Abstract
Successful immunotherapy with peptide vaccines depends on the in vivo generation of sufficient numbers of anti-tumor T cells with appropriate phenotypic and functional characteristics to mediate tumor destruction. Herein, we report the induction of high frequencies of circulating CD8+ T cells (4.8% to 38.1%) directed against the native gp100:209-217 peptide derived from the gp100 melanoma-melanocyte tumor antigen in five HLA-A*0201 patients at high risk of recurrence of melanoma after multiple courses of immunization with modified gp100:209-217(210M) peptide in IFA. Longitudinal peripheral blood mononuclear cell (PBMC) analysis revealed a phenotypic shift of native peptide-specific CD8+ T cells from an early effector to an effector memory (CD27- CD28- CD62L- CD45RO+) phenotype with repeated immunizations and functional maturation that correlated with gp100:209-217 peptide-specific T-cell precursor frequencies. Postimmunization PBMC exhibited direct ex vivo recognition of melanoma cell lines in ELISPOT analysis, showed lytic capability against peptide-pulsed target cells, and proliferated in response to native peptide stimulation. One year after final immunization, circulating vaccine-specific CD8+ T cells persisted in patients’ PBMC with a maintained effector memory phenotype. The results herein demonstrate the efficacy of a multiple course peptide-immunization strategy for the generation of high frequencies of tumor antigen-specific T cells in vivo, and further show that continued peptide immunization results in the escalating generation of functionally mature, tumor-reactive effector memory CD8+ T lymphocytes.
Keywords: human antigen/peptide/epitope, vaccination, tumor immunity, T lymphocytes, differentiation
The identification of numerous major histocompatibility complex (MHC) class I-restricted epitopes from human cancers over the last decade has facilitated the use of peptide-based vaccination as a new immunotherapeutic approach for the treatment of patients with cancer.1 In melanoma, the melanocytic lineage-specific antigen, gp100 protein, has been identified as a frequent target of tumor-infiltrating lymphocytes (TIL), and adoptive cell transfer of gp100-reactive TIL has been shown to mediate tumor regression in patients with metastatic melanoma.2 Although several peptides derived from gp100 are naturally processed and presented on human melanoma cells and associated with HLA-A*0201-restricted tumoricidal cytolytic T-cell (CTL) activity,3 two gp100 epitopes recognized by CTL, gp100:209-217 and gp100:280-288 (referred to g209 and g280, respectively), bind to HLA-A*0201 molecules with intermediate affinity due to suboptimal amino acid residues in key MHC-anchoring positions.4 Modification of the g209 peptide, by substituting a methionine for threonine at position 2, increased peptide-HLA-A*0201 binding affinity and enhanced the capacity for eliciting tumor antigen-reactive CTL through in vitro sensitization of peripheral blood mononuclear cells (PBMC) from patients with melanoma.5-7 Peptide-based vaccination with the modified g209 peptide (referred to as g209-2M) was more efficient than the g209 peptide in successfully immunizing patients with metastatic melanoma.7 Circulating native g209 peptide-specific CD8+ T cell frequencies were generally less than 1% in these patients.8 Despite the generation of reproducible, tumor antigen-specific T-cell responses after peptide vaccination, objective cancer responses were seldom observed in immunized patients.6,7,9
The primary goal of peptide vaccination for the treatment of patients with cancer is the in vivo induction or amplification of sufficient numbers of functionally mature, tumor antigen-specific cells capable of tumor cell obliteration and long-term protection. Current peptide vaccination strategies, which often vary in injection number and frequency, have shown limited effectiveness in generating circulating melanoma antigen-reactive T-cell frequencies greater than 2% of CD8+ T cells in vivo,8,10-12 more than one magnitude lower than antigen-specific CD8+ T-cell frequencies required for the elimination of acute viral infections.13-15 Although vaccine-induced T cells can display anti-melanoma reactivity both invitro and ex vivo, their limited numbers may be closely linked to the low rate of peptide vaccine-induced tumor regression observed in vivo. The detection of these modest populations has often involved in vitro antigen-driven expansion of vaccine-specific T cells. Because in vitro stimulation can hamper the accurate monitoring of vaccination-elicited T-cell frequency, phenotype, and functional differentiation in vivo, the generation of greater vaccine-specific T-cell frequencies in vivo would provide an improved means of direct immune monitoring.
To investigate whether a more aggressive vaccination protocol can facilitate the enhancement of functionally active melanoma antigen-reactive T-cell numbers in vivo and allow for the phenotypic and functional analysis of circulating lymphocytes without the need for in vitro stimulation, HLA-A*.0201 patients at high risk of recurrence of melanoma were vaccinated with the modified g209-2M peptide in adjuvant using a multiple-course vaccination strategy conducted during a 336-day period. Here, we longitudinally analyzed native g209 peptide-specific T-cell precursor frequency in the peripheral blood of five peptide-vaccinated patients using direct ex vivo peptide-specific tetramer and interferon-gamma (IFN-γ) ELISPOT assays over multiple courses of immunization. Multiparameter flow cytometric analysis was used to study the effect of the multiple course strategy upon changes in cell surface phenotype associated with T-cell differentiation. In addition, the functional characteristics of PBMC from immunized patients were evaluated for the expression of perforin and ex vivo cytokine production, proliferation, and specific cytolytic activity against g209 peptide-pulsed antigen-presenting cells (APC) and gp100-expressing melanoma cells. Lastly, the frequency, differentiation phenotype, and function of persisting vaccine-specific T cells were analyzed 1 year after the last immunization.
MATERIALS AND METHODS
Patient Selection
In the current study, five HLA-A*0201-positive patients between the ages of 38 and 65 received 1 mg modified gp100:209-217(210M) peptide (g209-2M) and 1 mg tyrosinase:368-376(370D) peptide in a protocol approved by the Institutional Review Board of the National Cancer Institute. Each peptide was emulsified separately in Montanide ISA 51 (IFA) adjuvant and injected subcutaneously in different extremities. All patients had completely resected melanoma with no clinical evidence of disease but were at high risk of recurrence. Patients 1, 2, and 3 received peptide vaccination every 3 weeks for a total of 16 injections (4 courses of 4 injections each). Patients 4 and 5 received 4 courses of peptide injection; each course consisted of 10 weekly injections followed by a 3-week break between courses. PBMC were obtained by cytapheresis before peptide vaccination, 3 weeks after each vaccination course and 1 year after the final immunization. Although the number of peptide injections per course differed between these vaccination arms, the time of leukapheresis was identical among cohorts with PBMC samples collected 84 days after the start of each new vaccination course. Collected cells were enriched for lymphocytes with lymphocyte separation medium (ICN Biomedicals Inc., Aurora, OH) and were cryopreserved.
Peptides
The nonamer modified gp100:209-217(210M) peptide (g209-2M, IMDQVPFSV) and the tyrosinase:368-376(370D) peptide (YMDGTMSQV) were produced to Good Manufacturing Practice grade by solid phase synthesis by Multiple Peptide Systems (San Diego, CA). The gp100:209-217 peptide (g209, ITDQVPFSV) and the gp100:280-288 peptide (g280, YLEPGPVTA) were provided by the National Cancer Institute Cancer Therapy Evaluation Program.
Cells and Culture Conditions
Before analysis, PBMC were thawed and rested overnight in complete media (CM) consisting of RPMI 1640 (Invitrogen Corp., Carlsbad, CA) supplemented with 2 mM glutamine (Biofluids, Rockville, MD), 25 mM HEPES buffer (Biofluids), 100 U/mL penicillin (Biofluids), 100 μg/mL streptomycin (Biofluids), 50 μM 2-mercaptoethanol (Invitrogen) and 10% heat-inactivated human AB sera (Gemini Bioproducts, Woodland, CA) to dgeplete adherent monocytes. The HLA-A*0201+ melanoma cell lines, 526 and 624, and the HLA-A*0201- melanoma lines, 624.28 and 888, were derived in this laboratory from tumor samples by enzymatic digestion as previously described.16
Tetramers and Monoclonal Antibodies
PE-labeled g209-2M, g209, and HIV gag (SLYNTVATL) peptide/HLA-A*0201 tetramer complexes were obtained from Beckman Coulter, Inc. (Fullerton, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-human CD27, CD8, and HLA-DR antibodies and biotin-conjugated CCR7 monoclonal antibodies (mAB) were obtained from BD Biosciences (San Jose, CA). Biotinylated CCR7 antibody was used with streptavidin-APC (BD Biosciences). FITC-conjugated anti-CD28, CD45RA, CD45RO, CD62L, CD25, perforin, mouse IgG2b, and APC-labeled CD8 antibodies were obtained from BD PharMingen (San Diego, CA)
Flow Cytometric Immunofluorescence Analysis
After 16-to 24-hour culture of thawed PBMC in CM to remove adherent monocytes, nonadherent cells were resuspended in fluorescent-activated cell sorter buffer consisting of phosphate-buffered saline (PBS) with 2% fetal bovine serum(Gemini Bioproducts) at 5 × 106 cells/mL and blocked with 10% normal mouse Ig (Caltag Labs, Burlingame, CA) for 15 minutes on ice. Cells (5 × 105) in 100 μL were stained with tetramer and FITC and APC-conjugated mABs at 4°C for 40 minutes in the dark. Cells were washed twice, briefly stained with propidium iodide for nonviable cell exclusion, and subsequently analyzed in a FACSCalibur (Becton Dickinson) For CCR7 staining, cells were first stained with biotinylated anti- CCR7 antibody, washed, and treated with streptavidin-APC. Subsequently, CCR7 antibody-stained cells were stained with tetramer and anti-CD8 antibody. For intracellular perforin staining, cells were stained with tetramer and anti-CD8 antibody and then permeabilized and fixed using the Cytofix/Cytoplug Plus with GolgiPlug kit (BD PharMingen). Cells were then stained with FITC conjugated perforin antibody, washed twice, and analyzed.
IFN-γ ELISPOT Assay
Nitrocellulose-lined ELISPOT plates (Millipore) were coated overnight at room temperature with 1 μg anti-human IFN-γ antibody per well (BioSource), washed 4 times, and then blocked with CM for 30 minutes at 37°C. HLA-A2 transfected CIR cells (C1RA2) were irradiated (20,000 rad) and pulsed with 1 μM peptide for 1 to 3 hours at 37°C. Then 1 × 105 peptide-pulsed or nonpulsed C1RA2 cells or 1 × 104 nonirradiated 526, 624, 624.28, or 888 melanoma cell lines were added to each well along with 1 × 105 rested patient’s PBMC in CM and incubated at 37°C, 5%, CO2 for 24 hours. Wells were washed six times and detection performed by the addition of 200ng biotinylated-anti human IFN-γ antibody (BD Pharmingen, San Diego, CA) followed by addition of avidin-alkaline-phosphatase (Invitrogen) and subsequent BCIP/NBT substrate treatment. Visualization and analysis of ELISPOTs was performed with the CTL IMMUNOSPOT Reader with the same settings for all samples.
Proliferation Assay
Freshly thawed or overnight rested PBMC were washed and resuspended at 2 × 106 cells/mL in CM. One hundred microliters of cell suspension (2 × 105) was then added per well to a 96-well U-bottom plate, each well containing 2 × 105 irradiated HLA-A2+ T2 cells (20,000 rad) pulsed with 1 μM of g209 peptide or g280 peptide in 100 μL CM, in triplicate. Cell cultures were then incubated at 37°C for 3 and 4 days and pulsed with 1 μCi [3H]-thymidine per well for the final 18 hours of incubation. Plates were harvested onto nylon filters using the Betaplate system and radioactivity quantified using a Betaplate counter. Results are expressed in counts per minute as the mean of triplicate cultures.
Chromium Release Assay
Pre- and postimmunization PBMC were thawed and incubated overnight in CM. T2 cell targets were pulsed or not with 1 μM g209 or g280 peptide for 1 hour and subsequently labeled with 100 μCi 51Cr at 37°C for 1.5 hours. Target cells were washed three times in PBS, resuspended in CM at 105 viable cells/mL, and 100 μL added per well of a 96-well U-bottom plate. Effector cells were washed twice in CM and added to wells at the given ratios. Plates were centrifuged and incubated at 37°C for 4 hours, after which time the supernatants were harvested and counted in a gamma counter. Percent specific lysis was calculated as (experimental - spontaneous lysis/maximal - spontaneous lysis) × 100.
RESULTS
Increasing Frequencies of Tumor Antigen-Specific CD8+ T Cells in Vaccinated Patients
To quantitate the number of circulating g209 peptide-specific CD8+ T cells after multiple courses of immunization with the class I-restricted g209-2M peptide, flow cytometric analysis with peptide/HLA tetramer and anti-CD8 antibody staining was performed ex vivo on PBMC collected before and 3 weeks after every vaccination course in each of the 5 vaccinated patients. The native peptide was always included as a target in all tetramer and ELISPOT assays.
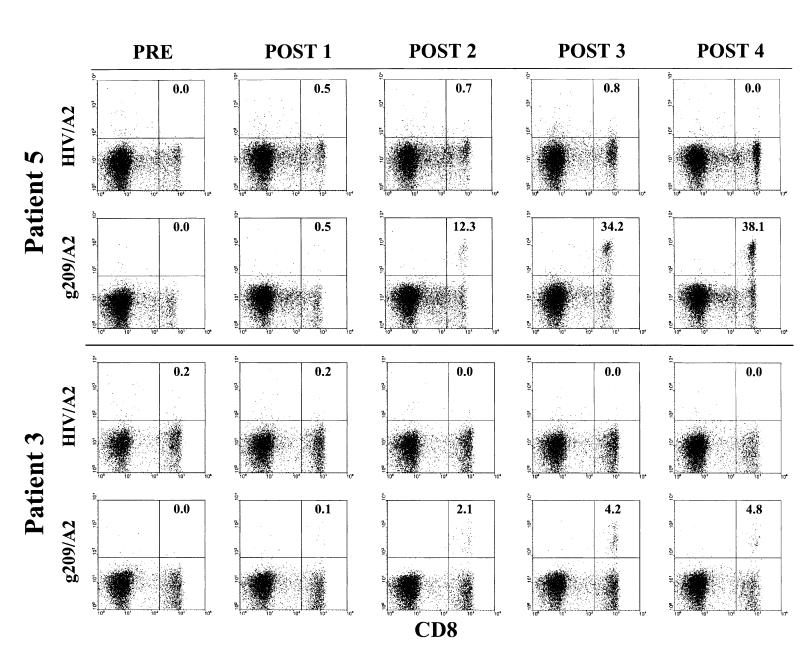
Control flow cytometry assays revealed that approximately 50% of PBMC with intermediate CD8 expression levels also expressed the NK cell-associated marker, CD16 (FcγRIIIa); however, CD8bright leukocytes showed a CD3+CD16-CD14-CD20- expression pattern, consistent with a true CD8+ T lymphocyte phenotype. Thus, this latter population was analyzed in all tetramer assays. Examples of the flow cytometric tetramer assays of PBMC from two patients are shown in Figure 1 using both the specific g209 tetramer and a control HIV tetramer. No gp100:209-217 peptide/HLA-A*0201 (g209/A2)-positive cells were detectable before immunization. Expansion of circulating g209-specific CD8bright T cells was observed in all 5 vaccinated patients upon completion of the 4-course immunization schedule. Tumor antigen-specific CD8+ T-cell frequencies ranged from 4.8% to 38.1% of circulating CD8+ T cells (Table 1). All patients were successfully immunized against the native g209 peptide after two immunization courses, although circulating g209-specific cells were evident in patients 1, 4, and 5 after a single immunization course. Melanoma epitope-specific T-cell frequencies generally increased in parallel with successive immunization courses, irrespective of the vaccination schedule administered. Staining of PBMC of patients 3 and 5 with modified g209-2M peptide/A2 tetramer revealed an antigen-specific expansion similar to the g209-specific CD8+ T-cell frequency measured. In contrast to the expansion of g209-specific cells, control tetramer staining of PBMC of patients 3 and 5 showed consistently low to undetectable frequencies of circulating HIV/A2-positive CD8+ T cells over this time (Fig. 1 and Table 1).
FIGURE 1.
Longitudinal ex vivo evaluation of peptide vaccine-induced CD8+ T-cell responses in the peripheral blood of patients 5 and 3 before (PRE) and 3 weeks after each vaccination course (POST 1-4). PBMC from patients 5 and 3 were stained with either gp100:209-217 peptide/HLA-A*0201 or HIV gag/HLA-A*0201 tetramers and anti-CD8FITC antibody after overnight incubation in CM and immediately analyzed by flow cytometric analysis. Dot plots are shown for propidium iodide-negative gated PBMC. Values correspond to the percentage of total CD8bright T cells that are g209/A2 tetramer-positive calculated as the number of CD8+ tetramer+ cells divided by the total number of CD8bright T cells minus the CD8[H11502] tetramer+ background [H11503]100.
TABLE 1.
Percentage of Tetramer Positive Cells in the CD8 bright T-Cell Population
| Number of Vaccination Courses | ||||||
|---|---|---|---|---|---|---|
| Patient | Tetramer | Preimmune | 1 | 2 | 3 | 4 |
| 1 | g209 | 0.0 | 2.6 | 14.4 | 8.6 | 13.2 |
| 2 | g209 | 0.0 | 0.0 | 0.8 | 8.0 | 6.4 |
| 3 | g209 | 0.0 | 0.1 | 2.1 | 4.2 | 4.8 |
| g209-2M | 0.0 | 0.0 | 2.6 | 5.2 | 4.9 | |
| HIV | 0.2 | 0.2 | 0.0 | 0.0 | 0.1 | |
| 4 | g209 | 0.0 | 1.8 | 9.8 | 15.0 | 19.1 |
| 5 | g209 | 0.0 | 0.5 | 12.3 | 34.2 | 38.1 |
| g209-2M | 0.3 | 0.7 | 9.6 | 35.9 | 31.0 | |
| HIV | 0.0 | 0.5 | 0.7 | 0.8 | 0.0 | |
Before flow cytometric analysis, cryopreserved patient PBMC were thawed and rested overnight in CM to allow cell recovery and facilitate the removal of adherent monocytes. Nonadherent cells were blocked with normal mouse immunoglobulin before g209:tHLA-A*0201 tetramer and mouse anti-human CD8 antibody staining and viable cells positively selected through PI gating. Values correspond to the number of gated CD8 bright tetramer+ cells divided by the number of total CD8bright cells minus the CD8- tetramer+ background (number of CD8- tetramer+ cells divided by total CD8-cell number) × 100.
Phenotypic Changes in Tumor Antigen-Specific CD8+ T cells with Increasing Immunizations
We next evaluated the phenotypic characteristics and differentiation status of melanoma epitope-specific T cells in vivo using multiparameter flow cytometric analysis of tetramer-positive cells. Longitudinal ex vivo analysis of vaccinated patient PBMC revealed that, at the earliest point of detection, g209-specific CD8+ T cells displayed an expression pattern most consistent with an early-stage effector phenotype (Fig. 2). The majority of g209/A2-positive CD8+ T cells ex-pressed CD45RO and CD27. A high frequency of g209/A2-positive cells in some but not all patients expressed CD28 and CD62L. Smaller but significant numbers of cells appeared to remain CD45RAbright throughout the immunization courses. CCR7, a secondary lymphoid organ homing receptor expressed on naive and central memory CD8+ T lymphocytes, was found on less than 8% of circulating g209-specific cells after the first immunization course.
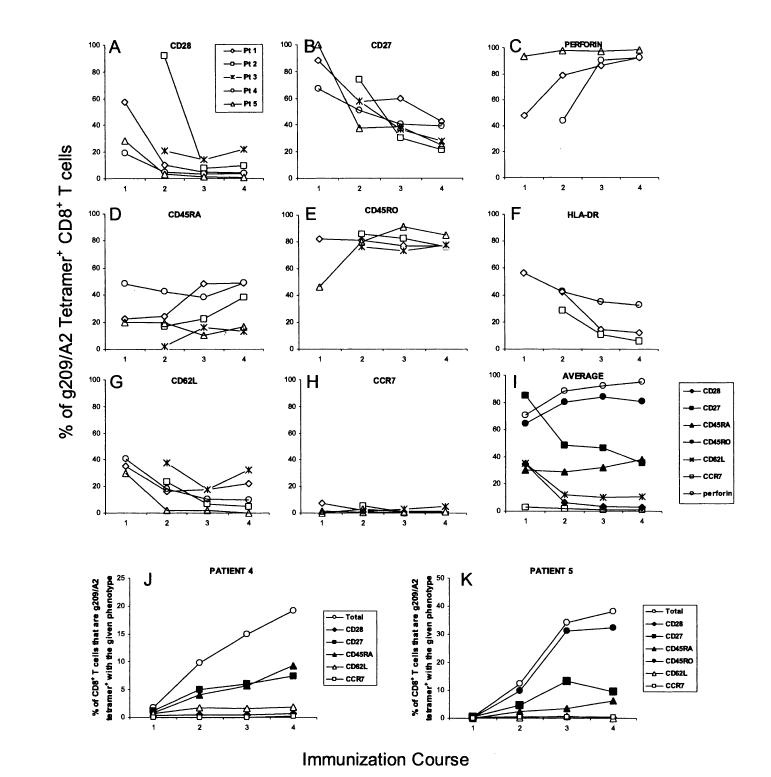
FIGURE 2.
Phenotypic maturation of native peptide-specific CD8+ T cells in vivo after multiple course peptide vaccination. PBMC from vaccinated patients were incubated overnight in culture media and stained for gp100:209-217 peptide/HLA-A*0201 tetramer, CD8, and either CD28 (A), CD27 (B), perforin (C), CD45RA (D), CD45RO (E), HLA-DR (F), CD62L (G), or CCR7 (H) using multiparameter flow cytometry. Propidium iodide staining was performed for viable cell gating in all cases with the exception of anti-perforin antibody-stained cells requiring fixation and permeabilization. Values correspond to the percentage of g209-specific CD8+ T cells positively expressing the given marker after each vaccination course. (I) Values represent the average percentage of tetramer-positive CD8+ T cells expressing the marker from patients with detectable g209/A2 tetramer-positive PBMC after the first vaccination course. (J) and (K), Values represent the percentage of the CD8+ T-cell population that are g209/A2+ and express the indicated marker from patients 4 and 5 PBMC, respectively.
A phenotypic shift occurred in the circulating tumor antigen-specific cell population in parallel with subsequent immunization courses, consistent with T-cell differentiation to a fully activated, memory phenotype. On average, the frequency of g209/A2-positive T cells expressing CD27, CD28, and CD62L steadily decreased with repeated immunization courses while the percentage of CD45RO+ g209-specific T cells remained high (Fig. 2I). Despite some variation among individual patient samples, the frequency of CD45RAbright g209/A2-positive cells remained constant over time. Although CD25 was undetectable at all time points tested (data not shown), another cell surface marker associated with activation, HLA-DR, was observed on tumor antigen-specific cells soon after immunization and diminished with successive immunization courses. Concomitant with the increased frequency of g209/A2-positive CD8+ T cells over the course of the vaccination schedule, the frequency of tetramer-positive CD8+ T cells with an effector memory phenotype escalated (CD45RO+ CD45RA- CD28- CD27- CD62L- CCR7-), exemplified by PBMC from patients 4 and 5 (Figs. 2J, K). Three weeks after the fourth and final immunization course, the majority of peptide vaccine-induced CD8+ T cells expressed an effector memory phenotype. Consistent with this effector phenotype, intracellular staining revealed an increasingly high level of perforin in g209-specific CD8+ T cells (Fig. 2C).
Functional Maturation of Circulating gp100:209-217 Peptide-Reactive CD8+ T Lymphocytes In Vivo
Because phenotypic changes observed in the g209-specific T-cell population suggested an increasing frequency of circulating tumor antigen-specific cells with effector function, we next assessed the functional characteristics of PBMC from immunized patients over the course of the vaccination schedule. An ELISPOT assay was performed to measure IFN-γ production in response to g209 epitope-specific stimulation. Insignificant numbers of IFN-γ-secreting cells were noted from prevaccination PBMC stimulated with either g209 (ITDQVPFSV) or g209-2M (IMDQVPFSV) peptide-pulsed APC (Table 2). The frequency of IFN-γ producing g209-reactive T cells increased in parallel with successive immunization courses, reaching frequencies ranging between 118 and 1587 cells per 105 PBMC after 3 immunization courses. With the exception of PBMC from patient 5, which responded to the immunizing tyrosinase:368-376(370D) peptide, ex vivo stimulation of vaccine-immune cells with APC pulsed with the tyrosinase or gp100:280-288 (g280; YLEPGPVTA) peptides failed to elicit specific cytokine secretion in any sample. Stimulation with the modified g209-2M peptide-pulsed APC resulted in T-cell precursor frequencies comparable to those observed in g209 peptide-stimulated cultures.
TABLE 2.
Longitudinal Enumeration of Peptide-Stimulated IFN-γ Secretion by g209-2M Peptide-Vaccinated PBMC Ex Vivo (Spots per 105 PBMC)
| Patient | Vaccination Course | g280 | g209 | g209-2M | tyr |
|---|---|---|---|---|---|
| 1 | Preimmune | 2 | 0 | 0 | 0 |
| Post 1 | 5 | 219 | 214 | 0 | |
| Post 2 | 0 | 883 | 1005 | 0 | |
| Post 3 | 0 | 393 | 422 | 5 | |
| Post 4 | n.d. | n.d. | n.d. | n.d. | |
| 2 | Preimmune | 0 | 0 | 0 | 0 |
| Post 1 | 0 | 0 | 0 | 0 | |
| Post 2 | 7 | 24 | 8 | 13 | |
| Post 3 | 0 | 118 | 192 | 3 | |
| Post 4 | 7 | 135 | 253 | 3 | |
| 3 | Preimmune | 0 | 0 | 0 | 0 |
| Post 1 | 0 | 15 | 17 | 0 | |
| Post 2 | 0 | 300 | 210 | 2 | |
| Post 3 | 0 | 452 | 370 | 0 | |
| Post 4 | 0 | 403 | 382 | 2 | |
| 4 | Preimmune | 5 | 8 | n.d. | 10 |
| Post 1 | 2 | 166 | n.d. | 5 | |
| Post 2 | 1 | 1840 | n.d. | 2 | |
| Post 3 | 1 | 1587 | n.d. | 2 | |
| Post 4 | n.d. | n.d. | n.d. | n.d. | |
| 5 | Preimmune | 0 | 7 | 6 | 6 |
| Post 1 | 0 | 4 | 11 | 10 | |
| Post 2 | 2 | 263 | 307 | 67 | |
| Post 3 | 0 | 1250 | 1932 | 453 | |
| Post 4 | 0 | 1373 | 1493 | 270 |
PBMC obtained before and 3 weeks after each vaccination course (Post 1-4) were tested for reactivity against 1 μM g280, g209, g209-2M, or tyrosi-nase (tyr) peptide stimulation. Values correspond to the number of IFN-γ-secreting cells per 105 PBMC from each patient over the course of the vaccination schedule measured by ex vivo ELISPOT analysis. Cytokine-secreting cell number was calculated as the average number of spots from six replicate wells. Values not determined are indicated n.d.
A correlation existed between the absolute number of IFN-γ-producing cells and g209/A2 tetramer-positive CD8+ T cells per 105 patient PBMC (Fig. 3, P < 0.001). On average, about 21% of circulating g209/A2-positive CD8+ T cells were capable of producing IFN-γ in response to peptide stimulation during the course of the immunization schedule.
FIGURE 3.
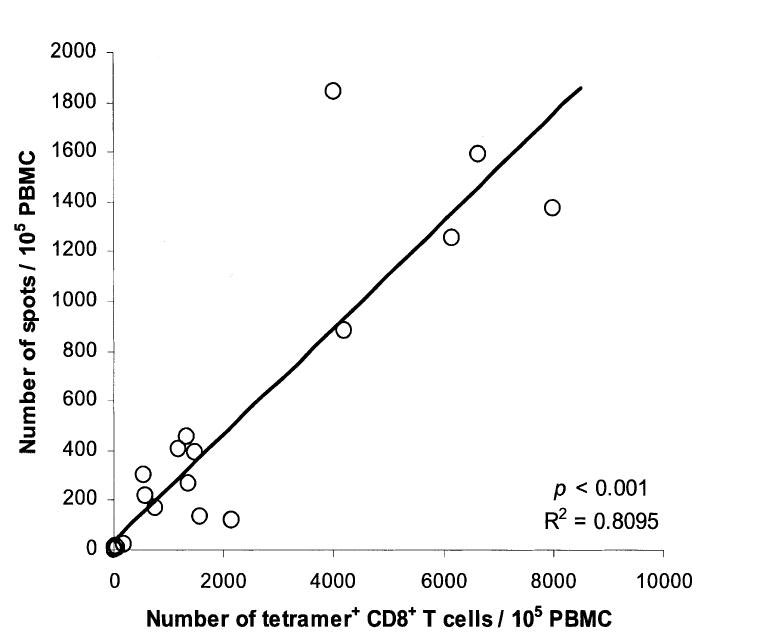
Functional maturation of tetramer-positive CD8+ T cells in the peripheral blood of g209-2M peptide-vaccinated patients. The absolute number of gp100:209-217 peptide/HLA-A*0201 tetramer-positive CD8+ T cells per 105 PBMC was deduced by applying the percentage of tetramer-positive CD8+ PBMC in the circulation (total number of tetramer-positive CD8+ PBMC/mL divided by total number of PBMC/mL [H11503] 100) to 105 PBMC for patient PBMC collected over the immunization course and compared with the number of IFNγ-secreting cells/105 PBMC after overnight incubation with 1 μM g209 peptide as measured by ELISPOT assay. Circles correspond to paired results for each PBMC sample tested. (P < 0.001).
To directly evaluate the immune potential of melanoma epitope-reactive PBMC against tumor, we measured the precursor frequency of IFN-γ producing PBMC ex vivo in response to HLA-matched or nonmatched melanoma cell stimulation by ELISPOT analysis. In 24-hour assays, postimmunization course 3 or 4 PBMC from 80% (4/5) of g209-2M-vaccinated patients secreted IFN-γ when stimulated with the HLA-A2-positive melanoma cells, 526 and 624, with T-cell precursor frequencies ranging from 32 to 198 and 31 to 228 IFN-γ-producing cells per 105 PBMC, respectively (Table 3). Stimulation with HLA-A2-negative melanoma cells failed to elicit significant responses in all PBMC tested. Postvaccination course 3 PBMC from patient 2 did not respond to stimulation with HLA-A2-matched or nonmatched tumor cells, a finding consistent with the low frequency of g209 peptide-stimulated, IFN-γ-producing PBMC found in this patient (Table 2).
TABLE 3.
Tumor Stimulated IFN-γ Secretion by g209-2M Peptide-Vaccinated PBMC Ex Vivo (Spots per 105 PBMC)/
| A2+ gp100+ Tumor Line | A2- gp100+ Tumor Line | |||||
|---|---|---|---|---|---|---|
| Patient | Vaccination Course | 526 | 624 | 624.28 | 888 | P* |
| 1 | Post 3 | 32 ± 5 | 31 ± 4 | 0 ± 1 | 15 ± 2 | 0.0004 |
| 2 | Post 3 | 2 ± 3 | 0 ± 1 | 0 ± 1 | 0 ± 1 | n.s. |
| 3 | Post 4 | 72 ± 6 | 44 ± 3 | 0 ± 1 | 1 ± 1 | 0.0003 |
| 4 | Post 4 | 198 ± 12 | 228 ± 8 | 0 ± 2 | 2 ± 2 | 0.0005 |
| 5 | Post 4 | 114 ± 19 | 99 ± 16 | 0 ± 1 | 1 ± 0 | 0.0003 |
PBMC (105) were tested for reactivity after overnight stimulation with 104 irradiated melanoma cells (20,000 rad). Values represent the number of IFN-γ-secreting cells per 105 PBMC from each patient at the indicated vaccination course measured by ex vivo ELISPOT analysis ± s.e.m. Cytokine-secreting cell number was calculated as the average number of spots from 4 replicate wells minus the average number of spots from 4 tumor control wells. Asterisk represents significance determined by Kruskal-Wallis test comparing reactivities against A2+ and A2- negative tumor groups. n.s., not significant.
Proliferative Capacity of g209-Reactive PBMC
To determine the proliferative capability of g209-reactive PBMC, pre- and postcourse 4 patient PBMC were rested overnight in CM and evaluated for proliferation against HLA-A2-expressing T2 cells pulsed with 1 μM g209 or g280 peptides in 3-day and 4-day [3H]-thymidine incorporation assays. In 3-day assays, no difference in proliferation was observed from prevaccination PBMC when stimulated with either g209 or g280 peptide-pulsed T2 cells (Table 4). In contrast, proliferation in response to g209 but not to g280 peptide stimulation was noted from 3 of 4 postvaccination PBMC (P < 0.05). Similar but overall higher levels of proliferation were noted in all day-4 postimmunization cultures stimulated with g209 peptide compared with preimmune cultures; however, increased proliferation was measured against g209 peptide in 2 preimmune samples.
TABLE 4.
Peptide-Stimulated Proliferation by g209-2M Peptide-Vaccinated PBMC
| Day 3 | Day 4 | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Patient | g280 | g209 | P | g280 | g209 | P |
| Preimmune | 1 | 7793 ± 237 | 6503 ± 86 | n.s. | 14,319 ± 770 | 14,057 ± 704 | n.s. |
| 2 | 11,619 ± 253 | 10,168 ± 612 | n.s. | 20,087 ± 266 | 21,634 ± 778 | n.s. | |
| 3 | 5664 ± 320 | 5388 ± 256 | n.s. | 10,634 ± 1667 | 17,243 ± 1231 | <0.05 | |
| 4 | 8141 ± 880 | 9173 ± 827 | n.s. | 13,977 ± 1090 | 21,130 ± 731 | <0.05 | |
| 5 | 11,955 ± 466 | 12,973 ± 900 | n.s. | 25,191 ± 1494 | 28,062 ± 610 | n.s. | |
| Course 4 | 1 | 8025 ± 454 | 18,400 ± 887 | <0.05 | 15,324 ± 881 | 34,838 ± 1675 | <0.05 |
| 2 | n.d. | n.d. | n.d. | 15,730 ± 742 | 22,892 ± 2269 | <0.05 | |
| 3 | 6918 ± 408 | 11,405 ± 971 | <0.05 | 13,505 ± 285 | 24,911 ± 1482 | <0.05 | |
| 4 | 5347 ± 275 | 18,652 ± 987 | <0.05 | 8156 ± 456 | 30,075 ± 1571 | <0.05 | |
| 5 | 8842 ± 202 | 10,393 ± 691 | n.s. | 14,474 ± 1631 | 18,675 ± 428 | <0.05 | |
Preimmune and postvaccination course 4 PBMC were rested overnight in CM and cocultured in 96-well plates (2 × 105 cells/well) with an equal number of irradiated T2 cells (20,000 rad) pulsed with 1 μM of g209 or g280 peptide in 3- and 4-day [3H]-thymidine incorporation assays. Values reflect the average of 3 replicate wells and indicate counts per minute ± s.e.m. Nonsignificant P values (>0.05) for g209 peptide-specific responses are indicated as n.s. Values not determined are indicated as n.d.
Cytolytic Activity Against Peptide-Pulsed Targets
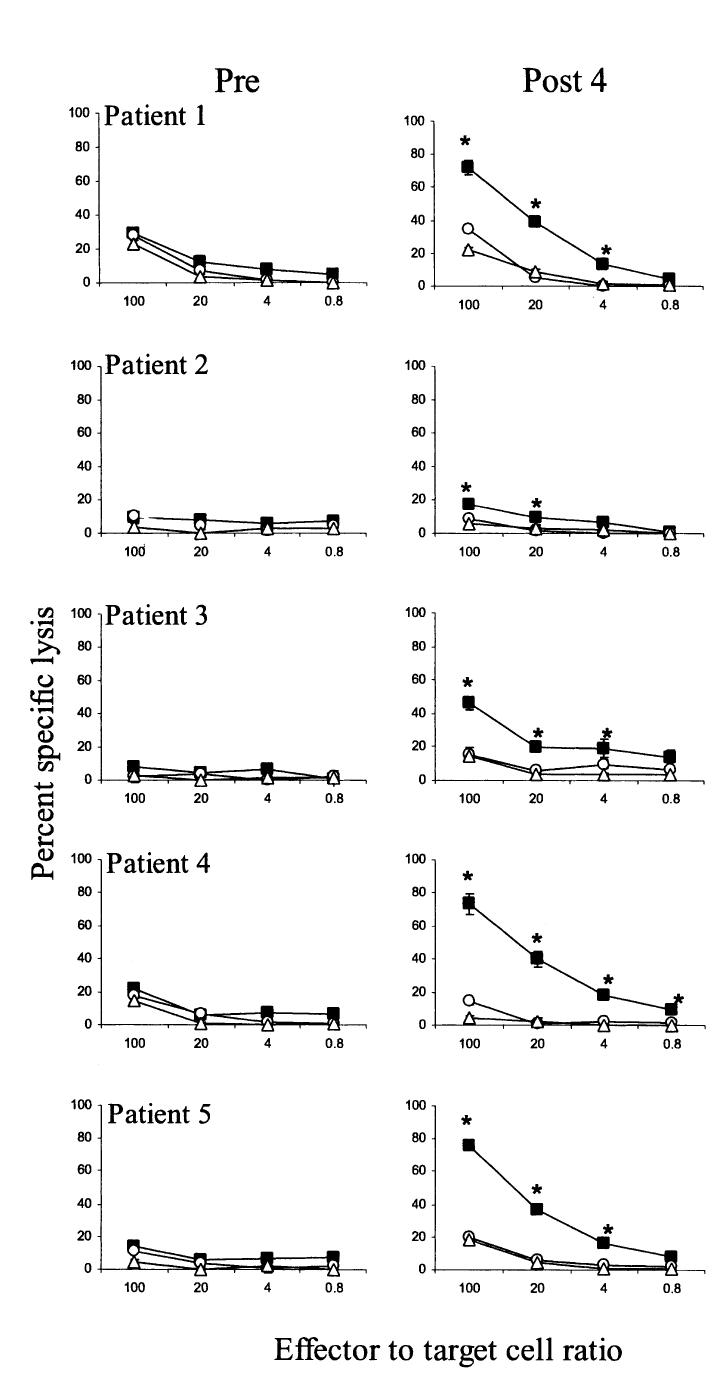
We next directly evaluated the cytolytic capacity of circulating g209-reactive cells using a 4-hour chromium release assay. In preimmune samples, there was no difference in the low lytic activity measured against T2 cells pulsed with either 1 μM g209 or g280 peptide at a 100:1 effector-to-target-cell ratio (Fig. 4). In contrast, all postimmunization PBMC exhibited antigen-specific lysis of g209 peptide-pulsed T2 target cells (P < 0.05). However, none of the postimmunization course 4 PBMC samples displayed significant lytic activity against either HLA-A2-negative 624.28 melanoma cells or HLA-A2-matched 624 melanoma cell targets at a 100:1 effector-to-target-cell ratio (not shown).
FIGURE 4.
Cytolytic capacity of PBMC after multiple courses of g209-2M peptide vaccination. Pre- (left panels) and postimmunization course 4 PBMC (right panels) from patients 1-5 were rested overnight in CM and analyzed for cytolytic activity against T2 target cells alone (triangles) or pulsed with 1 μM native g209 (squares) or g280 (circles) in a 4-hour 51Cr-release assay. Values represent the mean percent lysis from triplicate wells [H11506] SEM at the indicated effector-to-target-cell ratio. Asterisk indicates a significant difference (P < 0.05) between g209-specific and g280 control lysis determined by two-sided Kruskal-Wallis test.
Persistence of Circulating Vaccine-Specific T Cells
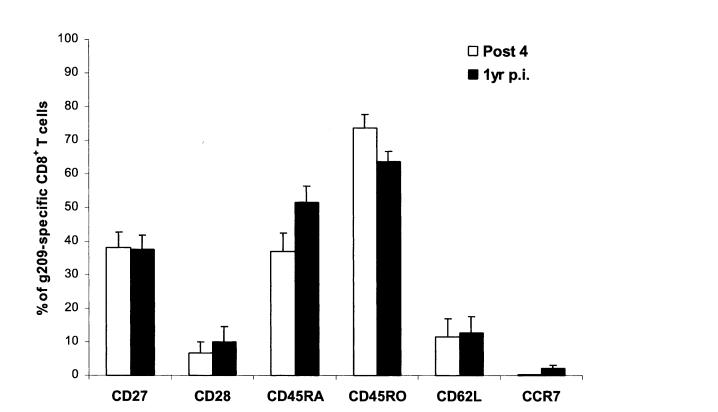
To evaluate the persistence and phenotype of g209 pep- tide-specific CD8+ T cells in patients receiving multiple courses of modified g209-2M peptide, multiparameter flow cytometric analysis was performed on PBMC collected from patients 1, 2, 3, and 5, 1 year after final immunization. Compared with post-course 4 PBMC, tetramer analysis revealed a reduced yet sustained presence of circulating g209 peptide-specific CD8+ T cells in all vaccinated patients with tumor antigen-specific T-cell frequencies ranging between 25.4% and 0.8% of CD8+ T cells (Table 5). To determine whether the persistent g209-specific T-cell population had undergone phenotypic changes, tetramer-positive CD8+ T cells from postcourse 4 and 1 year postimmunization PBMC were examined for expression of markers associated with T-cell differentiation. Consistent with the phenotype observed after 4 courses of peptide immunization, g209 peptide-specific T cells maintained an effector memory phenotype 1 year postimmunization with a small increase in the frequency of CD45RA expressing cells paralleling a decreased CD45RO expression (Fig. 5). The frequency of tumor antigen-specific cells expressing CD27, CD28, CD62L, and CCR7 remained unchanged. In assays performed simultaneously with preimmune and course 4 PBMC (Table 4 and Figure 4), antigen- specific lysis of g209 peptide-pulsed T2 cells by 1 year postimmunization PBMC was detected from 1 of 3 patients in a 4-hour chromium-release assay, while increased proliferation by all 1-year samples was measured in response to g209 peptide-pulsed T2 cells in a 3-day [3H]-thymidine incorporation assay (Table 5).
TABLE 5.
Analysis of g209-2M Peptide-Vaccinated Patient PBMC 1 Year After Immunization
| % g209-Specific of CD8+ T Cells* | Peptide-Induced Proliferation† | Cytolytic Activity‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Post 4 | 1 year | g280 | g209 | P | g280 | g209 | P |
| 1 | 12.4 | 5.0 | 11,905 ± 714 | 18,663 ± 1371 | <0.05 | 62.6 ± 3.4 | 63.6 ± 4.7 | n.s. |
| 2 | 5.6 | 0.8 | 10,208 ± 259 | 15,069 ± 1809 | <0.05 | 12.0 ± 2.1 | 11.1 ± 1.5 | n.s. |
| 3 | 6.6 | 2.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5 | 38.2 | 25.4 | 8646 ± 1366 | 25,259 ± 481 | <0.05 | 13.1 ± 0.8 | 51.8 ± 1.4 | <0.05 |
Tetramer and functional assays on 1-year postimmunization PBMC were performed concurrently with preimmune and course 4 samples.
Values correspond to the number of gated CD8+ tetramer+ cells divided by the number of total CD8bright cells minus the CD8- tetramer+ background (number of CD8- tetramer+ cells divided by total CD8- cell number) × 100.
Values reflect the average counts per minute ± s.e.m. of 3 replicate well cocultures containing 2 × 105 PBMC and an equal number of irradiated T2 cells (20,000 rad) pulsed with 1 μM of g209 or g280 peptide in a 3-day [3H]-thymidine incorporation assay.
Values represent the mean percent lysis from triplicate wells ± s.e.m. at a 100:1 effector to 1 μM peptide-pulsed target cell ratio in a standard 4-hour 51Cr-release assay. Significance values >0.05 are indicated as n.s.
FIGURE 5.
Phenotypic analysis of g209-specific CD8+ T cells from 1-year postimmunization PBMC. PBMC from vaccinated patients 1-year after final peptide vaccination were incubated overnight in culture media and were stained using gp100:209-217 peptide/HLA-A*0201 tetramer, anti-CD8 antibody, and antibodies specific for either CD27, CD28, CD45RA, CD45RO, CD62L, or CCR7 molecules for multiparameter flow cytometric analysis. Propidium iodide staining was performed for viable cell gating in all cases. Values correspond to the average percentage of g209-specific CD8+ T cells from patients 1, 2, 3, and 5 PBMC that positively express the indicated markers.
DISCUSSION
Peptide-based vaccines represent a promising approach for the immunotherapy of patients with melanoma. In multiple clinical trials using peptide injection to attempt to immunize patients against melanoma epitopes, rarely has ex vivo peptide/HLA tetramer-based analysis measured circulating vaccine-specific CD8+ T cell frequencies greater than 2%.8,10-12 In these studies of patients with metastatic melanoma, the number of injection cycles was generally limited by progression of disease, and thus it has been difficult to clearly assess the impact of repeated peptide vaccination. In tetramer-based analysis, the number of modified MART-1:26-35(27L) peptide-specific PBMC increased over the course of 6 native MART-1:26-35 peptide injection cycles in 1 of 5 vaccinated patients with metastatic melanoma, reaching 2.3% of circulating CD8+ T cells,17 exceeding common frequencies of naturally arising MART-1-specific CD8+ T cells in the peripheral blood and tumor-infiltrated lymph nodes of patients with melanoma.18 Similarly, a recent clinical trial in which patients with malignant melanoma were repetitively vaccinated with g209-2M peptide over 6 months demonstrated g209-2M peptide-specific CD8+ T-cell frequencies greater than 1% in 8 of 29 patients after vaccination, including 2 patients who exhibited modified peptide-specific frequencies of 4.96% and 8.86%; however, the frequency of T cells specific for native g209 peptide was not assessed.19
In the current study, we have investigated the longitudinal effect of multiple-course g209-2M peptide vaccinations on circulating g209 peptide-specific T-cell expansion, phenotype, and function in patients at high risk of recurrent melanoma. Unlike patients with metastatic melanoma, patients in the adjuvant setting who are clinically free of disease can be immunized over a long period. We selected for study 5 HLA-A*0201 patients who had completed the 4-course peptide immunization regimen with g209-2M peptide in IFA over 336 days. Peptide-reactive cells were undetectable in preimmunization PBMC from all 5 patients by tetrameric staining, ELISPOT analysis, and cytolytic activity assessment. Upon completion of the multiple course vaccination schedule, successful immunization was noted in all 5 patients. Frequencies of native peptide-specific PBMC increased in parallel with repeated modified peptide vaccination to high levels, ranging between 4.8% and 38.1% of circulating CD8+ T cells, reaching levels reportedly required for the successful elimination of acute viral infection.13-15 This demonstrates a marked improvement in the induction of heightened antigen-specific T- cell precursor frequencies over previous immunization strategies. PBMC from patients 1 and 4 demonstrated tetramer- positive staining and peptide-stimulated IFN-γ secretion 3 weeks after the initial vaccination course. All patients were successfully immunized after completion of the second immunization course, at which time patients had received either 8 or 20 peptide injections over 24 weeks. One year after immunization, circulating g209-specific CD8+ T cells persisted in 4 patients tested, albeit at lower frequencies, demonstrating a long-term impact of g209-2M peptide vaccination upon the persistence of the g209-specific T cells, as reported previously in in vitro stimulation studies,20 and at considerable frequencies of the CD8+ T-cell population.
Several investigators have proposed that the maturational pathway of CD8+ T cells may be delineated through the examination of cell surface molecules such as the costimulatory receptors CD27 and CD28, the CD45 isoforms, and the chemokine receptor CCR7.21,22 Additionally, correlations between cell surface marker expression and T-cell function have previously been demonstrated.21,23-25 Considerable confusion exists concerning the phenotypic characteristics of tumor antigen-specific cells in patients with melanoma.26 Immune function of tumor antigen-reactive CD8+ T cells appears to be linked to phenotype because CCR7- CD45RA- CD45RO+ MART-1 tetramer-positive PBMC from patients with melanoma, but not similar cells bearing a CCR7+ CD45RA+ CD45RO- phenotype, produced IFN-γ in response to peptide stimulation and lysed tumor cells when presorted ex vivo.27,28 Similarly, circulating MART-1 tetramer-positive cells from 1 patient receiving repeated peptide vaccination displayed strong functional activity ex vivo, with longitudinal analysis revealing a phenotypic shift from a naive CD28+ CD27+ CD45RAhigh to an activated/effector CD28- CD27- CD45RAlow phenotype in parallel with peptide immunization.17 In our study, the g209/A2 tetramer-positive CD8+ T- cell population displayed a cell surface phenotype consistent with an early effector differentiation state (CD27+ CD28+ CD45RAlow CD45RO+ HLA-DR+) at the initial point of detection. A phenotypic shift after continued vaccination resulted in a growing proportion of effector memory CD27- CD28- CD45RO+ CD62L- HLA-DR- perforin+ g209-reactive CD8+ T cells in vivo. Although loss of CD45RA expression appeared to coincide with T-cell receptor (TCR)-mediated proliferation, the percentage of CD45RA expressing g209-reactive T cells remained stable or slightly increased over the immunization regimen, similar to the reversion of CD45RA expression observed in the stabilized memory pool in acute EBV infection.29 Indeed, CMV-specific T cells reside in either preterminally (CD45RA- CD45RO+ CCR7-) or terminally differentiated (CD45RA+ CD45RO- CCR7-) CD8+ T-cell pools.22 In our study, the majority of vaccine-elicited g209-reactive PBMC were CD45RA- CD45RO+ CCR7-; however, both populations appear to comprise the circulating vaccine-specific population, and g209-reactive PBMC maintained the capacity to proliferate in response to ex vivo peptide pulsed APC-stimulation, indicating that the g209-specific population was not entirely at a terminal stage of differentiation.
The downregulation of CD62L and CCR7, molecules essential for lymphocyte migration to lymph nodes, on g209-specific memory cells may reflect the generation of effector memory T cells.21 Despite the coexpression and downregulation of these molecules on naive and effector memory cells, respectively, we found that CCR7 and CD62L displayed dissimilar kinetics of downregulation on vaccine-specific T cells in vivo. Although early CD62L expression was lost with successive vaccination courses, CCR7 was continually low to undetectable in the circulating g209-specific cell population. Upon completion of the immunization protocol, nearly all g209-specific cells were negative for CD62L and CCR7, consistent with the effector memory phenotype. One year after immunization, g209-specific T cells in the peripheral blood sustained their effector memory phenotype. This contrasts the recent demonstration of conversion of memory CD8+ T cells from effector memory (CD62L- CCR7-) to central memory (CD62L+ CCR7+) under homeostatic control and influenced by antigen dose in an acute viral infection system.30 Accordingly, increased numbers of antigen encounters and antigen dose, as provided by our vaccination strategy, may result in the preferential development of effector memory T cells. Importantly, the current longitudinal phenotypic analysis demonstrates the ability of the multiple course immunization strategy to generate increasing proportions of phenotypically mature tumor-antigen-reactive CD8+ T cells with correlative functional maturation.
Characterization of vaccine-induced T-cell function is important, because tetramer-positive staining does not strictly correlate with antigen-specific T-cell responsiveness.26 The high expression levels of intracellular perforin, a key effector protein for CTL-mediated cytolysis, by more than 90% of tetramer-positive cells were suggestive of a functional potential of peptide vaccine-induced g209-specific CD8+ T cells. Indeed, ELISPOT assays demonstrated an increasing number of antigen-specific IFN-γ producing PBMC with repeated vaccinations. The values of g209-specific PBMC detected by g209/A2 tetramer were nearly 5-fold higher than the number of cells producing IFN-γ in response to peptide stimulation, consistent with differences noted in acute viral infection.31 Corresponding with peptide-stimulated cytokine secretion, postimmunization PBMC from 4 of 5 patients secreted IFN-γ in response to HLA-matched tumor stimulation, demonstrating the direct immune reactivity of g209-specific PBMC against tumor ex vivo. Although cytolytic activity by postcourse 4 PBMC was detected against g209 peptide-pulsed target cells in 4-hour chromium release assays, cytolysis of melanoma cell targets was not observed. The demonstration of cytolytic activity against melanoma cells by Melan-A/MART-1-peptide vaccine-induced CTL in other studies required antigen-specific cell presorting with prolonged incubation or mitogen stimulation.17
In addition to g209-2M peptide, all patients also received multiple vaccination courses of tyrosinase 368-376(370D) peptide in IFA; however, with the exception of PBMC from patient 5, vaccination with the HLA-A2-restricted tyrosinase peptide failed to immunize, as shown previously.32 The tyrosinase epitope has a HLA-binding affinity similar to the native g209 peptide, which was less effective than the modified g209-2M peptide for successful immunization of patients with metastatic melanoma.4,7 Because the correlation between MHC-binding affinity of peptide and immunogenicity has illustrated the importance of the peptide association and dissociation rate with the HLA complex for induction of antigen-specific responses,33-35 synthetic modification of the tyrosinase peptide to increase HLA-binding affinity without altering TCR recognition of the peptide/HLA complex may thus allow for greater immunizing efficacy.
It is possible that lack of detectable lesions as a selective reservoir for vaccine-induced, tumor antigen-reactive cells in our patients may account in part for the enhanced T-cell precursor frequencies observed in the peripheral blood. In our prior clinical trials, the enhanced rate of tumor regression in patients with melanoma treated with g209-2M peptide and interleukin-2 was not associated with increased frequencies of tumor antigen-reactive cells in the peripheral blood,7 and analysis of fine-needle aspirates of melanoma lesions in vaccinated patients suggested tumor-specific T-cell localization to metastatic melanoma sites.8,36 In addition, naturally arising tumor antigen-specific CD8+ T-cell responses appear to differ both quantitatively and qualitatively in the peripheral blood versus the tumor site, with a higher frequency of antigen- experienced, tumor-specific cells at the lesion.18
The current analysis of preimmune and postimmunization PBMC from patients receiving multiple-course peptide immunization reveals an improved method for the generation of increased numbers of functionally and phenotypically mature tumor antigen-specific CD8+ T cells that persist in vivo. Vaccine-elicited T cells proliferated in response to peptide stimulation and produced IFN-γ in response to cognate peptide or tumor stimulation ex vivo; however, PBMC from immunized patients exhibited cytolytic activity against peptide-pulsed target cells but not against gp100-expressing melanoma cells ex vivo, suggesting that, in addition to the magnitude of the immune response, qualitative features of peptide vaccine-elicited T-cell responses may be equally as important for treatment of patients with melanoma. Although this study was not conducted to evaluate the impact of multiple courses of peptide vaccination upon clinical outcome, these 5 peptide-vaccinated patients remain free of recurrent melanoma 1 year after immunization; however, the long-term impact of this strategy upon tumor recurrence rate remains unknown. In addition, no autoimmune disease was observed in these patients despite high frequencies of circulating g209-reactive CD8+ T lymphocytes with cytolytic capability. Although the duration of the multiple immunization course protocol may hinder its application for the therapeutic treatment of patients with melanoma with progressive disease, the ability to generate strong and possibly protective tumor antigen-specific CD8+ T-cell responses in vivo in patients with no evident disease but at high risk of recurrence provides a basis on which future peptide immunization strategies may be designed in the adjuvant setting.
ACKNOWLEDGMENTS
The authors thank Shawn Farid and Arnold Mixon in the Flow Cytometry Facility of the Surgery Branch, National Cancer Institute for their helpful contribution to this study.
REFERENCES
- 1.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Delgado CH, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skipper JC, Gulden PH, Hendrickson RC, et al. Mass-spectrometric evaluation of HLA-A*0201-associated peptides identifies dominant naturally processed forms of CTL epitopes from MART- 1 and gp100. Int J Cancer. 1999;82:669–677. doi: 10.1002/(sici)1097-0215(19990827)82:5<669::aid-ijc9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami Y, Eliyahu S, Jennings C, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 5.Salgaller ML, Afshar A, Marincola FM, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides. Cancer Res. 1995;55:4972–4979. [PubMed] [Google Scholar]
- 6.Salgaller ML, Marincola FM, Cormier JN, et al. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–4757. [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KH, Wang E, Nielsen MB, et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292–6300. [PubMed] [Google Scholar]
- 9.Cormier JN, Salgaller ML, Prevette T, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART- 1/Melan A. Cancer J Sci Am. 1997;3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen MB, Monsurro V, Migueles SA, et al. Status of activation of circulating vaccine-elicited CD8+ T cells. J Immunol. 2000;165:2287–2296. doi: 10.4049/jimmunol.165.4.2287. [DOI] [PubMed] [Google Scholar]
- 11.Pittet MJ, Valmori D, Dunbar PR, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speiser DE, Lienard D, Pittet MJ, et al. In vivo activation of melanoma-specific CD8(+) T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32:731–741. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 15.Zinkernagel RM, Hengartner H. Antiviral immunity. Immunol Today. 1997;18:258–260. doi: 10.1016/s0167-5699(97)80017-5. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 17.Pittet MJ, Speiser DE, Lienard D, et al. Expansion and functional maturation of human tumor antigen-specific CD8+ T cells after vaccination with antigenic peptide. Clin Cancer Res. 2001;7:796s–803s. [PubMed] [Google Scholar]
- 18.Romero P, Dunbar PR, Valmori D, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JW, II, Walker EB, Fox BA, et al. Adjuvant immunization of HLA-A2-positive melanoma patients with a modified gp100 peptide induces peptide-specific CD8+ T-cell responses. J Clin Oncol. 2003;21:1562–1573. doi: 10.1200/JCO.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JHT, Rosenberg SA. Long-term survival of anti-tumor lymphocytes generated by vaccination of patients with melanoma with a peptide vaccine. J Immunother. 2000;23:401–404. doi: 10.1097/00002371-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 23.Azuma M, Phillips JH, Lanier LL. CD28-T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 24.Michie CA, McLean A, Alcock C, et al. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 25.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 27.Dunbar PR, Smith CL, Chao D, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumors-pecific immune response. J Immunol. 2000;165:6644–6652. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 28.Valmori D, Scheibenbogen C, Dutoit V, et al. Circulating tumor-reactive CD8(+) T cells in melanoma patients contain a CD45RA(+)CCR7(-) effector subset exerting ex vivo tumor-specific cytolytic activity. Cancer Res. 2002;62:1743–1750. [PubMed] [Google Scholar]
- 29.Dunne PJ, Faint JM, Gudgeon NH, et al. Epstein-Barr virus-specific CD8(+) T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–940. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003 doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 31.Tan LC, Gudgeon N, Annels NE, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 32.Schaed SG, Klimek VM, Panageas KS, et al. T-cell responses against tyrosinase 368-376(370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund’s adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8:967–972. [PubMed] [Google Scholar]
- 33.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 34.Sette A, Vitiello A, Reherman B, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 35.Lipford GB, Bauer S, Wagner H, et al. In vivo CTL induction with point- substituted ovalbumin peptides: immunogenicity correlates with peptide- induced MHC class I stability. Vaccine. 1995;13:313–320. doi: 10.1016/0264-410x(95)93320-9. [DOI] [PubMed] [Google Scholar]
- 36.Kammula US, Lee KH, Riker AI, et al. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–6875. [PubMed] [Google Scholar]