Abstract
The Pseudomonas aeruginosa efflux pumps MexAB-OprM, MexCD-OprJ, and MexEF-OprN play an important role in susceptibility to fluoroquinolones in vitro. To determine if levofloxacin MICs arising from different levels of expression of efflux pumps result in a proportional reduction in the response to levofloxacin in vivo, isogenic strains of P. aeruginosa were tested with levofloxacin in two mouse models of infection (sepsis and neutropenic mouse thigh models). The levofloxacin 50% effective doses (ED50s) increased proportionally with the MICs for most strains. Similarly, the 24-h area under the concentration-time curve (AUC)/MIC ratio that resulted in 90% of the maximum bactericidal activity (90% Emax) exceeded 75 for all strains except those with elevated MICs due to MexEF-OprN overexpression. In these strains, levofloxacin ED50s were 2- to 10-fold lower than the ED50/MIC ratios in the other strains and 90% Emax AUC/MIC ratios were 2- to 4-fold lower than those predicted from pharmacodynamic modeling of efficacy against other strains. These data show that while the MexEF-OprN efflux pump can provide P. aeruginosa resistance to levofloxacin in vitro, it appears to be less efficient in providing resistance to levofloxacin in animal models of infection.
Pseudomonas aeruginosa is an opportunistic pathogen in which four multicomponent multidrug-resistant efflux pumps have been identified. Overexpression of one or more of these efflux pumps, MexAB-OprM (15), MexCD-OprJ (14), MexEF-OprN (9), and MexXY-OprM (1), generates some degree of resistance to all known classes of antibiotics available for the treatment of infections caused by this pathogen, including fluoroquinolones, β-lactams, and aminoglycosides. In the case of fluoroquinolones, all four Mex pumps confer low-level resistance, with MICs ranging from 1 to 8 μg/ml; and mutants that overexpress three of these Mex pumps have been isolated among fluoroquinolone-resistant bacteria in clinical settings: nalB mutants that overexpress MexAB-OprM (4), nfxB mutants that overexpress MexCD-OprJ (18), and nfxC mutants that overexpress MexEF-OprN (6). Overexpression of MexXY-OprM has not been reported as a cause of fluoroquinolone resistance in the clinical setting. MexAB-OprM is the only pump expressed in wild-type cells at a level high enough to confer multidrug resistance. Deletion of any component of this pump renders P. aeruginosa more susceptible to various antibiotics, including fluoroquinolones (7, 12, 15, 17). However, overexpression of either the MexCD-OprJ or the MexEF-OprN efflux pump restores in vitro resistance to fluoroquinolones in strains lacking the MexAB-OprM efflux pump (8, 10, 11).
In the study described in this report, we evaluated the effects of the overexpression or deletion of various Mex pumps on the activity of levofloxacin in vivo using two mouse models of infection. We show that while the overexpression of MexEF-OprN increases the levofloxacin MIC in vitro, the proportional increase in in vivo resistance is not observed, suggesting that the MexEF-OprN efflux pump is less efficient than the MexAB-OprM or the MexCD-OprJ efflux pump at expelling levofloxacin in vivo.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., December 2001.)
MATERIALS AND METHODS
Antimicrobial agents.
Levofloxacin (Levaquin; Ortho McNeil) was obtained from commercial sources.
Bacterial strains.
Strains Pa 812, Pa 813, Pa 814, and Pa 815 were spontaneous single-step mutants of P. aeruginosa (Table 1) selected by the spiral gradient endpoint method (Spiral Biotech, Inc. Norwood, Mass.). Undiluted overnight cultures were applied to 150-mm Mueller-Hinton agar plates containing a levofloxacin concentration gradient ranging from 0.02 to 4 μg/ml either in the absence or in the presence of MC-4,609, a MexAB-selective efflux pump inhibitor, at 10 μg/ml. After 48 h, colonies growing at concentrations higher than the MIC were selected.
TABLE 1.
Pseudomonas aeruginosa strains used in this study
| Strain | Genotype or descriptiona | Source or reference | MIC (μg/ml) |
|---|---|---|---|
| Pa 040 | Clinical isolate | This study | 0.125 |
| Pa 064 | ATCC 27313 | This study | 0.25 |
| Pa 103 | Clinical isolate | This study | 1 |
| Pa 107 | Clinical isolate | This study | 0.125 |
| Pa 812 | nfxC (mexEF-oprN is overexpressed) from Pa 040 | This study | 3 |
| Pa 813 | nfxC (mexEF-oprN is overexpressed) from Pa 064 | This study | 1.75 |
| Pa 814 | nfxC (mexEF-oprN is overexpressed) from Pa 103 | This study | 4.75 |
| Pa 815 | nfxC (mexEF-oprN is overexpressed) from Pa 107 | This study | 7.25 |
| PAM 1001 | nfxC (mexEF-oprN is overexpressed) | This study | 3.25 |
| PAM 1014 | nfxC oprM::ΩHg from PAM 1001 | This study | 3.25 |
| PAM 1020 | PA01 prototroph | 1 | 0.25 |
| PAM 1032 | nalB (mexAB-oprM is overexpressed) | 1 | 1.5 |
| PAM 1033 | nfxB (mexCD-oprJ is overexpressed) | 1 | 2.25 |
| PAM 1034 | nfxC (mexEF-oprN is overexpressed) | 1 | 4 |
| PAM 1106 | mexA::Tc | 1 | 0.06 |
| PAM 1154 | oprM::ΩHg | 1 | 0.015 |
| PAM 1168 | nfxC oprM::ΩHg | This study | 3.25 |
| PAM 1176 | nfxB mexA::Tc | This study | 1 |
| PAM 1177 | nfxB oprM::ΩHg | 1 | 1 |
| PAM 1178 | nfxC mexA::Tc | This study | 3.5 |
| PAM 1409 | ΔmexCD-oprJ::Gm | 1 | 0.25 |
| PAM 1623 | ΔmexEF-oprN::ΩHg | 1 | 0.25 |
| PAM 1626 | ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | 1 | 0.015 |
| PAM 1723 | nalB ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | 2 | 1.5 |
| PAM 1738 | nfxB ΔmexAB-oprM::Cm ΔmexEF-oprN::ΩHg | 2 | 2.25 |
| PAM 1753 | nfxC ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm | 2 | 2.5 |
ΩHg, Hg resistance derivative of interposon Ω; Gm, gentamicin resistance; Cm, chloramphenicol resistance; Tc, tetracycline resistance.
Susceptibility testing.
MICs were determined by a broth microdilution assay according to CLSI (formerly NCCLS) reference methods, with the following exception: antibiotic dilutions were done in 0.25-μg/ml increments (i.e., 1.50, 1.25, 1.00, 0.75, 0.50, and 0.25 μg/ml). At concentrations below 0.25 μg/ml, antibiotic dilutions were twofold. Assays were performed in a final volume of 100 μl. The inocula were adjusted to yield a cell density of 5 × 105 CFU/ml. Antibiotics were prepared at a concentration equivalent to twofold the highest desired final concentration in culture medium and were then diluted directly into 96-well microtiter plates. Microtiter plates were incubated for 24 h at 35°C and were read by using a microtiter plate reader (Molecular Devices) at 650 nm as well as by visual observation by using a microtiter plate reading mirror. The MIC was defined as the lowest concentration of antibiotic at which the visible growth of the organism is completely inhibited.
Animal models. (i) Mouse model of sepsis.
All strains were grown overnight at 35°C in Mueller-Hinton broth (MHB). The following morning, they were subcultured to fresh MHB and incubated for 4 to 5 h at 37°C. The cells were washed twice with phosphate-buffered saline and adjusted to ca. 108 CFU/ml. The inoculum was mixed with an equal volume of sterile 14% hog gastric mucin (16) and kept in an ice bath until it was used (<1 h). Male Swiss mice (Charles Rivers, Hollister, CA) were infected with an intraperitoneal dose of 0.5 ml of the bacterial suspension (∼1.0 × 107 CFU/mouse for each strain). Antibiotics were administered subcutaneously at 0 and 2 h postchallenge. The total dose required for survival at 72 h (the 50% effective dose [ED50]) was determined by the probit method (13). All strains tested in this model produced 50% mortality at between 1.0 × 104 and 5.0 × 104 CFU/mouse and 100% mortality at between 1.0 × 106 and 5.0 × 106 CFU/mouse (data not shown).
(ii) Neutropenic mouse thigh model.
All strains were grown overnight at 35°C in MHB. On the following morning, they were subcultured to fresh MHB and incubated for 4 h at 35°C. The inocula were adjusted to ∼5.0 × 106 CFU/ml. Male Swiss mice were made neutropenic by the intraperitoneal injection of cyclophosphamide (Cytoxan; Mead Johnson) at 150 mg/kg of body weight on days −4 and −1. On day 0, the mice were infected by the intramuscular injection of 0.1 ml of inoculum in each thigh (four thighs per group per time point). Levofloxacin was given intraperitoneally every 4 h over a 24-h period at doses ranging from 0.31 to 100 mg/kg (1.9 to 600 mg/kg/day). At 24 h after the initiation of therapy, both thighs were removed aseptically and homogenized in 4 ml of ice-cold phosphate-buffered saline. Serial 10-fold dilutions of the homogenized material were plated on Mueller-Hinton agar, and the colonies were counted. The change in bacterial counts was determined by subtracting the bacterial counts in the treatment groups from the bacterial counts in the untreated controls at the start of therapy.
Pharmacokinetics.
Male Swiss mice were made neutropenic by an intraperitoneal injection of 150 mg/kg cyclophosphamide (Cytoxan; Mead Johnson) on days −4 and −1. On day 0, the mice were administered a single intraperitoneal dose of levofloxacin at 10, 30, or 100 mg/kg. Groups of three mice were killed at 0.08, 0.16, 0.25, 0.5, 0.75, 1.0, 2.0, 3.0, and 4.0 h after dosing. Blood samples (one sample per animal) were collected by cardiac puncture. Serum concentrations were fit by using WinNonlin (Pharsight, Mountain View, Calif.). Levofloxacin analytical standards (0.05 to 100 mg/liter) were prepared in fresh pooled mouse serum collected from untreated animals. The serum samples or standards were mixed with double the volume of 4% trichloroacetic acid, vortexed, and then centrifuged at 12,000 rpm for 10 min by using a refrigerated Eppendorf 5415c centrifuge set at 4 to 10°C. Aliquots of the supernatant (25 μl) were injected directly onto a high-pressure liquid chromatograph by using a temperature-controlled autoinjector set at 10°C. A standard curve of the peak area versus standard concentration was constructed, and the data were fit by using weighted linear regression (MK model, version 5.0; Biosoft, Ferguson, Mo.). The concentration of levofloxacin in the serum samples was calculated from this standard curve.
Pharmacodynamic modeling.
The relationship between each pharmacokinetic (PK) and pharmacodynamic (PD) parameter (i.e., the percentage of the time over 24 h that the concentration was greater than the MIC, the 24-h area under the concentration-time curve [AUC]/MIC ratio, and the maximum concentration in serum [Cmax]/MIC ratio) and the reduction in the log number of CFU per thigh between time zero and 24 h after the start of treatment were analyzed by using the sigmoid maximum reduction (Emax) pharmacodynamic model (equation 1):
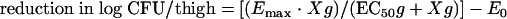 |
(1) |
where Emax is the maximum reduction in the log number CFU/thigh, X is the PK-PD parameter being examined (e.g., 24-h AUC/MIC), EC50 is the X value corresponding to 50% of the Emax, E0 is the effect when X is equal to 0 (untreated control animals), and g is a sigmoidicity factor which controls the steepness of the curve.
The best model for each data set was established by using the Akaike criterion (2).
RESULTS
Susceptibility studies.
The MICs for each strain are listed in Table 2. Levofloxacin MICs varied from 0.015 μg/ml to 7.25 μg/ml.
TABLE 2.
Serum pharmacokinetic parameters of levofloxacin following administration of intraperitoneal doses in male neutropenic mice
| Parametera | PK parameter value at the following levofloxacin dose (mg/kg):
|
||
|---|---|---|---|
| 10 | 30 | 100 | |
| Mean wt (kg) | 0.022 | 0.021 | 0.024 |
| V/F (liter/kg) | 0.77 | 0.84 | 0.92 |
| Cmax (mg/liter) | 9.06 | 25.47 | 79.04 |
| Tmax (h) | 0.3 | 0.24 | 0.26 |
| AUC (mg · h/liter) | 5.03 | 14.98 | 47.80 |
| CL/F (liter/h/kg) | 1.99 | 2.00 | 2.09 |
| t1/2 (h) | 0.27 | 0.29 | 0.30 |
V/F, volume of distribution; Tmax, time to Cmax; CL/F, clearance; t1/2, half-life.
Systemic infection studies.
The activities of levofloxacin against strains of P. aeruginosa in a systemic infection model are shown in Table 3 and Fig. 1. Levofloxacin ED50s increased linearly for MICs between 0.25 and 2.25 μg/ml and produced ED50/MIC ratios of approximately 40 to 60. For MICs of 0.015 μg/ml (strains PAM 1154 and PAM 1626), ED50/MIC ratios were 93 and 160, respectively. A linear regression of the data for strains without MexEF-OprN overexpressed had an R2 value of 0.96. For strains with MexEF-OprN overexpression (MICs, 3.25 to 4.0 μg/ml), the ED50/MIC ratios were 3.8 to 16.1.
TABLE 3.
Activity of levofloxacin against strains of P. aeruginosa in a systemic infection model
| Strain | MIC (μg/ml) | ED50 (μg/kg) | 95% confidence limit | ED50/MIC ratio |
|---|---|---|---|---|
| PAM 1154 | 0.015 | 2.4 | 1.5-3.3 | 160 |
| PAM 1626 | 0.015 | 1.4 | 0.6-2.2 | 93.3 |
| PAM 1020 | 0.25 | 13.6 | 8.0-20 | 54.4 |
| PAM 1409 | 0.25 | 10.6 | 5.0-16 | 42.4 |
| PAM 1623 | 0.25 | 15.1 | 7.0-23 | 60.4 |
| PAM 1032 | 1.50 | 65.6 | 32-97 | 43.7 |
| PAM 1723 | 1.50 | 63.2 | 46-79 | 42.1 |
| PAM 1033 | 2.25 | 100.4 | 76-142 | 44.6 |
| PAM 1177 | 1.00 | 38.2 | 24-53 | 38.2 |
| PAM 1034 | 4.00 | 64.4 | 48-81 | 16.1 |
| PAM 1168 | 3.25 | 33.2 | 19-47 | 10.2 |
| PAM 1001 | 3.25 | 24.1 | 16-33 | 6.0 |
| PAM 1014 | 3.25 | 15.1 | 3.0-27 | 3.8 |
FIG. 1.
Activity of levofloxacin against strains of P. aeruginosa in a systemic infection model. ♦, strains without MexEF-OprN overexpressed; ▪, strains with MexEF-OprN overexpressed.
Pharmacokinetics.
The pharmacokinetic parameters of levofloxacin in neutropenic mice are shown in Table 2. Levofloxacin was absorbed rapidly after an intraperitoneal injection, with the times to Cmax (Tmax) ranging from 0.24 to 0.30 h. The AUC values increased linearly with the dose.
Neutropenic mouse thigh model.
The activities of levofloxacin against all strains tested in the neutropenic mouse thigh model are presented in Table 4 and Fig. 2 and 3. Levofloxacin AUC/MIC ratios for strains without MexEF-OprN overexpression were fit to an Emax model with an R2 value of 0.86. Static, 1-log-drop, 2-log-drop, and 90% Emax AUC/MIC ratios were 50, 70, 95, and 125, respectively. Strains with MexEF-OprN overexpression were fit to an Emax model with an R2 value of 0.87. Static, 1-log-drop, 2-log-drop, and 90% Emax AUC/MIC ratios were 20, 27, 35, and 50, respectively.
TABLE 4.
Activity of levofloxacin against strains of P. aeruginosa in a neutropenic mouse thigh model
| Strain | MIC (μg/ml) | Static AUC/MIC ratio | 90% Emax AUC/MIC |
|---|---|---|---|
| PAM 1154 | 0.015 | 48 | 125 |
| PAM 1626 | 0.015 | 52 | 125 |
| PAM 1106 | 0.06 | 42 | 80 |
| PAM 1020 | 0.25 | 50 | 120 |
| PAM 1409 | 0.25 | 50 | 90 |
| PAM 1623 | 0.25 | 48 | 90 |
| Pa 064 | 0.25 | 80 | 120 |
| PAM 1176 | 1.00 | 30 | 75 |
| PAM 1177 | 1.00 | 40 | 90 |
| Pa 103 | 1.00 | 45 | 120 |
| PAM 1032 | 1.50 | 50 | 90 |
| PAM 1723 | 1.50 | 20 | 90 |
| PAM 1033 | 2.25 | 67 | 120 |
| PAM 1738 | 2.25 | 60 | 100 |
| Pa 813 | 1.75 | 17 | 34 |
| PAM 1753 | 2.50 | 10 | 40 |
| Pa 812 | 3.00 | 15 | 50 |
| PAM 1168 | 3.25 | 28 | 40 |
| PAM 1178 | 3.50 | 30 | 45 |
| PAM 1034 | 4.00 | 23 | 45 |
| Pa 814 | 4.75 | 16 | 45 |
| Pa 815 | 7.25 | 30 | 41 |
FIG. 2.
Levofloxacin AUCs required to achieve 90% Emax in the neutropenic mouse thigh infection model. ♦, strains without MexEF-OprN overexpressed; ▪, strains with MexEF-OprN overexpressed
FIG. 3.
Pharmacodynamic (Emax) model fits of neutropenic mouse thigh infection model data. •, strains without MexEF-OprN overexpressed; ▪, strains with MexEF-OprN overexpressed
DISCUSSION
These data show that while the MexEF-OprN efflux pump provides the highest levofloxacin MICs against P. aeruginosa in vitro, it was the least efficient efflux pump in these two animal models of infection. In the mouse sepsis model, ED50/MIC ratios for levofloxacin were 40 to 160, except when MexEF-OprN was overexpressed, in which case the ratios dropped to 4 to 16. In the mouse thigh infection model, 90% Emax was obtained at levofloxacin AUC/MIC ratios of 80 to 125, except when MexEF-OprN was overexpressed, in which case the ratios dropped to 35 to 50. The differences in the PK-PD parameter for efficacy (AUC/MIC ratio) in these models suggest that target values may differ in strains with reduced susceptibilities to levofloxacin due to MexEF-OprN overexpression. Fluoroquinolones have been described to require an AUC/MIC ratio of 125 in order to reach optimal clinical and microbiological outcomes against infections caused by gram-negative bacteria (5). In the case of P. aeruginosa strains with MexEF-OprN overexpression, an AUC/MIC ratio of 50 achieved 90% of Emax, which is more akin to the AUC/MIC ratios required for the treatment of Streptococcus pneumoniae infections (3) than for those caused by gram-negative bacteria, such as P. aeruginosa.
Elevated MICs due to efflux pumps, which may or may not have a correlate effect on the in vivo response, may have implications for susceptibility breakpoints and will require additional study.
Acknowledgments
The work described in this paper was conducted as part of a research collaboration with Daiichi Pharmaceutical Co., Ltd.
REFERENCES
- 1.Aires, J. R., T. Kohler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike, H. 1978. Posterior probabilities for choosing a regression model. Ann. Inst. Stat. Math. 30A:9-14. [Google Scholar]
- 3.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H. Y., M. Yuan, and D. M. Livermore. 1995. Mechanisms of resistance to beta-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J. Med. Microbiol. 43:300-309. [DOI] [PubMed] [Google Scholar]
- 5.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda, H., M. Hosaka, S. Iyobe, N. Gotoh, T. Nishino, and K. Hirai. 1995. nfxC-type quinolone resistance in a clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:790-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotoh, N., N. Itoh, H. Tsujimoto, J. Yamagishi, Y. Oyamada, and T. Hishino. 1994. Isolation of OprM-deficient mutants of Pseudomonas aeruginosa by transposon insertion mutagenesis: evidence of involvement in multiple antibiotic resistance. FEMS Microbiol. Lett. 122:267-273. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh, N., H. Tsujimoto, M. Tsuda, K. Okamoto, A. Nomura, T. Wada, M. Nakahashi, and T. Nishino. 1998. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. K. Curty, and J. C. Pechere. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 10.Lee, A., W. Mao, M. Warren, A. Mistry, K. Hoshino, R. Okumura, H. Ishida, and O. Lomovskaya. 2000. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J. Bacteriol. 182:3142-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomovskaya, O., A. Lee, K. Hoshino, H. Ishida, A. Mistry, M. S. Warren, E. Boyer, S. Chamberland, and V. J. Lee. 1999. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1340-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasiello, A. P., J. M. Essigman, and G. N. Wogan. 1977. Rapid and accurate determination of median lethal dose (LD50) and its error with a small computer. J. Toxicol. Environ. 3:797-809. [DOI] [PubMed] [Google Scholar]
- 14.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 15.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng, N. H., H. S. Kaplan, J. M. Herbert, C. Moore, H. Douglas, A. Wunderlich, and A. I. Braude. 1975. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc. Natl. Acad. Sci. USA 82:1790-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneyama, H., A. Ocaktan, M. Tsuda, and T. Nakae. 1997. The role of mex-gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 233:611-618. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida, T., T. Muratani, S. Iyobe, and S. Mitsuhashi. 1994. Mechanisms of high-level resistance to quinolones in urinary tract isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 38:1466-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]





