Abstract
Pseudomonas aeruginosa is an opportunistic human pathogen. Treatment is complicated by frequent acquired resistance to antipseudomonal therapies. Polyamines (cadaverine, putrescine, spermidine, and spermine) are ubiquitous polycationic compounds essential for all living organisms. In a dose-dependent manner, polyamines increased the susceptibility of P. aeruginosa to 14 β-lactam antibiotics, chloramphenicol, nalidixic acid, and trimethoprim as demonstrated by a reduction in MIC of up to 64-fold. This effect was partially antagonized (25 to 50%) by the presence of 10 mM of Mg2+ or Ca2+. In contrast, the effects of the outer membrane permeabilizers, polymyxin B nonapeptide and EDTA, were completely abolished by 3 mM Mg2+ or Ca2+. Changes on the outer membrane barrier by these compounds were assessed by activity measurements of periplasmic β-lactamase. The results showed that while EDTA and polymyxin B serve as outer membrane disorganizing agents as expected, exogenous spermidine and spermine did not exhibit any apparent effect on outer membrane permeability or rupture. In summary, these results strongly suggest that the increased antibiotic susceptibility by polyamines is exerted by a mechanism that differs from that of EDTA and polymyxin B. Polyamines might be potentially useful in antipseudomonal therapies by increasing the effectiveness of certain β-lactam antibiotics.
Pseudomonas aeruginosa is a gram-negative human pathogenic bacterium responsible for severe nosocomial infections, life-threatening infections in immunocompromised persons, and chronic infections in cystic fibrosis patients (6, 23, 29, 30). Antimicrobial treatment is often difficult because of development of resistant strains (12). While tremendous efforts have been devoted to deciphering molecular details of resistance mechanisms, little has been done to identify methods for increasing antibiotic susceptibility to available drugs. Antibiotic susceptibility enhancement was first reported in the late 1950s (21, 31) and was related to outer membrane permeabilization of gram-negative bacteria by cationic and chelating agents. The outer membrane of gram-negative bacteria consists of an asymmetric double layer of polyanionic lipopolysaccharide (LPS) molecules (outer leaflet) and glycerophospholipids (inner leaflet). LPS molecules are electrostatically linked by divalent cations (e.g., Mg2+ and Ca2+), forming a “tiled-roof” structure with strong integrity that functions as an effective permeability barrier against hydrophobic antibiotics, detergents, dyes, and macromolecules (20, 25, 27). However, this structure can be weakened by removing divalent ions or replacing them with other cationic agents. This results in an increase of outer membrane permeability and sensitizes the bacteria to hydrophobic antibiotics, detergents, or dyes (27). Several such compounds (e.g., EDTA, polymyxin B nonapeptide [PMBN], lysine polymers, and protamine) have been reported to sensitize gram-negative bacteria to antimicrobial agents in this manner (27).
Vaara and coworkers have extensively investigated cationic agents that increase outer membrane permeability (20, 25, 27). PMBN is the one of the best-characterized cationic outer membrane permeabilizers and sensitizes enteric bacteria to hydrophobic antibiotics. However, PMBN is extremely nephrotoxic, and thus, its use in clinical applications is markedly reduced (27).
Natural polyamines, including cadaverine, putrescine, spermidine, and spermine, are a group of ubiquitous cationic compounds found in all living organisms. Spermine is present in eukaryotic cells whereas the others are present in both prokaryotic and eukaryotic cells. Polyamines are essential for optimal cell growth and viability, and intracellular concentrations of polyamines are at millimolar levels in both prokaryotic and eukaryotic cells (7). In prokaryotic cells, polyamines have been reported as potential regulatory molecules in DNA replication, transcription, translation, and enzyme activities (8). A recent report suggested that in Escherichia coli, polyamines enhance the expression of a set of regulatory genes at the level of translation and subsequently stimulate the transcription of hundreds of genes required for optimal cell growth and viability (33). Other reports suggest a role for polyamines in the protection of cells from external toxic conditions, such as oxidative stress (9, 26), radiation (10), acidic pH (22, 24), and other toxic agents (2, 16). Polyamines are also involved in control of membrane permeability by blocking outer membrane porin channels (e.g., OmpF and OmpC) in E. coli (3). In contrast, the synthetic polyamine analogues naphthylacetylspermine and methoctramine were reported to increase the outer membrane permeability by disruption of LPS integrity, resulting in increased susceptibility of E. coli to hydrophobic antibiotics (32). An early study conducted by Vaara and Vaara concluded that cadaverine, spermidine, and spermine at submillimolar concentrations had neither bactericidal nor sensitizing activity to antibiotics in E. coli (28).
We previously studied arginine metabolisms and polyamine utilization in P. aeruginosa PAO1 (14) to explore the physiological roles of polyamines in this organism. We reported that exogenous natural polyamines can enhance the susceptibility of P. aeruginosa PAO1 to multiple antibiotics, including β-lactams, chloramphenicol, nalidixic acid, and trimethoprim, but not to erythromycin, novobiocin, and fusidic acid. We also presented data in support of the notion that the mechanism of antibiotic susceptibility by polyamines is fundamentally different from that associated with EDTA or PMBN.
MATERIALS AND METHODS
Growth conditions of P. aeruginosa.
P. aeruginosa PAO1 was grown on LB or cation-adjusted Mueller-Hinton (MH) (Oxoid, Ogdensburg, N.Y.) medium at 37°C for antibiotic susceptibility testing of P. aeruginosa PAO1. All antibiotics and chemicals, including polyamines (spermidine, spermine, putrescine, and cadaverine), EDTA, and PMBN, were purchased from Sigma (St. Louis, MO). Polyamines or antibiotic stock solutions were prepared in double-distilled water or the solvents suggested by the manufacturer and filtered through 0.4-μm disposable Millipore membranes (Billerica, Mass.). The final pH value of prepared medium was 7.5 after appropriate adjustments.
Antibiotic susceptibility testing.
Antibiotic susceptibility was tested by the standard broth dilution methods according to the guidelines of the CLSI (formerly NCCLS) (17, 18). Briefly, each stock solution of antibiotics was added to cation-adjusted MH broth to achieve serial twofold concentrations between 0.031 and 1,024 μg/ml and suspended into sterile 17- by 100-mm snapped-cap Falcon culture tubes (1 ml/tube; Fisher Scientific). Fresh overnight cultures of P. aeruginosa PAO1 were diluted in saline to an optical density at 600 nm of 0.09 to 0.1 (approximately 1 × 108 viable cells per ml, which were confirmed by colony counts on LB agar plates after appropriate dilutions). A portion of the adjusted cell suspension (2 to 5 μl for ∼105 cells) was inoculated to MH broth containing antibiotics as indicated. The cell cultures were then incubated overnight (14 to 16 h) at 37°C. The MIC was defined as the lowest concentration of each antibiotic that completely inhibited the growth of the inoculum.
β-Lactamase assay.
The overnight culture of P. aeruginosa PAO1 was diluted 100-fold in 20 ml of MH broth with or without the supplements as indicated. The diluted cell cultures were grown at 37°C for 4 h with shaking at 350 rpm. Carbenicillin (50 μg/ml) was then added, and the cell growth continued for an additional hour. Cells were harvested, washed, and resuspended in 5 ml of 50 mM potassium phosphate buffer (pH 7.0). Cells were broken by French press at 55.6 × 105 Pa (800 lb/in2), and the cell-free crude extract was collected after centrifugation at 15,000 × g for 15 min. The protein concentration of the crude extracts was determined by the Bradford method (1), using bovine serum albumin as the standard.
Τhe enzymatic activity of β-lactamase was determined using nitrocefin (Oxoid) as the substrate. The reaction mixture (2 ml) contained 100 μg/ml of nitrocefin in 0.1 M sodium phosphate buffer (pH 7.0). The activity of β-lactamase was monitored at 30°C by absorbance changes at 486 nm in a Cary 3E spectrophotometer (Varian). One unit of activity was defined as the amount of β-lactamase that digests 1 μmol of nitrocefin per minute at 37°C. The molar extinction coefficient of nitrocefin is 20,500 M−1 cm−1 at 486 nm.
Construction of a lacZ::ampC promoter fusion.
The genomic DNA of P. aeruginosa PAO1 was extracted and used as template to amplify the promoter region of ampC (PA4110) (13) with the following pair of primers: 5′-GGAAGTCCTCCAGCCGCGGCAG-3′ and 5′-GGCGTCCTTTGTCGTTGGCTGC-3′. The 500-bp PCR product was purified by QIAGEN spin columns (Chatsworth, Calif.) and inserted into the SmaI site of a broad-host-range transcriptional fusion vector, pQF50 (5). The orientation and the DNA sequences of the insert in the resulting plasmid, pAU16R, were confirmed by nucleotide sequencing reactions at the Biotechnology Core Facility of Georgia State University. For measurements of β-galactosidase activities, o-nitrophenyl-β-d-galactopyranoside (ONPG) was used as the substrate as described previously (15).
Outer membrane permeability/disruption assay.
The outer membrane permeability/disruption assay was performed by examining the activity distribution of periplasmic β-lactamase (11). Briefly, an overnight culture of P. aeruginosa PAO1 harboring pQF50 (5) was diluted 60-fold into 20 ml prewarmed LB broth. Cell growth was continued in an incubator with shaking (350 rpm) at 37°C until an optical density at 600 nm reached 1.0. Cells were divided into 2-ml aliquots and then were treated with indicated concentrations of EDTA, polymyxin B, spermine, or spermidine for 10 min at room temperature. For each aliquot of treated cells, a cell-free filtrate was collected from 1 ml of the cell suspension after passing through a 0.4-μm membrane filter (Billerica, Mass.). Measurements of β-lactamase activities in cell-free filtrates and cell suspensions were determined as described above, and cell-only activities were derived by subtraction of each pair of measurements.
RESULTS AND DISCUSSION
Polyamines increased susceptibility to various antibiotics.
We have reported that exogenous polyamines induce expression of the oprH-phoPQ operon and increase MICs of cationic peptides, aminoglycosides, quinolones, and fluorescent dyes against P. aeruginosa (10a). To our surprise, MICs of another set of antibiotics were decreased by the presence of exogenous polyamines (spermine, spermidine, putrescine, and cadavarine). As shown in Table 1, MICs of β-lactams, chloramphenicol, nalidixic acid, and trimethoprim were decreased up to 64-fold in the presence of the polyamines. MICs of erythromycin, novobiocin, and fusidic acid were not affected by the presence of polyamines. MICs of all the antibiotics tested were decreased in the presence of EDTA or PMBN. In the negative control, there was no change in MICs in the presence of l-arginine, which can serve as the precursor of putrescine biosynthesis.
TABLE 1.
MICs of antibiotics to P. aeruginosa PAO1 in the presence of polyamines, EDTA, and PMBN
| Antibiotic | MIC (μg/ml) in presence ofa:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No compound | Spd | Spn | Put | Cad | EDTA | PMBN | Arg | |
| Ampicillin | >1,024 | 64 | 64 | 128 | 128 | 32 | 16 | >1,024 |
| Azlocillin | 4 | 1 | 1 | 2 | 2 | 1 | 1 | 4 |
| Aztreonam | 4 | 0.5 | 0.5 | 1 | 0.5 | 1 | 0.5 | 4 |
| Carbenicillin | 64 | 4 | 4 | 16 | 16 | 4 | 2 | 64 |
| Cefoperazone | 4 | 2 | 2 | 2 | 2 | 1 | 1 | 4 |
| Ceftazidime | 2 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.25 | 2 |
| Ceftriaxone | 16 | 0.25 | 0.25 | 4 | 0.25 | 2 | 0.5 | 16 |
| Cephaloridine | >1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 256 | 128 | >1,024 |
| Cephalothin | >1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 256 | 128 | >1,024 |
| Cloxacillin | >1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | >1,024 |
| Moxalactam | 8 | 0.25 | 0.25 | 2 | 0.25 | 1 | 1 | 8 |
| Penicillin G | >1,024 | 512 | 512 | 512 | 512 | 128 | 128 | >1,024 |
| Piperacillin | 8 | 2 | 2 | 2 | 2 | 2 | 2 | 8 |
| Ticarcillin | 16 | 2 | 2 | 4 | 0.5 | 4 | 4 | 16 |
| Chloramphenicol | 128 | 32 | 32 | 64 | 64 | 8 | 2 | 128 |
| Nalidixic acid | 128 | 64 | 64 | 64 | 64 | 64 | 4 | 128 |
| Trimethoprim | 256 | 128 | 64 | 128 | 128 | 32 | 4 | 256 |
| Erythromycin | 128 | 128 | 64 | 128 | 128 | 32 | 1 | 128 |
| Novobiocin | >1,024 | >1,024 | >1,024 | >1,024 | >1,024 | 512 | 256 | >1,024 |
| Fusidic acid | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 128 | 2 | 1,024 |
MIC measurements in MH broth were repeated three times with identical results. Compound concentrations were as follows: 20 mM spermidine (Spd), putrescine (Put), cadaverine (Cad), and arginine (Arg); 1 mM spermine (Spn) and EDTA; and 1 μg/ml PMBN.
To further examine the effects of polyamines on antibiotic susceptibility, the MIC of carbenicillin was determined in the presence of different concentrations of spermidine and spermine. As shown in Table 2, it was found that 0.5 mM of spermine or 1 mM of spermidine is sufficient to decrease the MIC of carbenicillin 4-fold and that spermine was more effective than spermidine in achieving the maximal 16-fold sensitization of P. aeruginosa to carbenicillin. This concentration-dependent susceptibility effect was also observed with EDTA and PMBN (Table 2).
TABLE 2.
MICs of carbenicillin against P. aeruginosa PAO1 in the presence of different concentrations of polyamines, EDTA, and PMBNa
| Compound | MIC (μg/ml) of carbenicillin at compound concn of:
|
|||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 20 | |
| Spermidine | 64 | 32 | 16 | 16 | 8 | 4 |
| Spermine | 64 | 16 | 4 | 4 | 4 | 4 |
| Putrescine | 64 | 64 | 32 | 32 | 32 | 16 |
| Cadaverine | 64 | 64 | 32 | 32 | 32 | 16 |
| EDTA | 64 | 32 | 4 | 4 | NG | NG |
| PMBN | 64 | 4 | 2 | NG | NG | NG |
MIC measurements in MH broth were repeated three times with identical results. The concentration of PMBN is in μg/ml while other concentrations are in mM. NG, no growth.
It has been proposed that spermine, a natural polyamine of eukaryotes, might possess an antibacterial activity in accordance with its high concentration in human semen (4). We have tested this idea by monitoring the cell growth in the presence of various concentrations of spermine and found that up to 20 mM of exogenous spermine did not show any retardation effect on cell growth in either minimal medium or LB broth. Similar results were also obtained for spermidine and putrescine. In contrast, cell growth was retarded significantly by 5 mM of EDTA or 3 μg/ml of PMBN.
Effects of divalent magnesium and calcium ions on polyamine-mediated antibiotic susceptibility.
It has been reported that the effects of EDTA and PMBN are inhibited by exogenous divalent cationic ions (Mg2+ or Ca2+) (27). To test whether the effect of polyamines was also inhibited by the exogenous cationic ions, the MIC of carbenicillin was measured in the presence of various concentrations of Mg2+ or Ca2+. As shown in Table 3, the addition of up to 10 mM Mg2+ exerted a partial effect in antagonizing the effects of spermine or spermidine. In contrast, the effects of EDTA or PMBN were completely abolished by as little as 3 mM of Mg2+ (Table 3). Similar results were also observed when Mg2+ was replaced with Ca2+ (data not shown).
TABLE 3.
MICs of carbenicillin against P. aeruginosa PAO1 in the presence of different concentrations of Mg2+
| Compound (concn) | MIC (μg/ml) of carbenicillin at Mg2+ concn (mM) of:
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 10 | |
| None | 64 | 64 | 64 | 64 | 64 |
| Spermidine (20 mM) | 4 | 8 | 16 | 16 | 16 |
| Spermine (1 mM) | 4 | 8 | 16 | 16 | 16 |
| Putrescine (20 mM) | 16 | 32 | 32 | 32 | 32 |
| Cadaverine (20 mM) | 16 | 32 | 32 | 32 | 32 |
| EDTA (1 mM) | 4 | 32 | 64 | 64 | 64 |
| PMBN (1 μg/ml) | 2 | 16 | 64 | 64 | 64 |
No change on outer membrane permeability or rupture by polyamines.
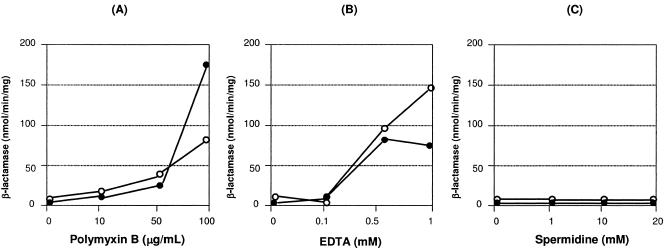
Measurements of β-lactamase activities in cell-free filtrates or whole cells have been used to assess whether changes on the outer membrane barrier are associated with increased antibiotic susceptibility (11, 19). When cells were treated with polymyxin B, a cationic peptide antibiotic known to increase outer membrane permeability, significant activities of β-lactamase were detected in the whole cells when 100 μg/ml of this antibiotic was applied to the cell suspension (Fig. 1A). As shown in Fig. 1B, release of periplasmic β-lactamase into the suspension solution as the result of outer membrane rupture can be detected following 0.5 mM of EDTA and increased with 1 mM of EDTA, and outer membrane permeability was also increased as evidenced by the activities detected from whole cells. In comparison, only a very low level of β-lactamase activity was detected following the addition of 20 mM spermidine (Fig. 1C) or spermine (data not shown). These results indicate that it is very unlikely that polyamines exert their effects by rupturing the outer membrane or by changing outer membrane permeability.
FIG. 1.
Outer membrane permeabilization assays. Cells were treated with indicated concentrations of polymyxin B (A), EDTA (B), and spermidine (C). Activities of periplasmic β-lactamase were determined by enzymatic measurements as described in Materials and Methods. Filled circles, cell-free filtrates; empty circles, whole cells.
Polyamines have no effect on either transcriptional regulation of ampC or the enzymatic activity of β-lactamase.
The polyamine effect on increased susceptibility to β-lactams could be mediated by reduced expression of ampC encoding β-lactamase and/or by an inhibitory effect on the enzymatic activity of β-lactamase. The first possibility was tested by measurements of β-galactosidase activities from a recombinant strain of P. aeruginosa PAO1 harboring pAU16R, an ampC::lacZ promoter fusion, as described in Materials and Methods. The results revealed no effect on the level of ampC expression in the presence (900 nmol/min/mg) or absence (890 nmol/min/mg) of 20 mM spermidine. Total β-lactamase activities from cells grown in the presence or absence of 20 mM spermidine were also measured and revealed no change (data not shown). Furthermore, the addition of 20 mM spermidine to the reaction mixture showed no effect on the activity of β-lactamase. All these results indicated that the effect of polyamines on the increased susceptibility to β-lactam antibiotics was not due to the reduced β-lactamase activity at the genetic or protein level.
In conclusion, we found that polyamines at the millimolar levels can increase the susceptibility of P. aeruginosa to a variety of antibiotics (Table 1). These data differ from the conclusion of Vaara and Vaara, who examined roles of the polyamines in antibiotic susceptibility of enteric bacteria and concluded that polyamines had neither bactericidal nor sensitizing activity (28). The discrepancy could be due to the concentrations of polyamines, as they used only submillimolar concentrations of polyamines, or due to different bacteria used in the study. Although polyamines are polycationic, a characteristic common to many outer membrane permeabilizers (PMBN and other cationic peptide antibiotics), the results presented here do not support the hypothesis that polyamines are outer membrane disorganizing agents. We showed that polyamines significantly enhanced the effectiveness of 14 β-lactam antibiotics, including several penicillins, cephalosporins, and monobactams. Polyamines theoretically hold a great potential in increasing the effectiveness of current antimicrobial therapies for P. aeruginosa infections. Considering the potential impact of this discovery on clinical applications, elucidation of the molecular mechanism of polyamines on antibiotic susceptibility and the possible linkage of this effect to polyamine metabolism warrants further investigation.
Acknowledgments
This work was supported by National Science Foundation grants 0316005 and 0415608.
We are grateful to David Y. Graham for critical review of the manuscript.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay, M. K., C. W. Tabor, and H. Tabor. 2003. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 100:2261-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dela Vega, A. L., and A. H. Delcour. 1996. Polyamines decrease Escherichia coli outer membrane permeability. J. Bacteriol. 178:3715-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fair, W. R., and N. Wehner. 1971. Antibacterial action of spermine: effect on urinary tract pathogens. Appl. Microbiol. 21:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 7.Gugliucci, A. 2004. Polyamines as clinical laboratory tools. Clin. Chim. Acta 344:23-35. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi, K., and K. Kashiwagi. 2000. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 271:559-564. [DOI] [PubMed] [Google Scholar]
- 9.Jung, I. L., T. J. Oh, and I. G. Kim. 2003. Abnormal growth of polyamine-deficient Escherichia coli mutant is partially caused by oxidative stress-induced damage. Arch. Biochem. Biophys. 418:125-132. [DOI] [PubMed] [Google Scholar]
- 10.Kim, I. G., and T. J. Oh. 2000. SOS induction of the recA gene by UV-, gamma-irradiation and mitomycin C is mediated by polyamines in Escherichia coli K-12. Toxicol. Lett. 116:143-149. [DOI] [PubMed] [Google Scholar]
- 10a.Kwon, D. H., and C.-D. Lu. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 50:1615-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, X. Z., L. Zhang, and K. Poole. 2000. Interplay between the MexA-MexB-OprM multidrug efflux system and the outer membrane barrier in the multiple antibiotic resistance of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:433-436. [DOI] [PubMed] [Google Scholar]
- 12.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 13.Lodge, J. M., S. D. Minchin, L. J. Piddock, and J. W. Busby. 1990. Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC beta-lactamase. Biochem. J. 272:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, C.-D., Y. Itoh, Y. Nakada, and Y. Jiang. 2002. Functional analysis and regulation of the divergent spuABCDEFGH-spuI operons for polyamine uptake and utilization in Pseudomonas aeruginosa PAO1. J. Bacteriol. 184:3765-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Minton, K. W., H. Tabor, and C. W. Tabor. 1990. Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc. Natl. Acad. Sci. USA 87:2851-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement. M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repaske, R. 1958. Lysis of gram-negative organisms and the role of versene. Biochim. Biophys. Acta 30:225-232. [DOI] [PubMed] [Google Scholar]
- 22.Samartzidou, H., M. Mehrazin, Z. Xu, M. J. Benedik, and A. H. Delcour. 2003. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J. Bacteriol. 185:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samer, Q., B. A. Cunha, P. Dua, and K. D. Lessnau. 2004. Pseudomonas aeruginosa infections. [Online.] http://www.emedicine.com/med/topic1943.htm.
- 24.Soksawatmaekhin, W., A. Kuraishi, K. Sakata, K. Kashiwagi, and K. Igarashi. 2004. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51:1401-1412. [DOI] [PubMed] [Google Scholar]
- 25.Sukupolvi, S., and M. Vaara. 1989. Salmonella typhimurium and Escherichia coli mutants with increased outer membrane permeability to hydrophobic compounds. Biochim. Biophys. Acta 988:377-387. [DOI] [PubMed] [Google Scholar]
- 26.Tkachenko, A., L. Nesterova, and M. Pshenichnov. 2001. The role of the natural polyamine putrescine in defense against oxidative stress in Escherichia coli. Microbiol. Mol. Biol. Rev. 176:155-157. [DOI] [PubMed] [Google Scholar]
- 27.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaara, M., and T. Vaara. 1983. Polycations sensitize enteric bacteria to antibiotics. Antimicrob. Agents Chemother. 24:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Delden, C. 2004. Virulence factors in Pseudomonas aeruginosa. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 30.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [Online.] http://www.cdc.gov/ncidod/eid/vol4no4/vandelden.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren, G. H., J. Gray, and J. A. Yurchenco. 1957. Effect of polymyxin on the lysis of Neisseria catarrhalis by lysozyme. J. Bacteriol. 74:788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda, K., C. Ohmizo, and T. Katsu. 2004. Mode of action of novel polyamines increasing the permeability of bacterial outer membrane. Int. J. Antimicrob. Agents 24:67-71. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, M., K. Kashiwagi, A. Shigemasa, S. Taniguchi, K. Yamamoto, H. Makinoshima, A. Ishihama, and K. Igarashi. 2004. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 279:46008-46013. [DOI] [PubMed] [Google Scholar]