Abstract
The macrolide antibiotic azithromycin improves lung function and prognosis among patients with cystic fibrosis or diffuse panbronchiolitis, independently of bacterial eradication. Anti-inflammatory effects have been implicated, but data from in vivo studies are scarce, and the link between abnormal electrolyte content in airway surface liquid and bronchial infections remains uncertain.
In the present study, we treated human airway epithelia on filter supports with azithromycin and monitored transepithelial electrical resistance. We found that azithromycin increased transepithelial electrical resistance of airway epithelia in a dose-dependent manner. Immunocytochemistry and Western blotting showed that addition of azithromycin changed the locations of proteins in cell cultures and induced processing of the tight junction proteins claudin-1 and claudin-4, occludin, and junctional adhesion molecule-A. These effects were reversible, and no effect was seen when cells were treated with penicillin or erythromycin. The data indicate that azithromycin increases the transepithelial electrical resistance of human airway epithelia by changing the processing of tight junction proteins. The results are novel and may help explain the beneficial effects of azithromycin in patients with cystic fibrosis, diffuse panbronchiolitis, and community-acquired pneumonia.
Respiratory infections remain an important cause of morbidity and mortality despite the development of novel antimicrobial agents. New infectious agents emerge, and multiresistant bacteria are a growing problem, leading to increased interest in host defense research that could provide tools in the fight against pulmonary infections. Epidemiological studies have generated interesting results regarding lung defense mechanisms. An example is the effect of macrolides, a class of commonly prescribed antibiotics, on patients with respiratory infections. Treatment with macrolide antibiotics improved 5- and 10-year survival rates among patients with diffuse panbronchiolitis (10, 20). This observation set the stage for large studies evaluating the effect of macrolide antibiotics on patients with cystic fibrosis (CF). Three recent randomized, placebo-controlled trials indicate that azithromycin significantly improves lung function by increasing the forced expiratory volume per second (5, 18, 28). Interestingly, improvement in lung function did not correlate with reduction of Pseudomonas aeruginosa or Staphylococcus aureus in sputum, suggesting that the favorable effect of azithromycin did not require bacterial eradication (5, 18, 28). Other studies found that the combination of a macrolide and a cephalosporin antibiotic improved the prognosis of patients with pneumococcal pneumonia, compared to that of patients receiving single-antibiotic treatment (12, 26). Cephalosporins are active against Streptococcus pneumoniae, but the beneficial effect of additional macrolide therapy on patients with this common type of pneumonia is largely unexplained. Speculation regarding the mechanism by which azithromycin improves clinical outcomes in diffuse panbronchiolitis, CF, and pneumonia include an anti-inflammatory effect and effects on sputum rheology, biofilm formation, bacterial adherence, and flagellin expression (16, 17, 24) Several studies have demonstrated the importance of the bronchial epithelium in lung defense (13, 23). In addition to being a mechanical barrier, it regulates electrolyte content of the airway surface liquid (ASL) (25). In cystic fibrosis, loss of cystic fibrosis transmembrane conductance regulator chloride channel activity produces abnormal ASL electrolyte and water content. Data also indicate that this may decrease lung defense against infections (23). If the electrolyte and water content of the ASL does affect lung defense, regulation of ion transport through paracellular pathways could be important in preventing lung infections. Tight junctions (TJs) located in the apicolateral membranes of epithelia form a barrier between adjacent cells and regulate the movement of ions and solutes across the paracellular space. TJs vary among different epithelia in barrier properties, meeting different functional requirements for each tissue type. The TJ complex consists of three types of transmembrane proteins—claudins, occludin, and junctional adhesion molecules (JAMs)—as well as zonula occludens, proteins that serve as adaptors to the actin cytoskeleton at the cytoplasmic face of TJs (14). Claudins and occludin are tetraspan transmembrane proteins with two extracellular loops and cytoplasmic C and N termini. There are at least 24 members of the claudin family, predicted to range in size from 20 to 27 kDa, and they show distinct tissue expression patterns. Occludin is considerably larger at ∼65 kDa and is widely expressed at TJs. JAM-A is a single-pass transmembrane protein of ∼40 kDa and a member of the immunoglobulin superfamily.
The importance of claudins as regulators of paracellular ion transport is evident for several human diseases. Simon et al. showed that mutations in the gene encoding claudin-16 is the cause of recessive renal hypomagnesemia. Their data further suggested that claudins could form a selective paracellular ion channel (22). Other studies indicate that claudin-3 and -4 are receptors for Clostridium perfringens enterotoxin (9), a common cause of food poisoning. Mutations in claudin-14 are associated with a recessive form of deafness, where the ionic environment in the cochlear duct is altered (27). To our knowledge, no data are available regarding a role for occludin or JAM-A in electrolyte transport.
In this study, we show that azithromycin increases transepithelial electrical resistance (TER) in human airway epithelia in vitro and affects both localization and processing of the tight junction proteins claudin-1 and claudin-4, occludin, and JAM-A. These effects of azithromycin on TJ proteins were specific and reversible, but no effects were found on the adherens junction protein E-cadherin or after treatment with other antibiotics, such as penicillin. The results show novel biological effects of a commonly used antibiotic on key proteins that maintain respiratory epithelial integrity and could be the initial step in explaining the clinical benefit from azithromycin treatment in CF, diffuse panbronchiolitis, and community-acquired pneumonia.
MATERIALS AND METHODS
Cell culture.
Primary bronchial epithelial cells (a gift from Michael J. Welsh, University of Iowa, Iowa City, Iowa) were cultured on plastic flasks coated with Vitrogen 100 (Cohesion, Palo Alto, Calif.) in serum- and antibiotic-free bronchial epithelial growth medium with supplements (item no. CC-3170; Cambrex, East Rutherford, N.J.). We established an immortalized cell line, VA-10. Transduction of normal human bronchial epithelial cells was performed with sterile filtered supernatant from the PA317 LXSN packaging cell lines, containing a retroviral construct with human papillomavirus type 16, E6 and E7 (CRL-2203; American Type Culture Collection, Rockville, Md.) and the neomycin resistance gene. Transduction was done in the presence of 8 μg/ml polybrene (Sigma-Aldrich). Transfected cells were selected by cultivation in the presence of 500 μg/ml neomycin (Life Technologies, Gaithersburg, Md.). For immunocytochemistry, cells were grown on Chamber slides (Nalge Nunc, Naperville, Ill.). For TER experiments, cells were grown on Transwell permeable support filters (Corning Costar Corporation, Acton, Mass.) and cultured for the first day in a 50:50 concentration of Dulbecco’s modified Eagle medium-Ham's F-12 medium (Gibco, Burlington, Canada) in 5% fetal bovine serum (Gibco). On the day after seeding, the cells were cultured and maintained in a 50:50 concentration of DMEM-Ham's F-12 medium supplemented with 2% Ultroser G (Biosepra, Cergy-Saint-Christophe, France).
Antibiotics.
Azithromycin (Zitromax; Pfizer ApS, Ballerup, Denmark), erythromycin lactobionate (Abboticin; Abbot, Solna, Sweden), and penicillin G (Penicillin Leo; Leo, Ballerup, Denmark) were dissolved as instructed by the manufacturer and then further diluted to the desired concentrations.
Measurement of transepithelial electrical resistance.
A Millicell-ERS voltohmmeter (Millipore, Billerica, Mass.) was used to measure the TER value of confluent filters. All measurements were done in triplicate, and TER values were normalized for the area of the filter and were obtained after background subtraction.
Growth curve.
Analysis of cell growth was performed using a standard protocol. Cells were plated onto 24-well plates and cultured at 37°C in a humidified 5% CO2 atmosphere with or without 40 μg/ml azithromycin. After 24 h, three wells of both cultures were trypsinized and counted by using a hemocytometer. This was repeated daily for 7 days, and the results were plotted as a growth curve.
Immunocytochemistry.
Immunofluorescent staining was performed on methanol-fixed cells. The primary antibodies used were rabbit anti-JAM-A and anti-claudin-1 and mouse anti-claudin-4, anti-occludin, and anti-E-cadherin. The antibodies were purchased from Zymed Laboratories (San Francisco, Calif.). We used iso-type-specific Alexa Fluor secondary antibody conjugates from Molecular Probes (Eugene, Oreg.). Images were captured by a Zeiss LSM 5 Pa confocal microscope (Carl Zeiss AG, Munich, Germany).
Western blotting.
Equal amounts of proteins, as determined by the Bradford method (2), were loaded and run on a NuPAGE 10% Bis-Tris gel (Invitrogen, Carlsbad, Calif.) and transferred to a polyvinylidene difluoride membrane (Invitrogen). The blots were blocked in 5% nonfat milk and subsequently incubated with the primary antibody overnight, followed by incubation with the secondary antibodies, horseradish peroxidase-conjugated anti-mouse or anti-rabbit, (Amersham Biosciences United Kingdom Ltd., Little Chalfont, England) for 1 h. Protein bands were visualized using the enhanced chemiluminescence system and Hyperfilm (Amersham Biosciences).
Statistical analysis.
Statistical analysis was performed using Student′s t test. The data are given as means ± the standard errors of the means (SEM). P values of <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Azithromycin increases transepithelial electrical resistance in human airway epithelia in vitro.
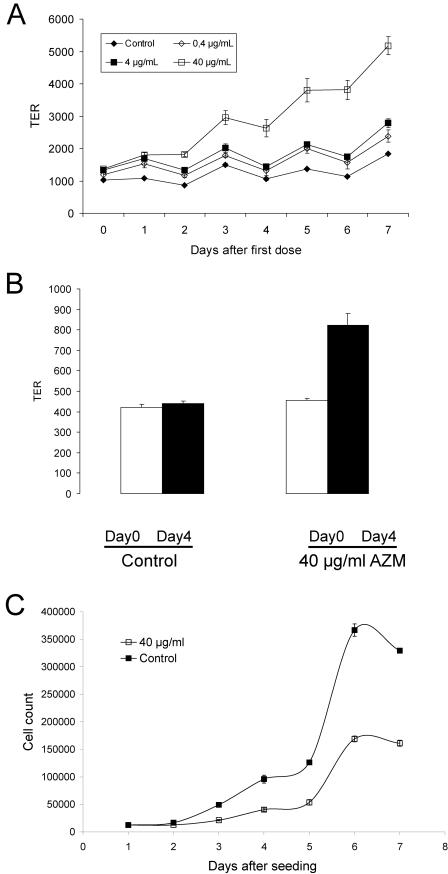
We measured TER across airway epithelia after treatment with azithromycin. We used 0.4, 4, and 40 μg/ml of azithromycin, based on clinical studies showing that, for patients receiving 250 mg azithromycin daily for 4 weeks, the median sputum concentration of azithromycin was 9.5 μg/ml (range, 0.6 to 79.3 μg/ml) (1). We found that the addition of 40 μg/ml azithromycin to the basolateral side of the epithelium increased TER from 1234 ± 29 (control) to 2920 ± 195 Ω/cm2 ± SEM (P < 0.05, n = 24) (Fig. 1A). Addition of azithromycin to the apical side had no effect on TER (data not shown). Figure 1B shows that a single dose of 40 μg/ml azithromycin daily over 4 days increased TER by approximately 80%. Erythromycin (30 μg/ml) or penicillin (20 μg/ml) had no effect on TER (data not shown). To explore the possibility that azithromycin produced multiple layers of epithelial cells, we generated a growth curve and found that azithromycin treatment resulted in fewer cells (Fig. 1C), suggesting that proliferation of epithelial cells does not explain the observed increase in TER. Azithromycin did not affect viability, and no effect on apoptosis was observed, as measured by immunostaining and Western blotting for cleaved caspase-3 (data not shown).
FIG. 1.
(A) Effect of azithromycin on TER of human airway epithelia in vitro. Human airway epithelial cells were cultured on Transwell filters. After reaching confluence, azithromycin (0.4, 4.0, and 40 μg/ml) was added to the basolateral side of epithelia every 48 h for 8 days. TER was measured using a Millicell-ERS electrical resistance system. Data are given as means ± SEM (n = 3). Azithromycin increased TER in a dose-dependent manner. (B) Azithromycin increases TER in human airway epithelia. Measurements were made at day 0 (open bars), before any treatment, and at day 4 (solid bars), after four doses of 40 μg/ml azithromycin. Data are given as means ± SEM (n = 6). Forty micrograms per milliliter of azithromycin added daily increased TER significantly (P < 0.0001). (C) Growth curve. Human airway epithelial cells were cultured on 24-well plates and treated continuously with 40 μg/ml azithromycin. Data are given as means ± SEM (n = 3). Azithromycin (40 μg/ml) decreases cell proliferation. AZM, azithromycin.
Azithromycin changes the processing of claudin-1 and -4.
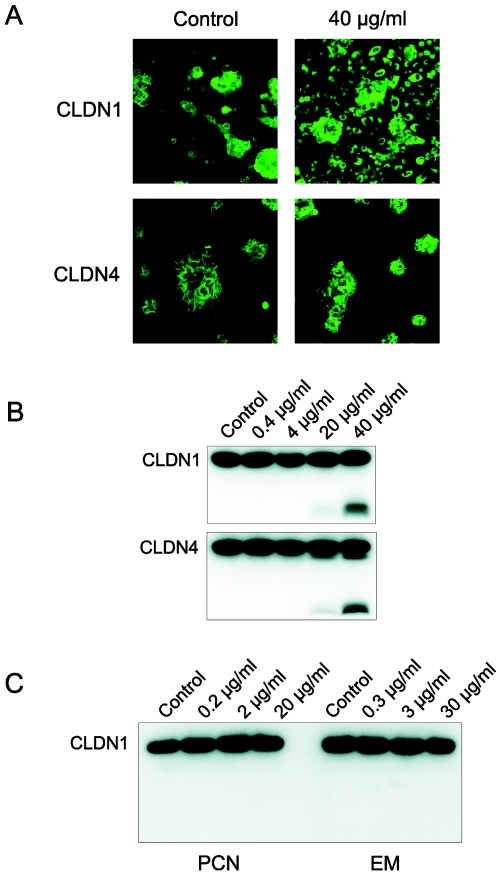
Tight junction proteins are required for epithelial integrity, a key component of structural and functional lung defense (15). We used specific antibodies to determine the cellular locations of claudin-1 and -4. Epithelia were cultured on glass slides and treated with azithromycin continuously from seeding or after reaching confluence. Under both conditions, immunocytochemistry suggested that azithromycin shifted claudin-1 and -4 to an intracellular location (Fig. 2A). To further characterize this effect, we used Western blotting. Lysates from the cells produced a band consistent with the molecular mass of claudins (∼23 kDa). Interestingly, in addition to the expected 23-kDa band (Fig. 2B), a rapidly migrating band (∼10 kDa) was produced after treatment with azithromycin (20 and 40 μg/ml). The smaller band was detected with both claudin-1 and claudin-4. These data indicate that azithromycin affects the processing of claudin-1 and -4. Azithromycin produced the same processing pattern for claudin-1 in two other cell lines, the alveolar epithelial line A549 and the breast luminal epithelial line D382 (data not shown), suggesting that this effect is general in epithelial cells. The processing of TJ proteins could affect lung defense mechanisms such as the mechanical barrier function or the regulation of airway surface liquid electrolytes. Interestingly, earlier studies suggest that azithromycin improves outcome in patients with CF, diffuse panbronchiolitis, and pneumonia independently of antibacterial effects. The data presented here might help explain some of the beneficial clinical effects of azithromycin. Unlike azithromycin, neither penicillin nor erythromycin affected the processing of claudin-1 (Fig. 2C).
FIG. 2.
(A) Immunocytochemical analyses of the effects of azithromycin on the expression of claudin-1 and -4. Human airway epithelial cells were cultured on chamber slides. Green indicates expression of claudin-1 or -4. The left row is the control. The right row shows cells after treatment with 40 μg/ml azithromycin. (B) Western blot of the effects of azithromycin on the expression of claudin-1 and -4. Equal amounts of protein from cells treated with different concentrations of azithromycin were subjected to Western blotting. Blotting for claudin-1 and -4 revealed a rapidly migrating band in lysates from cells treated with 40 μg/ml azithromycin. (C) Effect of penicillin and erythromycin on the expression of claudin-1. Equal amounts of protein from human airway epithelial cells treated with penicillin or erythromycin were subjected to Western blotting. Unlike azithromycin, neither penicillin nor erythromycin produced a rapidly migrating band. CLDN, claudin; PCN, penicillin; EM, erythromycin.
One potential mechanism by which azithromycin might alter the processing of tight junction proteins is activation of proteolytic enzymes. The size of the rapidly migrating band in the claudin experiments could be consistent with a cleavage site in the cytoplasmic loop. This could affect the structure or location of the extracellular loops of claudins that have been shown to determine charge selectivity and TER (3, 4). However, several other possibilities exist, and the origin of the rapidly migrating band requires further analysis by immunoprecipitation and amino acid sequencing.
Azithromycin changes the processing of occludin and JAM-A.
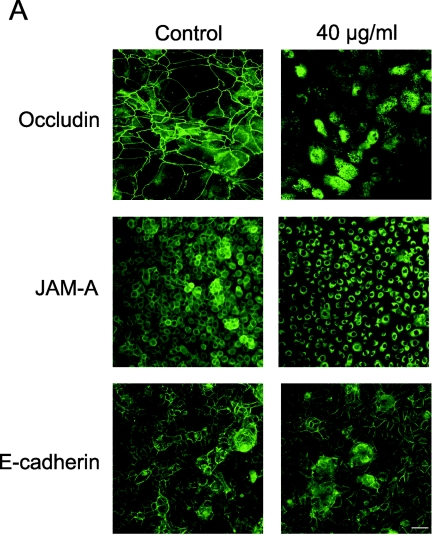
To test the possibility that azithromycin affected the processing of occludin, JAM-A, or E-cadherin, we used immunocytochemistry and Western blotting. Figure 3A indicates that azithromycin induces the intracellular localization of occludin and JAM-A but does not affect the localization of E-cadherin. Analysis of occludin protein expression revealed a ∼65 kDa band, consistent with the molecular mass of full-length occludin (Fig. 3B). Interestingly, a shift towards a smaller band of ∼40 kDa was observed in lysates from cells treated with azithromycin. Azithromycin also affected the expression of JAM-A, producing two rapidly migrating bands in addition to the expected 36- to 41-kDa band. In contrast, the Western blot of E-cadherin was unaffected by azithromycin (Fig. 3B).
FIG.3.
Effect of azithromycin on the expression of occludin, JAM-A, and E-cadherin. (A) Immunocytochemistry. Human airway epithelial cells were cultured on chamber slides. Green indicates expression of junctional molecules. The left row is the control. The right row shows cells after treatment with 40 μg/ml azithromycin. (B) Equal amounts of protein from cells treated with different concentrations of azithromycin were subjected to Western blotting. Blotting for occludin and JAM-A revealed a rapidly migrating band in lysates from cells treated with 40 μg/ml azithromycin.
The intracellular accumulation of occludin, claudins, and JAM-A after treatment with azithromycin is a puzzling phenomenon. Protein retention in endoplasmic reticulum or in Golgi apparatus, which allows cleavage, is a potential explanation for the effect of azithromycin on the processing of TJ proteins (Fig. 2A and 3A). Recent studies by Howe et al. demonstrate that treating cells with transforming growth factor β (TGFβ) resulted in perinuclear accumulation of the cystic fibrosis transmembrane conductance regulator chloride channel in epithelial cells. This was shown to be dependent on reorganization of the actin cytoskeleton. Exposure to TGFβ caused reorganization of F-actin into elongated stress fibers, in marked contrast to the more diffuse F-actin in control epithelial cells (8). Since the TJ complex is linked to the actin cytoskeleton through zonula occludens proteins, future studies should address this issue.
The expression of nonjunctional cell adhesion molecules may be affected by azithromycin. Semaan et al. found no significant effect of azithromycin on levels of soluble intracellular adhesion molecule (sICAM) in plasma from patients with coronary artery disease (21). In contrast, Hillis et al. showed that a 5-day azithromycin course for patients recovering from an acute coronary syndrome reduced serum levels of sICAM-1 (7). By studying the effect of azithromycin on nonjunctional cell adhesion molecules, we could have made our observations more specific. However, our data showing that azithromycin does not affect the processing of E-cadherin suggest that its effect on claudin-1 and -4, occludin, and JAM-A is specific. In addition, our model focuses on transepithelial electrolyte transport and tight junction proteins.
Interestingly, erythromycin affected neither TER nor the processing of TJ proteins. This suggests that, unlike the anti-inflammatory effects of macrolides, the effects on TER and the processing of TJ proteins found in our study are specific to azithromycin. Azithromycin is derived from erythromycin; the chemical difference is a methyl-substituted nitrogen atom incorporated into the lactone ring. Whether this is required in the macrolide chemical structure to affect the processing of TJ proteins should be further investigated. The successful management of diffuse panbronchiolitis (DPB) with erythromycin has been explained by its antibacterial and anti-inflammatory effects. The etiology of DPB remains unknown. Unlike CF, DPB has not been shown to be caused by defects in transepithelial electrolyte transport. Therefore, the clinical effect of azithromycin in CF patients could be caused by its common macrolide effects in addition to its specific effects on transepithelial electrolyte transport.
The effect of azithromycin on the processing of claudin-1 and occludin is reversible.
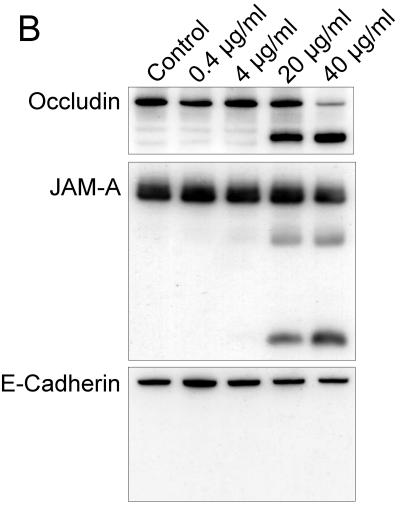
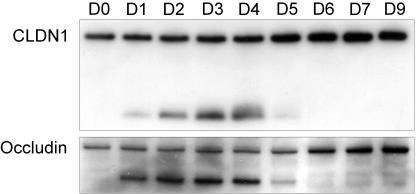
To test if the effect of azithromycin on the processing of claudin-1 and occludin was reversible, we applied azithromycin to epithelia daily for 4 days and maintained the culture without azithromycin. Protein was extracted before treatment and then daily. The data show that the effect of azithromycin on claudin-1 and occludin is evident 24 h after the first exposure to azithromycin. The effect is reversed at day 5, 24 h after removal of azithromycin (Fig. 4).
FIG. 4.
Reversible effect of azithromycin on claudin-1 and occludin. Confluent cells were treated with 40 μg/ml azithromycin daily for 4 days. After day 4, cells were cultured with medium alone. Equal amounts of protein were subjected to Western blotting. Protein was extracted before addition of azithromycin and then daily for 7 days and again on day 9. CLDN1, claudin-1.
Conclusion.
The study shows that azithromycin increases TER and affects the processing of tight junction proteins in human airway epithelia in vitro. The data do not define an association between altered protein processing and TER modification. However, such an association is suggested by various earlier studies that found that the extracellular loops of claudins contain charged amino acids (19), that the expression of different claudins increases or decreases TER (6), and that claudins create charge-selective channels in certain epithelial paracellular pathways (4).
The effects of azithromycin reported here are novel and may have implications for lung defense. Lee et al. (11) found that confluent low-TER airway epithelia bound 25 times more P. aeruginosa than confluent high-TER airway epithelia and that the bacterium bound frequently at cell borders, indicating that tight junctions might be involved. Claudins or other tight junction proteins are potential therapeutic targets in CF and other diseases of abnormal transepithelial ion transport. Future studies might attempt to better define the effect of azithromycin and other antibiotics on the function of tight junction proteins. Such work could be important in light of recent international pneumonia epidemics and increasing bacterial resistance to multiple antibiotics.
Acknowledgments
Grant support was provided by the Icelandic Research Council Thematic Program in Postgenomic Biomedicine (RANNIS), the St. Joseph's Hospital Landakot Science Fund, the University of Iceland, Landspitali University Hospital, and Actavis.
The authors thank Michael J. Welsh of the University of Iowa, Iowa City, Iowa, for providing airway epithelial cells and for helpful advice and discussions, Magnus Gottfredsson and other laboratory colleagues for useful discussions, and Kristín L. Steinadóttir for assistance with TER measurements.
REFERENCES
- 1.Baumann, U., M. King, E. M. App, S. Tai, A. Konig, J. J. Fischer, T. Zimmermann, W. Sextro, and H. von der Hardt. 2004. Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can. Respir. J. 11:151-155. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Colegio, O. R., C. Van Itallie, C. Rahner, and J. M. Anderson. 2003. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284:C1346-C1354. [DOI] [PubMed] [Google Scholar]
- 4.Colegio, O. R., C. M. Van Itallie, H. J. McCrea, C. Rahner, and J. M. Anderson. 2002. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 283:C142-C147. [DOI] [PubMed] [Google Scholar]
- 5.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 6.Furuse, M., K. Furuse, H. Sasaki, and S. Tsukita. 2001. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 153:263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillis, G. S., C. V. Pearson, S. A. Harding, S. Sutherland, C. A. Ludlam, J. C. Marioni, R. J. Prescott, K. A. Fox, and A. D. Flapan. 2004. Effects of a brief course of azithromycin on soluble cell adhesion molecules and markers of inflammation in survivors of an acute coronary syndrome: a double-blind, randomized, placebo-controlled study. Am. Heart J. 148:72-79. [DOI] [PubMed] [Google Scholar]
- 8.Howe, K. L., A. Wang, M. M. Hunter, B. A. Stanton, and D. M. McKay. 2004. TGFbeta down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp. Cell Res. 298:473-484. [DOI] [PubMed] [Google Scholar]
- 9.Katahira, J., H. Sugiyama, N. Inoue, Y. Horiguchi, M. Matsuda, and N. Sugimoto. 1997. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J. Biol. Chem. 272:26652-26658. [DOI] [PubMed] [Google Scholar]
- 10.Keicho, N., and S. Kudoh. 2002. Diffuse panbronchiolitis: role of macrolides in therapy. Am. J. Respir. Med. 1:119-131. [DOI] [PubMed] [Google Scholar]
- 11.Lee, A., D. Chow, B. Haus, W. Tseng, D. Evans, S. Fleiszig, G. Chandy, and T. Machen. 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277:L204-L217. [DOI] [PubMed] [Google Scholar]
- 12.Martinez, J. A., J. P. Horcajada, M. Almela, F. Marco, A. Soriano, E. Garcia, M. A. Marco, A. Torres, and J. Mensa. 2003. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 36:389-395. [DOI] [PubMed] [Google Scholar]
- 13.Matsui, H., B. R. Grubb, R. Tarran, S. H. Randell, J. T. Gatzy, C. W. Davis, and R. C. Boucher. 1998. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95:1005-1015. [DOI] [PubMed] [Google Scholar]
- 14.Matter, K., and M. S. Balda. 2003. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 4:225-236. [DOI] [PubMed] [Google Scholar]
- 15.Mullin, J. M., N. Agostino, E. Rendon-Huerta, and J. J. Thornton. 2005. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov. Today 10:395-408. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, T., S. G. Louie, P. M. Beringer, and M. A. Gill. 2002. Potential role of macrolide antibiotics in the management of cystic fibrosis lung disease. Curr. Opin. Pulm. Med. 8:521-528. [DOI] [PubMed] [Google Scholar]
- 17.Saiman, L. 2004. The use of macrolide antibiotics in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 10:515-523. [DOI] [PubMed] [Google Scholar]
- 18.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 19.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 20.Schultz, M. J. 2004. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J. Antimicrob. Chemother. 54:21-28. [DOI] [PubMed] [Google Scholar]
- 21.Semaan, H. B., P. A. Gurbel, J. L. Anderson, J. B. Muhlestein, J. F. Carlquist, B. D. Horne, and V. L. Serebruany. 2000. The effect of chronic azithromycin therapy on soluble endothelium-derived adhesion molecules in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 36:533-537. [DOI] [PubMed] [Google Scholar]
- 22.Simon, D. B., Y. Lu, K. A. Choate, H. Velazquez, E. Al-Sabban, M. Praga, G. Casari, A. Bettinelli, G. Colussi, J. Rodriguez-Soriano, D. McCredie, D. Milford, S. Sanjad, and R. P. Lifton. 1999. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103-106. [DOI] [PubMed] [Google Scholar]
- 23.Smith, J. J., S. M. Travis, E. P. Greenberg, and M. J. Welsh. 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85:229-236. [DOI] [PubMed] [Google Scholar]
- 24.Southern, K. W., and P. M. Barker. 2004. Azithromycin for cystic fibrosis. Eur. Respir. J. 24:834-838. [DOI] [PubMed] [Google Scholar]
- 25.Tang, V. W., and D. A. Goodenough. 2003. Paracellular ion channel at the tight junction. Biophys. J. 84:1660-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterer, G. W., G. W. Somes, and R. G. Wunderink. 2001. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch. Intern. Med. 161:1837-1842. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox, E. R., Q. L. Burton, S. Naz, S. Riazuddin, T. N. Smith, B. Ploplis, I. Belyantseva, T. Ben-Yosef, N. A. Liburd, R. J. Morell, B. Kachar, D. K. Wu, A. J. Griffith, and T. B. Friedman. 2001. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104:165-172. [DOI] [PubMed] [Google Scholar]
- 28.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]