu, Y. Lu, and M. Honda. 1999. A highly pathogenic simian/human immunodeficiency virus with genetic changes in cynomolgus monkey. J. Gen. Virol. 8:1231-1240. [DOI] [PubMed] [Google Scholar]
- 38.Someya, K., D. Cecilia, Y. Ami, T. Nakasone, K. Matsuo, S. Burda, H. Yamamoto, N. Yoshino, M. Kaizu, S. Ando, K. Okuda, S. Zolla-Pazner, S. Yamazaki, N. Yamamoto, and M. Honda. 2005. Vaccination of rhesus monkeys with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif. J. Virol. 79:1452-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Someya, K., Y. Ami, T. Nakasone, Y. Izumi, K. Matsuo, S. Horibata, K.-Q. Xin, H. Yamamoto, K. Okuda, N. Yamamoto, and M. Honda. 2006. Induction of positive cellular and humoral immune responses by a prime-boost vaccine encoded with simian immunodeficiency virus gag/pol. J. Immunol. 176:1784-1795. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, H., Y. Suzuki, M. Tatsumi, H. Hoshino, E. S. Daar, and Y. Koyanagi. 2002. Isolation and characterization of an infectious HIV type 1 molecular clone from a patient with primary infection. AIDS Res. Hum. Retrovir. 18:1127-1133. [DOI] [PubMed] [Google Scholar]
- 41.Trkola, A., A. B. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. Barbas III, D. R. Burton, D. D. Ho, and J. P. Moore. 1995. Cross-clade neutralization of primary isolate of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Gunthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 43.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 44a.World Health Organization. 2004. WHO laboratory biosafety manual, 3rd ed. World Health Organization, Geneva, Switzerland.
- 45.Yamanaka, T., Y. Fujimura, S. Ishimoto, A. Yoshioka, M. Konishi, N. Narita, J. Mimaya, T. Meguro, T. Nakasone, Y. Okamoto, H. Yoshizaki, K. Yamada, and M. Honda. 1997. Correlation of titer of antibody to principal neutralizing domain of HIV MN strain with disease progression in Japanese hemophiliacs seropositive for HIV type 1. AIDS Res. Hum. Retrovir. 13:317-326. [DOI] [PubMed] [Google Scholar]