Abstract
The incidence of malaria is increasing, and there is an urgent need to identify new drug targets for both prophylaxis and chemotherapy. Potential new drug targets include Plasmodium proteases that play critical roles in the parasite life cycle. We have previously shown that the major surface protein of Plasmodium sporozoites, the circumsporozoite protein (CSP), is proteolytically processed by a parasite-derived cysteine protease, and this processing event is temporally associated with sporozoite invasion of host cells. E-64, a cysteine protease inhibitor, inhibits CSP processing and prevents invasion of host cells in vitro and in vivo. Here we tested allicin, a cysteine protease inhibitor found in garlic extracts, for its ability to inhibit malaria infection. At low concentrations, allicin was not toxic to either sporozoites or mammalian cells. At these concentrations, allicin inhibited CSP processing and prevented sporozoite invasion of host cells in vitro. In vivo, mice injected with allicin had decreased Plasmodium infections compared to controls. When sporozoites were treated with allicin before injection into mice, malaria infection was completely prevented. We also tested allicin on erythrocytic stages and found that a 4-day regimen of allicin administered either orally or intravenously significantly decreased parasitemias and increased the survival of infected mice by 10 days. Together, these experiments demonstrate that the same cysteine protease inhibitor can target two different life cycle stages in the vertebrate host.
Malaria is one of the most important infectious diseases in the world. Each year 300 to 500 million new cases are diagnosed and approximately 1.5 million people die of the disease, the majority of whom are children (11). More than 40% of the world's population lives in malaria-endemic areas and is at risk of contracting the disease (11). Infection is initiated when Plasmodium sporozoites are injected into the skin of a vertebrate host by an infected anopheline mosquito. The sporozoites enter the bloodstream and travel to the liver, where they invade hepatocytes, differentiate, and divide asexually to produce exoerythrocytic forms. Upon maturation, exoerythrocytic forms rupture and release merozoites that invade erythrocytes and initiate the blood stage of infection, which is responsible for the symptoms of the disease.
The incidence of malaria is increasing due to several factors, including resistance of the parasite to currently available antimalarial drugs, and there is an urgent need to develop new drugs for both the prophylaxis and treatment of malaria (11). Among the targets being explored for the development of new drugs are the proteases of Plasmodium, which play critical roles in the parasite's life cycle and can be targeted with specific inhibitors (reviewed in references 3, 20-22, and 36).
We have recently found that the major surface protein of the sporozoite, the circumsporozoite protein (CSP), is proteolytically processed by a parasite cysteine protease during invasion and that E-64, a cysteine protease inhibitor, inhibits CSP processing as well as sporozoite infectivity in vitro and in vivo (6). Other groups studying the erythrocytic stages of Plasmodium have found that parasite cysteine proteases play critical roles in hemoglobin degradation (39, 40) and merozoite release from erythrocytes (37). Taken together, these data suggest that cysteine protease inhibitors may target both preerythrocytic and erythrocytic stages of Plasmodium and may therefore be good drug candidates for the prevention and treatment of malaria.
The anti-infective properties of garlic have long been known to Chinese and Indian civilizations and were first described in Europe by Louis Pasteur (13). Garlic has an unusually high concentration of sulfur-containing compounds, and its antibacterial properties are largely due to one particular class of sulfur-containing compounds, the thiosulfinates (18). The thiosulfinate structure [S(=O)S] appears to be essential for the bactericidal, antifungal, and antiprotozoal properties of garlic, likely reacting with SH-containing enzymes of these pathogens (34, 45). Allicin is the most abundant thiosulfinate found in garlic and is generated when the enzyme alliinase reacts with its substrate alliin (18, 41). Enzyme and substrate are located in different compartments of the clove, so that allicin is generated only when the clove is crushed (18, 41). Many lines of evidence indicate that allicin is primarily responsible for garlic's anti-infective properties (1, 5, 15, 34, 38, 43), although studies have also found that ajoene, a metabolite of allicin found when garlic is crushed specifically in oil, also has some antibacterial properties (27). In fact, one study found that ajoene has an inhibitory effect on the erythrocytic stages of Plasmodium (30).
The precise mechanism of action of the thiosulfinates has, in many cases, not been demonstrated. However, when used at low concentrations, allicin appears to react specifically with the free sulfhydryl group present in the active site of cysteine proteases (32). Experiments with the intestinal parasite Entamoeba histolytica have shown that pure allicin inhibits both the cytopathological effects associated with infection (2) and the growth of the parasite (23) via its inhibitory effect on the parasite's cysteine proteases. Because of allicin's inhibitory activity on cysteine proteases and Plasmodium's requirement for cysteine protease activity during various life cycle stages, we set out to test the effects of allicin on the preerythrocytic and erythrocytic stages of Plasmodium.
MATERIALS AND METHODS
Parasites.
Plasmodium berghei (NK65 and ANKA strains) and Plasmodium yoelii (17XNL) sporozoites were grown in Anopheles stephensi mosquitoes as previously described (26) and were obtained from infected salivary glands on the day of the experiment.
Antibodies.
Monoclonal antibody (MAb) 3D11, directed against the repeat region of P. berghei CSP (46), was conjugated to Sepharose (14) and biotinylated using d-biotinoyl-ɛ-aminocaproic acid-N-hydroxysuccinimide ester as outlined in the manufacturer's protocol (Roche Applied Science).
Allicin preparation.
Pure allicin was prepared by passing the synthetic substrate alliin (24) through an immobilized alliinase column (38). The concentration of allicin was determined using a spectrophotometric assay with a chromogenic thiol (25) and confirmed by high-performance liquid chromatography (7). Dilute aqueous allicin solutions (1.8 mg/ml) were stored in the dark at 4°C for <3 months. When used in experiments with parasites, allicin was diluted in medium without Cys/Met, since these amino acids react with and inactivate the drug.
Metabolic labeling, immunoprecipitation, and SDS-polyacrylamide gel electrophoresis analysis.
P. berghei sporozoites were metabolically labeled for 1 h in Dulbecco's modified Eagle medium (DMEM) with l-[35S]Cys/Met as previously described (6) and chased in the presence of 10 μM E-64 or the indicated concentrations of allicin. Labeled sporozoites were lysed in 1% Triton X-100-150 mM NaCl-50 mM Tris-HCl, pH 8.0, with protease inhibitors, and lysates were incubated with 3D11-Sepharose overnight at 4°C. CSP was eluted and run on a 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel under nonreducing conditions. The gel was fixed, enhanced with Amplify (Amersham Pharmacia), dried, and exposed to film.
Cell contact assay.
P. berghei sporozoites transgenic for green fluorescent protein (GFP) (8) were incubated in DMEM with or without 50 μM allicin for 10 min at 28°C, diluted 12-fold to 4.2 μM allicin with DMEM, and then centrifuged (300 × g) onto coverslips with Hepa 1-6 cells (CRL-1830; American Type Culture Collection) at 4°C. Coverslips were then incubated at 37°C for 2 min, fixed with 4% paraformaldehyde, and stained with polyclonal antiserum that stains only full-length CSP (6), followed by antirabbit immunoglobulin conjugated to Texas Red. Sporozoites were counted using a Nikon Eclipse E600 fluorescence microscope, and each field was viewed with two filters so that GFP sporozoites and Texas Red staining sporozoites could be enumerated.
Allicin toxicity assay.
P. berghei sporozoites were incubated with the indicated concentrations of allicin for 10 or 60 min at 28°C, washed with DMEM, and then incubated with 1 μg/ml propidium iodide for 5 min at 25°C. The number of fluorescent sporozoites in each sample was counted using a Nikon Eclipse E600 microscope. Control samples consisted of sporozoites that were incubated for 60 min at 28°C in DMEM alone to assess background level of propidium iodide uptake and sporozoites that were heat killed at 65°C for 10 min to insure that the assay was working.
Gliding motility assay.
Glass eight-chambered Lab-Tek wells (Nalgene) were coated with 10 μg/ml MAb 3D11 in phosphate-buffered saline (PBS) overnight at 25°C and then washed three times with PBS. Precoating the wells with antibody captures shed CSP onto the slide for better visualization of the trails. P. berghei (2 × 104/well) sporozoites were incubated with 50 μM allicin in DMEM without Cys/Met for 10 min at 28°C. The medium was removed and replaced with DMEM-3% bovine serum albumin (BSA) containing either 50 μM or 4.2 μM allicin before sporozoites were added to the coated Lab-Tek wells. Sporozoites were incubated for 1 h at 37°C, the medium was removed, and the wells were fixed with 4% paraformaldehyde, washed, and blocked with PBS-1% BSA. To visualize the CSP-containing trails, the wells were then incubated with biotinylated MAb 3D11 followed by Streptavidin-fluorescein isothiocyanate (1:100 dilution; Amersham Pharmacia). All incubations were performed at 37°C for 1 h. Controls included untreated sporozoites and sporozoites added to wells in the presence of 1 μM cytochalasin D. For each group, gliding motility was quantified by counting the number of sporozoites associated with trails and, for those sporozoites with trails, counting the number of circles in each trail.
Sporozoite invasion assays.
Invasion assays were performed as previously described (31) with some modifications. P. berghei sporozoites were preincubated with the indicated concentrations of allicin for 10 min at 28°C, diluted 12-fold with DMEM-BSA, and added to Hepa 1-6 cells. Sporozoites were plated in each well of semiconfluent cells (5 × 104/well). After 1 h at 37°C, cells were washed and fixed, and sporozoites were stained with a double staining assay that distinguishes between intracellular and extracellular sporozoites (33).
Assay for sporozoite infectivity in vivo.
Female Swiss Webster mice, 5 to 6 weeks old, were injected intravenously (i.v.) with either 5 or 8 mg/kg of body weight of allicin (in DMEM without Cys/Met) 60 min, 30 min, or immediately before i.v. injection of 104 P. yoelii sporozoites. Forty hours later, livers were harvested, total RNA was isolated, and malaria infection was quantified using reverse transcription followed by real-time PCR with primers that recognize P. yoelii-specific sequences within the 18S rRNA as previously described (4). Ten-fold dilutions of a plasmid construct containing the P. yoelii 18S rRNA gene were used to create a standard curve. For allicin preincubation experiments, P. yoelii sporozoites were preincubated with or without 50 μM allicin (in DMEM without Cys/Met) for 10 min at 28°C and diluted 12-fold with medium before i.v. injection into mice. All in vivo data were analyzed using the Student t test for unpaired samples. All experiments were performed twice with six mice per group per experiment.
Assay for efficacy against erythrocytic stages in vivo.
The standard 4-day suppression test (29) with some modifications was used to assess the efficacy of allicin against malaria erythrocytic stages in vivo. Female Swiss Webster mice, 5 to 6 weeks old, were injected i.v. with 2 × 105 GFP-expressing P. berghei parasites (8), and 1 h later mice were injected i.v. with either 8 mg/kg of allicin (in DMEM without Cys/Met) or medium alone. Mice were treated with allicin or buffer once daily for an additional 3 days. For the experiments in which allicin was administered orally, Swiss Webster mice were infected with GFP-expressing parasites as above, and 1 h later, allicin (diluted in water) or water alone was administered by gavage. Total daily dosage was either 3 mg/kg/day or 9 mg/kg/day, administered in two doses (one in the morning and one in the evening) to decrease irritation to the gastric mucosa. Following treatment, survival of the mice was monitored and parasitemia was determined by fluorescence-activated cell sorting (FACS) analysis. For FACS, 2 μl of blood was diluted in 1 ml PBS containing 1% fetal calf serum and 0.01% NaN3, and the number of fluorescent cells was determined using the FACS Calibur System with CellQuest Software (Becton Dickinson). Statistical significance was determined using the Student t test for unpaired samples. All experiments were performed twice with five mice per group per experiment.
RESULTS
Inhibition of CSP cleavage.
We have previously shown that the cysteine protease inhibitor E-64 prevents proteolytic cleavage of CSP, the major surface protein of Plasmodium sporozoites (6). Because allicin has been shown to react with free sulfhydryl groups and reversibly inhibit cysteine proteases (2), we tested whether allicin would inhibit CSP cleavage. Pulse-chase metabolic labeling experiments, in which allicin was included in the chase, indicate that CSP cleavage was inhibited by 10, 25, and 50 μM allicin (Fig. 1A). The degree of inhibition was comparable to that observed with 10 μM E-64. In addition, chasing with 50 μM allicin for 10 min followed by dilution to 4.2 μM for the remainder of the chase prevented CSP cleavage to the same extent as when 50 μM allicin was present during the entire chase. The pulse-chase metabolic labeling experiments are performed with sporozoites in the absence of host cells. Under these conditions, the half-life of full-length CSP is on the order of hours (6, 46). However, when sporozoites are added to cells, cleavage occurs in minutes (6). Likely this reflects leaky microneme secretion of the protease in the absence of cells versus triggered secretion when sporozoites contact cells. We also tested the effect of allicin on CSP cleavage in the presence of host cells. For this, we added sporozoites to cells for 2 min and then counted the number of sporozoites that stained for full-length CSP. As shown in Fig. 1B, allicin also inhibits cleavage of CSP when sporozoites are in the presence of cells, indicating that it rapidly interacts with the CSP protease.
FIG. 1.
Allicin prevents cleavage of CSP. (A) P. berghei sporozoites were metabolically labeled with [35S]Cys/Met and kept on ice (labeled 0) or chased for 2 h in the absence of protease inhibitors (labeled 2), in the presence of 10 μM E-64 (labeled E-64), or in the presence of the indicated concentrations of allicin (labeled 10, 25, and 50). The lane labeled 50dil represents labeled sporozoites chased in the presence of 50 μM allicin for 10 min, which was then diluted to 4.2 μM for the remainder of the chase. After 2 h, the parasites were lysed and CSP was immunoprecipitated and analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. (B). P. berghei sporozoites were incubated in the presence or absence of 50 μM allicin for 10 min and then added to Hepa 1-6 cells for 2 min before being fixed and stained with antisera specific for full-length CSP. Two hundred sporozoites/well were counted, and means ± standard deviations for duplicate samples are shown.
Allicin toxicity.
In order to determine if the effect of allicin on CSP cleavage was due to a toxic effect on the sporozoites, we incubated parasites for 10 or 60 min with different concentrations of allicin and then added propidium iodide, a dye that is excluded by viable cells but penetrates the cell membranes of dead or dying cells. When sporozoites were incubated with either 1 or 10 μM allicin for up to 60 min, the percentage of sporozoites that took up the dye was no different from that of the untreated control (Fig. 2). A 10-min incubation with 50 μM allicin also did not kill sporozoites; however, when the incubation time was increased to 60 min, the number of sporozoites taking up the dye increased 1.5-fold, indicating that longer exposures to 50 μM allicin had a toxic effect on the sporozoites. Treatment of sporozoites with 50 μM allicin for 10 min, followed by dilution of the allicin to 4.2 μM for an additional 50 min, did not increase the number of fluorescent sporozoites compared to the untreated control. At concentrations higher than 50 μM, allicin was toxic to the sporozoites after only a 10-min exposure.
FIG. 2.
Toxicity of allicin for Plasmodium sporozoites. P. berghei sporozoites were incubated with the indicated concentrations of allicin for 10 min (gray bars) or 60 min (black bars) before the addition of propidium iodide. The “50 dil” bar indicates that sporozoites were incubated with 50 μM allicin for 10 min, followed by a 50-min incubation in 4.2 μM allicin. Control sporozoites were incubated in the absence of allicin for 60 min (white bar) or were heat killed (diagonally striped bar). For each sample, 200 sporozoites were counted and the percentage staining with propidium iodide is shown. This experiment was repeated three times, and a representative experiment is shown.
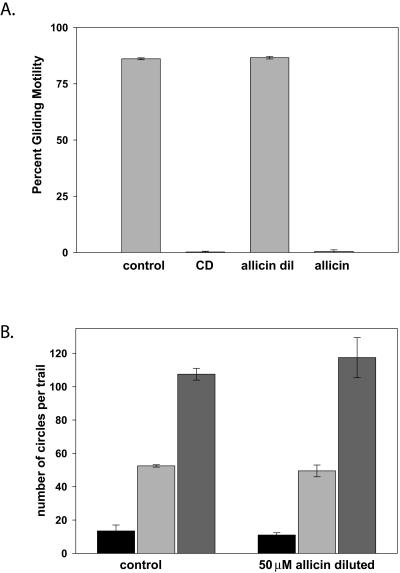
Allicin does not inhibit gliding motility.
Since uptake of propidium iodide is a terminal event, we also tested whether sporozoites incubated with allicin were still motile. Plasmodium sporozoites exhibit a unique form of substrate-dependent locomotion, termed gliding motility, which is required for cell invasion (42). We reasoned that if sporozoites were motile in the presence of allicin, then the compound was not affecting the overall health and metabolic activity of the parasites. When we tested allicin in motility assays, we found that preincubation with 50 μM allicin for 10 min followed by dilution to 4.2 μM had no effect on gliding motility (Fig. 3A). In addition, both the percentage of sporozoites that exhibited gliding motility and the number of circles per trail were the same in the allicin-treated sporozoites and in controls (Fig. 3B). However, if allicin was not diluted and sporozoites were kept in 50 μM allicin for the duration of the assay, gliding motility was completely inhibited (Fig. 3A). This is consistent with the toxicity profile of allicin that we observed using propidium iodide: prolonged incubations in 50 μM allicin were toxic, whereas a 10-min incubation in 50 μM allicin followed by an incubation in 4.2 μM was not.
FIG. 3.
The effect of allicin on gliding motility. P. berghei sporozoites were preincubated in buffer alone, 1 μM cytochalasin D (CD), or 50 μM allicin for 10 min and then added to wells for 1 h at 37°C, after which gliding motility was quantified. The sporozoites pretreated with allicin were either kept in 50 μM allicin during the motility assay (allicin) or diluted 12-fold so that the final concentration of allicin was 4.2 μM (allicin dil). Shown are (A) the percentage of sporozoites that exhibited gliding motility and (B) the number of gliding sporozoites exhibiting 1 (black bars), 2 to 10 (light gray bars), or >10 (dark gray bars) circles per trail. Each point was performed in triplicate, 200 sporozoites/well were counted, and the means ± standard deviations are shown.
Allicin prevents cell invasion.
Since allicin inhibited CSP cleavage and our previous studies showed that cleavage is associated with cell invasion, we next tested whether allicin would inhibit invasion of host cells. For these experiments, P. berghei sporozoites were pretreated with 10, 25, and 50 μM allicin for 10 min, the allicin was diluted 12-fold, and then sporozoites plus the diluted allicin were added to cells. As shown in Fig. 4, allicin inhibited sporozoite invasion of cells in a dose-dependent manner. At the lowest concentration tested (10 μM), allicin inhibited invasion by 37%. When sporozoites were pretreated with 50 μM allicin, invasion was inhibited by 89%, a result similar to that seen when sporozoites are pretreated with E-64 (6). Pretreatment of host cells with 50 μM allicin had no effect on invasion (Fig. 4).
FIG. 4.
Allicin inhibits sporozoite invasion of host cells. P. berghei sporozoites were pretreated for 10 min with the indicated concentrations of allicin, which was then diluted 12-fold before sporozoite addition to cells. After 1 h, cells were fixed and stained, and the numbers of intracellular and extracellular sporozoites were determined. “50*” indicates that Hepa 1-6 cells were preincubated with 50 μM allicin for 1 h and washed, and untreated sporozoites were then added to the cells. Each point was performed in triplicate, ≥50 fields/well were counted, and the means ± standard deviations are shown. Inhibition of invasion was calculated based on the invasion rate for sporozoites pretreated with buffer alone, which was 57%.
Inhibition of sporozoite infectivity in vivo.
We then tested the ability of allicin to inhibit sporozoite infectivity in vivo using the rodent malaria parasite P. yoelii. Mice were injected with allicin or buffer alone at different times before injection of sporozoites. Forty hours after sporozoite injection, the parasite burden in the liver was determined by reverse transcription followed by real time PCR. It should be noted that only sporozoites which invade and undergo many cycles of replication are detected in this assay, since the small amount of rRNA present in the sporozoite inoculum is below the sensitivity of the assay. As shown in Fig. 5A, mice injected with allicin had decreased levels of infection, and inhibition of infection was correlated with the length of time between allicin injection and sporozoite injection. When allicin was administered just before injection of sporozoites, it significantly decreased sporozoite infectivity compared to untreated controls (P < 0.001). Allicin injected 30 min prior to injection of sporozoites also resulted in decreased infectivity compared to the untreated mice (P < 0.001), but the protective effect was not as great as that seen when allicin was administered just before sporozoite injection. Administration of allicin 60 min prior to sporozoite injection yielded little protection (P < 0.25). We also found that protection was dose dependent, with 8 mg/kg having a larger effect than 5 mg/kg. Both doses, however, resulted in significantly decreased infections compared to controls (Fig. 5B) (P < 0.001).
FIG. 5.
Allicin decreases sporozoite infectivity in vivo. Mice were injected with allicin or buffer alone before injection of P. yoelii sporozoites. Forty hours later, mice were sacrificed, total liver RNA was extracted, and malaria infection was determined by quantitative PCR. Infection is expressed as the number of copies of P. yoelii 18S rRNA. (A) Mice were injected i.v. with 8 mg/kg allicin 1, 30, and 60 min before injection of sporozoites. (B) Mice were injected i.v. with 5 mg/kg allicin, 8 mg/kg allicin, or buffer alone 1 min before injection of sporozoites. (C) Sporozoites were preincubated with 50 μM allicin for 10 min, diluted 12-fold with buffer, and injected into mice. For all three graphs, results represent two independent experiments with six mice per group per experiment.
The decrease in efficacy of allicin over time is likely a consequence of its rapid decomposition in vivo (9). In order to test the inhibitory activity of allicin in vivo, before its metabolism in the blood, we performed a second set of experiments in which mice were injected with P. yoelii sporozoites that had been preincubated for 10 min with 50 μM allicin or buffer alone. As shown in Fig. 5C, mice injected with the allicin-pretreated sporozoites showed no evidence of malaria infection (P < 0.001).
Inhibition of erythrocytic stages in vivo.
Last, we tested the effect of allicin on the erythrocytic stages of P. berghei. Mice were injected with 2 × 105 erythrocytic-stage P. berghei parasites and then treated with allicin or buffer alone administered intravenously once daily for 4 days, beginning on the day of parasite injection (day 0). In the standard 4-day suppression test, parasitemia on the day after treatment termination is an indicator of drug potency. We found that 1 day after the last dose of allicin, the allicin-treated mice had a 94% decrease in parasitemia compared to controls (Fig. 6A) (P < 0.001). In addition, allicin treatment also prolonged the average survival time of the mice by ∼10 days, although the drug was administered for only 4 days (Fig. 6C). We then went on to test whether oral administration of allicin would also inhibit growth of erythrocytic-stage parasites. In these experiments, allicin was diluted in water and administered by gavage, and control mice were given water alone. Parasitemias on the day after the last dose of drug were significantly decreased in the allicin-treated mice compared to controls (Fig. 6B) (P < 0.001). In addition, mice given 9 mg/kg/day by mouth survived significantly longer than controls, whereas those given 3 mg/kg/day had an intermediate survival curve (Fig. 6C). Overall these data show that allicin is active against erythrocytic stages of Plasmodium when administered either orally or intravenously. In both sets of experiments (oral and intravenous), we continued to monitor parasitemias daily until the mice died: in the control mice, parasitemias were 13% ± 2.2% just prior to death, whereas parasitemias in the allicin-treated mice were 70% ± 5.2% just prior to death.
FIG. 6.
Allicin increases survival of mice infected with erythrocytic stages of Plasmodium. Mice were injected i.v. with GFP-expressing erythrocytic-stage P. berghei and 1 h later treated with allicin delivered intravenously (A and C) or orally (B and C). Treatment was continued once daily for an additional 3 days. (A) Parasitemias in control (buffer alone administered intravenously) and allicin-treated (8 mg/kg administered intravenously) groups on day 4. (B) Parasitemias in control (water administered orally) and allicin-treated (3 mg/kg and 9 mg/kg administered orally) groups on day 4. (C) Survival curves of mice receiving buffer administered intravenously, filled circles; water administered orally, filled triangles; 3-mg/kg/day allicin administered orally, unfilled triangles; 9-mg/kg/day allicin administered orally, filled squares; 8-mg/kg/day allicin administered intravenously, unfilled circles. Arrows indicate the days that mice were treated. Results represent two independent experiments with five to seven mice per group per experiment.
DISCUSSION
Here we show that allicin, a naturally occurring compound generated when garlic cloves are crushed, can inhibit malaria infection. Previous studies with allicin have shown that it has inhibitory effects on a wide range of bacteria, as well as some fungi and a few protozoans (1, 5, 12, 15, 16, 23, 38, 43). At high doses its toxicity is likely due to a variety of thiolation reactions; however, at low doses it acts more selectively as a cysteine protease inhibitor (2, 23, 32). We found that at low concentrations, the effects of allicin on sporozoites parallel those of E-64, a highly specific cysteine protease inhibitor: allicin inhibited proteolytic cleavage of CSP as well as cell invasion but did not affect gliding motility, suggesting that it is an inhibitor of the sporozoite cysteine protease(s) required for infectivity in the mammalian host.
Although the preerythrocytic stages of Plasmodium are the focus of the work presented here, there are difficulties inherent in solely targeting this stage with drugs. Most importantly, inhibition of preerythrocytic stages must be 100% effective, because a single successful sporozoite can lead to malaria. This prompted us to also examine the effect of allicin on the erythrocytic stages, and we found a significant inhibitory effect on these stages as well. We have not investigated allicin's mechanism of action in the erythrocytic stages. However, previous studies showing that cysteine proteases are required for growth of erythrocytic stages (39, 40) and inhibition of these proteases can decrease parasitemias in vivo (28, 35) suggest that allicin may be inhibiting cysteine protease function in the erythrocytic stages. If true, these data raise the possibility that the same cysteine protease inhibitor may be effective against different life cycle stages of Plasmodium, thus extending the usefulness of these potential drugs.
Interestingly, our data also show that at death, the allicin-treated mice had much higher parasitemias than control mice. Previous studies have shown that the ANKA strain of P. berghei causes a rodent form of cerebral malaria and leads to death at relatively low parasitemias (10, 19, 44). Our untreated mice died 6 to 9 days after infection with low parasite densities, indicating that the Swiss Webster mice we used in our experiments are susceptible to cerebral malaria. In contrast, the allicin-treated mice survived 10 to 15 days longer and their parasitemias reached very high levels, indicating that allicin treatment enabled them to escape the early death that is normally seen with this strain of P. berghei. One possibility is that slower growth of the parasite in the presence of allicin changes the nature of the immune response and thereby alters the outcome of the infection. However, it is also possible that allicin is modulating the immune response of the host (reviewed in reference 34) and thereby preventing the cascade of events that leads to early death seen with P. berghei ANKA.
Importantly, at the doses used to inhibit Plasmodium, we saw no toxic effects of allicin on the mammalian host: mice treated with allicin had activity levels and weight gain similar to those of the untreated controls. Although allicin likely reacts with host cysteine proteases, there are several reasons why mammalian cells may be more resistant to the effects of protease inhibitors than single-celled protozoan parasites (20, 21). Complex animals have a redundancy in protease function that does not exist in protozoan parasites, which are of necessity more genetically streamlined organisms (20, 21). In addition, parasite proteases may be more accessible to inhibitors than host enzymes, which are found within intracellular compartments. This may be, in part, because parasites actively import small compounds from the extracellular environment, as occurs with the erythrocytic stages of Plasmodium (21), and in the case of the sporozoite, it may be because the parasite protease acts extracellularly to cleave surface proteins during invasion (6). Higher doses of allicin would have eliminated this relative selectivity for the pathogen. In fact, previous studies in mice have shown that at high doses allicin is toxic, with a 50% lethal dose of 60 mg/kg after i.v. injection and 120 mg/kg after subcutaneous administration (17). In our study, a daily dose of 8 mg/kg i.v. was found to be toxic to the parasite with no obvious effects on the host.
Although allicin had a dramatic effect on both the sporozoite stage and erythrocytic stages, in experiments where mice were treated with the drug, neither stage was completely eradicated. Likely this is because in the blood circulation, allicin is rapidly metabolized to allyl-mercaptoglutathion, diallyl disulfide, diallyl trisulfide, and other various thiosulfinate products (9, 18). This is supported by our finding that the interval between allicin administration and sporozoite injection correlated well with allicin's inhibitory activity against sporozoites. These metabolites have not been found to be active against other pathogens, and our data suggest that they also are not active against Plasmodium. The doses we used in vivo were greater than what was required to see an effect in vitro, and this likely reflects the rapid metabolism of allicin discussed above, as well as the possibility that it is reacting with other free sulfhydryl groups present in a variety of serum proteins. Importantly, we found that allicin is also active after oral administration, supporting previous findings that allicin is not altered by passage through the digestive tract (9). We do not know what levels of allicin can be achieved after ingestion of garlic; however, we are currently testing whether frequent ingestion of garlic could lead to blood levels of allicin that are inhibitory to Plasmodium.
In conclusion, we have shown that allicin, a cysteine protease inhibitor present in freshly crushed garlic cloves, significantly inhibits sporozoite infectivity in vivo and decreases parasite loads in mice with blood-stage infections. These experiments demonstrate the feasibility of using the same cysteine protease inhibitor to target two different life cycle stages in the vertebrate host and support the idea that cysteine protease inhibitors may be useful drugs for the prophylaxis and treatment of malaria.
Acknowledgments
This work was supported by the National Institutes of Health, RO1 AI056840 (P.S.) and Training Grant 5T32 AI07180 (A.C.), and by grants from the Drake Family Foundation and by Yeda Co at the Weizmann Institute of Science (D.M.).
We thank Dabeiba Bernal and Jean Noonan for their expert assistance with mosquito rearing and infection and Daniel Eichinger for his critical reading of the manuscript.
REFERENCES
- 1.Ankri, S., and D. Mirelman. 1999. Antimicrobial properties of allicin from garlic. Microbes Infect. 1:125-129. [DOI] [PubMed] [Google Scholar]
- 2.Ankri, S., T. Miron, A. Rabinkov, M. Wilchek, and D. Mirelman. 1997. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrob. Agents Chemother. 41:2286-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J. 2000. Proteases involved in erythrocyte invasion by the malaria parasite: function and potential as chemotherapeutic targets. Curr. Drug Targets 1:59-83. [DOI] [PubMed] [Google Scholar]
- 4.Bruna-Romero, O., J. C. R. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury, A. K., M. Ahsan, S. N. Islam, and Z. U. Ahed. 1991. Efficacy of aqueous extract of garlic and allicin in experimental shigellosis in rabbits. Indian J. Med. Res. 93:33-36. [PubMed] [Google Scholar]
- 6.Coppi, A., C. Pinzon-Ortiz, C. Hutter, and P. Sinnis. 2004. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J. Exp. Med. 201:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eilat, S., Y. Oestraicher, A. Rabinkov, D. Ohad, D. Mirelman, A. Battler, M. Eldar, and Z. Vered. 1995. Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron. Artery Dis. 6:985-990. [PubMed] [Google Scholar]
- 8.Franke-Fayard, B., H. Trueman, J. Ramesar, J. Mendoza, M. van der Keur, R. van der Linden, R. E. Sinden, A. P. Waters, and C. J. Janse. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23-33. [DOI] [PubMed] [Google Scholar]
- 9.Freeman, F., and Y. Kodera. 1995. Garlic chemistry: stability of S-(2-propenyl)2-propene-1-sulfinothionate (allicin) in blood, solvents, and simulated physiological fluids. J. Agric. Food Chem. 43:2332-2338. [Google Scholar]
- 10.Grau, G. E., P.-F. Piguet, J. D. Engers, J. A. Louis, P. Vassali, and P.-H. Lambert. 1986. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 137:2348-2354. [PubMed] [Google Scholar]
- 11.Greenwood, B. M., K. Bojang, C. J. Whitty, and G. A. Targett. 2005. Malaria. Lancet 365:1487-1498. [DOI] [PubMed] [Google Scholar]
- 12.Gupta, K. C., and R. Viswanathan. 1955. Combined action of streptomycin and chloramphenicol with plant antibiotics against tubercle bacilli. I. Streptomycin and chloramphenicol with cepharanthine. II. Streptomycin and allicin. Antibiot. Chemother. 5:24-27. [PubMed] [Google Scholar]
- 13.Hahn, G. 1996. History, folk medicine and legendary uses of garlic, p. 1-24. In H. P. Koch and L. D. Lawson (ed.), Garlic: the science and therapeutic application of Allium sativum L. and related species. Williams & Wilkins, Baltimore, Md.
- 14.Harlow, E., and D. Lane. 1988. Antibodies, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Harris, L. C., S. L. Cottrel, S. Plummer, et al. 2001. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 57:282-286. [DOI] [PubMed] [Google Scholar]
- 16.Jonkers, D., J. Sluimer, and E. Stobberingh. 1999. Letter. Antimicrob. Agents Chemother. 43:3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch, H. P. 1996. Toxicity, side effects, and the unwanted effects of garlic, p. 221-228. In H. P. Koch and L. D. Lawson (ed.), Garlic: the science and therapeutic application of Allium sativum L. and related species. Williams & Wilkins, Baltimore, Md.
- 18.Lawson, L. D. 1996. The composition and chemistry of garlic cloves and processed garlic, p. 38-39. In H. P. Koch and L. D. Lawson (ed.), Garlic: the science and therapeutic application of Allium sativum L. and related species., 2nd ed. Williams & Wilkins, Baltimore, Md.
- 19.Lou, J., R. Lucas, and G. E. Grau. 2001. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin. Microbiol. Rev. 14:810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKerrow, J. H. 1999. Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg. Med. Chem. 7:639-644. [DOI] [PubMed] [Google Scholar]
- 21.McKerrow, J. H. 1999. Development of cysteine protease inhibitors as chemotherapy for parasitic diseases: insights on safety, target validation, and mechanism of action. Int. J. Parasitol. 29:833-837. [DOI] [PubMed] [Google Scholar]
- 22.McKerrow, J. H., E. Sun, P. J. Rosenthal, and J. Bouvier. 1993. The proteases and pathogenicity of parasitic protozoa. Annu. Rev. Microbiol. 47:821-853. [DOI] [PubMed] [Google Scholar]
- 23.Mirelman, D., D. Monheit, and S. Varon. 1987. Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic (Allium sativum). J. Infect. Dis. 156:243-244. [DOI] [PubMed] [Google Scholar]
- 24.Miron, T., T. Bercovici, A. Rabinkov, M. Wilchek, and D. Mirelman. 2004. [3H]allicin: preparation and applications. Anal. Biochem. 331:364-369. [DOI] [PubMed] [Google Scholar]
- 25.Miron, T., I. Shin, G. Feigenblat, L. Weiner, D. Mirelman, M. Wilchek, and A. Rabinkov. 2002. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: reaction of 4-mercaptopyridine with thiosulfinates. Anal. Biochem. 307:76-83. [DOI] [PubMed] [Google Scholar]
- 26.Myung, J. M., P. Marshall, and P. Sinnis. 2004. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol. Biochem. Parasitol. 133:53-59. [DOI] [PubMed] [Google Scholar]
- 27.Naganawa, R., N. Iwata, K. Ishikawa, H. Fukuda, T. Fujino, and A. Suzuki. 1996. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 62:4238-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson, J. E., G. K. Lee, A. Semenov, and P. J. Rosenthal. 1999. Antimalarial effects in mice of orally administered peptidyl cysteine protease inhibitors. Bioorg. Med. Chem. 7:633-638. [DOI] [PubMed] [Google Scholar]
- 29.Peters, W. 1975. The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 69:155-171. [PubMed] [Google Scholar]
- 30.Perez, H. A., M. de la Rosa, and R. Apitz. 1994. In vivo activity of ajoene against rodent malaria. Antimicrob. Agents Chemother. 38:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinzon-Ortiz, C., J. Friedman, J. Esko, and P. Sinnis. 2001. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J. Biol. Chem. 276:26784-26791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinkov, A., T. Miron, L. Konstantinovski, M. Wilchek, D. Mirelman, and L. Weiner. 1998. The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1379:233-244. [DOI] [PubMed] [Google Scholar]
- 33.Renia, L., F. Miltgen, Y. Charoenvit, T. Ponnudurai, J. P. Verhave, W. E. Collins, and D. Mazier. 1988. Malaria sporozoite penetration: a new approach by double staining. J. Immunol. Methods 112:201-205. [DOI] [PubMed] [Google Scholar]
- 34.Reuter, H. D., H. P. Koch, and L. D. Lawson. 1996. Therapeutic effects and applications of garlic and its preparations., p. 135-213. In H. P. Koch and L. D. Lawson (ed.), Garlic: the science and therapeutic application of Allium sativum L. and related species. Williams and Wilkins, Baltimore, Md.
- 35.Rosenthal, P. J., G. K. Lee, and R. E. Smith. 1993. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J. Clin. Investig. 91:1052-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal, P. J., P. S. Sijwali, A. Singh, and B. R. Shenai. 2002. Cysteine proteases of malaria parasites: targets for chemotherapy. Curr. Pharm. Des. 8:1659-1672. [DOI] [PubMed] [Google Scholar]
- 37.Salmon, B. L., A. Oksman, and D. E. Goldberg. 2001. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proc. Natl. Acad. Sci. USA 98:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shadkchan, Y., E. Shemesh, D. Mirelman, T. Miron, A. Rabinkov, M. Wilchek, and N. Osherov. 2004. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J. Antimicrob. Chemother. 53:832-836. [DOI] [PubMed] [Google Scholar]
- 39.Shenai, B. R., P. S. Sijwali, A. Singh, and P. J. Rosenthal. 2000. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase. J. Biol. Chem. 275:29000-29010. [DOI] [PubMed] [Google Scholar]
- 40.Sijwali, P. S., B. R. Shenai, J. Gut, A. Singh, and P. J. Rosenthal. 2001. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem. J. 360:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoll, A., and E. Seebeck. 1951. Chemical investigations on alliin, the specific principle of garlic. Adv. Enzymol. 11:377-400. [DOI] [PubMed] [Google Scholar]
- 42.Sultan, A. A., V. Thathy, U. Frevert, K. J. H. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 43.Uchida, Y., T. Takahashi, and N. Sate. 1975. The characteristics of the antibacterial activity of garlic. Jpn. J. Antibiot. 28:638-642. [PubMed] [Google Scholar]
- 44.Voza, T., A. M. Vigario, E. Belnoue, A. C. Gruner, J. C. Deschemin, M. Kayibanda, F. Delmas, C. J. Janse, B. Franke-Fayard, A. P. Waters, I. Landau, G. Snounou, and L. Renia. 2005. Species-specific inhibition of cerebral malaria in mice coinfected with Plasmodium spp. Infect. Immun. 73:4777-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willis, E. 1956. Enzyme inhibition by allicin, the active principle of garlic. Biochem. J. 63:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida, N., P. Potocnjak, V. Nussenzweig, and R. S. Nussenzweig. 1981. Biosynthesis of Pb44, the protective antigen of sporozoites of Plasmodium berghei. J. Exp. Med. 154:1225-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]