Abstract
Emtricitabine (FTC) is approved for the treatment of human immunodeficiency virus. FTC and clevudine (CLV) have activity against hepatitis B virus (HBV). This report summarizes the results of a double-blind, multicenter study of patients with chronic hepatitis B who had completed a phase 3 study of FTC and were randomized 1:1 to 200 mg FTC once daily (QD) plus 10 mg CLV QD or 200 mg FTC QD plus placebo for 24 weeks with 24 weeks of follow-up. One hundred sixty-three patients were treated (82 with FTC plus CLV [FTC+CLV] and 81 with FTC); 72% were men, 53% were Asian, 47% were Caucasian, and 52% were hepatitis B e antigen positive, and the median baseline HBV DNA level was 6 log10 copies/ml. After 24 weeks of treatment, 74% (FTC+CLV) versus 65% (FTC alone) had serum HBV DNA levels of <4,700 copies/ml (P = 0.114) (Digene HBV Hybrid Capture II assay). Twenty-four weeks posttreatment, the mean change in serum HBV DNA levels from baseline was −1.25 log10 copies/ml (FTC+CLV), 40% had undetectable viremia (versus 23% for FTC alone), and 63% had normal alanine aminotransferase levels (versus 42% for FTC alone) (P ≤ 0.025 for all endpoints). The safety profile was similar between arms during treatment, with less posttreatment exacerbation of hepatitis B in the combination arm. In summary, after 24 weeks of treatment, no significant difference between arms was observed, but there was a significantly greater virologic and biochemical response 24 weeks posttreatment in the FTC+CLV arm.
Chronic infection with hepatitis B virus (HBV) remains the most common cause of cirrhosis and hepatocellular carcinoma worldwide and affects an estimated 400 million persons (20). Currently approved therapies for chronic hepatitis B (CHB) include pegylated and standard interferon, lamivudine, adefovir dipivoxil, and entecavir. Interferon produces a response in terms of persistent normalization of alanine aminotransferase (ALT) levels, clearance of hepatitis B e antigen (HBeAg), and sustained loss of HBV DNA in approximately a quarter of HBeAg-positive patients (6) and produces a loss of hepatitis B s antigen in 5 to 10% of patients within 1 year (5). In HBeAg-negative chronic hepatitis B, peginterferon alfa-2a demonstrated prolonged and continued viral suppression (HBV DNA level of <400 copies/ml) in 19% of patients 24 weeks after the end of treatment (23). However, alfa interferon produces treatment-limiting side effects and significant tolerability issues.
Lamivudine, adefovir dipivoxil, and entecavir produce potent suppression of viral replication, but the treatment benefit from lamivudine is temporally limited by the development of resistance mutations (14% to 32% after 1 year) (13). Adefovir dipivoxil and entecavir both have activity against lamivudine-resistant viruses, but for all oral drugs, rebound viremia occurs in most patients after therapy is withdrawn. The limitations of currently available treatment options and the search for the ultimate cure have encouraged the clinical development of novel drugs for the treatment of CHB and the exploration of combination strategies.
Emtricitabine (FTC) {5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine)} is a nucleoside analog approved for the treatment of human immunodeficiency virus infection at a dose of 200 mg once daily (QD). In studies of patients with CHB, 200 mg FTC QD produced viral suppression and histologic improvement in a phase 3 study, which was similar to results for lamivudine and adefovir at 1 year (8, 9, 11, 19, 22). FTC selects for the same resistance mutations as lamivudine in the YMDD motif of the HBV polymerase (10).
Clevudine (CLV) [1-(2-deoxy-2-fluoro-β-l-arabinofuranosyl)thymine (l-FMAU)] is a nucleoside analogue of the unnatural β-l configuration, which has demonstrated potent in vitro activity against HBV (4). An additive to synergistic activity against HBV was observed in HepG2 2.2.15 cells when CLV was combined with FTC (14, 15). Additional in vitro data suggested that CLV more potently inhibited hepadnavirus polymerase associated with the DNA template (plus-strand DNA synthesis) rather than the RNA template (negative-strand synthesis), (27), theoretically representing a mechanism of action complementary to other antivirals such as FTC, which could translate into enhanced clinical activity when they are given in combination.
Potent and sustained suppression of woodchuck hepatitis virus (WHV) in infected woodchucks has been reported in studies of CLV treatment alone or in combination with FTC (12, 29). A notable preclinical attribute of CLV was prolonged suppression of viral replication following the cessation of treatment in woodchucks infected with WHV. In this model, significant reductions in intrahepatic covalently closed circular WHV DNA were observed in addition to a dose-related delay in rebound viremia with significant suppression in at least half of the treated animals for a period of 10 to 12 weeks posttreatment (26). A similar effect was observed in a clinical phase 2 dose escalation study of CLV treatment in 32 patients with CHB, which demonstrated a median decrease from the baseline of at least 1.2 log10 copies/ml 6 months after the end of 28 days of CLV dosing (25). The concept of augmenting this effect through a combination strategy was particularly attractive, and study FTCB-204 was conceived to evaluate treatment with FTC plus CLV (FTC+CLV). A treatment duration of 6 months was selected as sufficient to demonstrate the benefit of combination treatment and in view of the limited clinical experience with CLV at that time. Modeling of the effect of various CLV doses on serum HBV DNA levels indicated that 10 mg CLV once daily achieved 77% of the maximal predicted activity, which was considered satisfactory for a proof-of-concept study of combination treatment.
MATERIALS AND METHODS
Objectives.
The objective of this study was to compare the safety, efficacy, and tolerability of 24 weeks of treatment with 200 mg FTC QD in combination with 10 mg CLV QD to treatment with 200 mg FTC QD as monotherapy followed by 24 weeks of treatment-free follow-up by evaluating HBV DNA suppression, serologic response, changes in ALT levels, and the adverse-experience profile during and after therapy.
Study design.
This double-blind trial was conducted at 21 sites in the United States, Canada, Singapore, Bulgaria, and the Czech Republic in compliance with the Declaration of Helsinki and was approved by ethics committees and appropriate regulatory authorities. Patients were enrolled between 8 May 2002 and 30 June 2003, and all patients provided written informed consent. An interactive voice response system (ClinPhone, Inc., Nottingham, England) was used to centrally randomize (1:1) patients to receive 200 mg FTC plus 10 mg CLV QD orally or 200 mg FTC QD plus a placebo identical to CLV. Randomization was stratified by previous exposure to FTC and by geographic region: North America, Asia, and Europe. A block size of six was employed for balance.
At weeks 1, 2, 4, 6, and 8 and subsequently every 4 weeks, the following parameters were evaluated: serum HBV DNA, ALT, aspartate aminotransferase (AST), total bilirubin, alkaline phosphatase, albumin, lactate (monthly during treatment), and blood chemistries (creatine kinase, lactate dehydrogenase, blood urea nitrogen, creatinine, glucose, amylase, Na+, K+, Ca2+, Cl−, and bicarbonate). A complete blood count with differential was performed monthly through week 32 and every 8 weeks thereafter, and hepatitis e and s antigens and antibodies were evaluated every 12 weeks. Covance Laboratories performed safety laboratory tests.
This study underwent three reviews by a data and safety monitoring board that included blinded evaluation of adverse events (AEs) and safety laboratory results. There were no recommendations from the data and safety monitoring board to discontinue or modify the study.
Patients.
Patients with CHB who had completed a phase 3 study of FTC versus placebo for 48 weeks (FTCB-301) were eligible. Exclusion criteria included previous nucleoside analog treatment outside the context of study FTCB-301, ongoing antiviral or immunomodulatory therapy, clinical evidence of decompensated liver disease, coinfection with human immunodeficiency virus or hepatitis C virus, elevated alpha-fetoprotein, creatinine clearance of <60 ml/min, elevation of alanine or aspartate aminotransferase levels >10 times the upper limit of normal (ULN), pregnancy, and other concurrent significant medical disease. There was no entry criterion for HBV DNA to assure that patients would not be excluded from active treatment following the completion of study FTCB-301.
Virology.
For primary statistical analysis of the data, serum HBV DNA levels were measured using the Digene HBV Hybrid Capture II assay (Digene Corporation, Gaithersburg, MD), which is a signal amplification hybridization microplate technology using chemiluminescence for detection and quantitation (limit of detection [LOD], 4,700 copies/ml) with a dynamic range extending to 1.7 × 109 copies/ml.
In addition, a cross-sectional analysis of serum HBV DNA levels using a PCR assay at the end of treatment (week 24) was performed at Gilead Sciences, Durham, NC. The experimental assay used the ABI Prism 7000 sequence detection system, which employs real-time PCR (RT-PCR) with TaqMan technology to determine the amount of HBV DNA present in the sample (LOD, 250 copies/ml) (3, 7). The primers used for amplification targeted a conserved region of the X gene and reliably quantified viral loads for genotypes (A to E) over a 5-log dynamic range.
Resistance surveillance was performed at baseline and at week 24 on patient sera with detectable viremia (≥4,700 copies/ml) using dideoxy sequencing technology (ABI Prism 3100; Applied Biosystems, Foster City, CA) (2). The primers used for amplification allowed for sequence analysis of domains A through E (amino acids rt75 through rt255) of the HBV polymerase and for amino acids 67 through 226 of the small surface antigen. For this report, data are presented using the nomenclature where the methionine found in the YMDD motif of the HBV polymerase is referred to as position rt204. Sequence analysis, which included an evaluation of all genotypic changes from the consensus sequence, was performed for all baseline and week 24 samples using ClustalAlignment.
Statistical analysis.
The primary population for efficacy and safety analyses was the intent-to-treat population consisting of all randomized patients who were dispensed at least 1 dose of study medication. For efficacy analyses of categorical data, patients with missing data were considered to be nonresponders, i.e., a missing-equals-failure approach.
Efficacy parameters included serum HBV DNA levels, ALT levels, serology for HBeAg, and the proportion of patients developing new mutations associated with resistance to FTC (rtM204I/V with or without rtL180M and rtV173L) (17, 18, 27). Serology testing was performed using the DiaSorin Plus assay (DiaSorin Inc., Stillwater, MN).
The efficacy analysis included two coprimary endpoints, which were (i) the proportion of patients with serum HBV DNA levels below the assay LOD (4,700 copies/ml) at the end of treatment (week 24) and (ii) the proportion of patients whose levels were below the LOD at week 24 and then sustained, without therapy, suppression of HBV DNA levels below 105 copies/ml for 24 weeks.
The primary safety analysis included all AEs through week 36; serious AEs (SAEs) were recorded throughout the study. The severity of AEs and laboratory abnormalities were graded using criteria defined by the National Institute of Allergy and Infectious Diseases (1). Posttreatment exacerbations of hepatitis B were prospectively defined as either a 10-fold increase in ALT and/or AST levels from on-treatment nadir or an increase to 20 times the ULN. Serious AEs were prospectively defined as hospitalization, permanent disability or incapacity, death, congenital anomaly, life-threatening events, or an important medical event at immediate risk of producing one of these outcomes. In addition, exacerbations of hepatitis B (previously defined) associated with grade 4 elevations of ALT or AST levels or any grade 3 or 4 elevation of bilirubin levels or prothrombin time were also considered serious AEs. Comparisons between treatment arms for safety endpoints were performed using a Fisher's exact test.
Comparisons of the FTC+CLV group with the FTC group were controlled for randomization strata (geographic region and previous treatment with FTC) using the Cochran-Mantel-Haenszel test (21) for categorical variables (e.g., proportion with HBV DNA levels of <4,700 copies/ml, normal ALT levels, and a composite endpoint of both parameters) and the van Elteren test (28) for continuous variables (e.g., HBV DNA level change from baseline). All significance tests were two sided, using an α level of 0.05 with no adjustments for multiple comparisons.
The sample size of approximately 80 patients per arm provides approximately 70% power at the 5%, two-sided significance level to detect a 20% difference in virologic response between treatment arms, assuming that the probability of virologic response is 50% in the monotherapy arm.
RESULTS
Patient disposition and baseline characteristics.
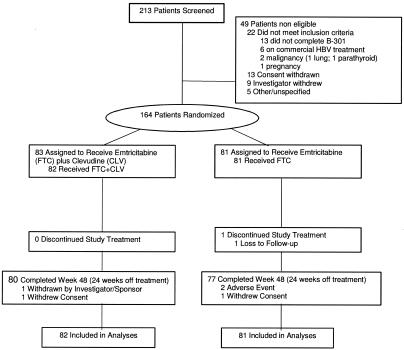
A total of 163 patients were randomized to either combination treatment with FTC+CLV (n = 82) or monotherapy with FTC (n = 81) and commenced treatment. Overall, 157 patients (96%) completed the study (80 in the FTC+CLV group and 77 in the FTC group), and all patients finished the 24-week treatment phase, except for one patient in the FTC arm who was lost to follow-up (Fig. 1). During the 24-week treatment-free follow-up phase, three patients discontinued to initiate commercial therapy to avoid the potential risk of posttreatment exacerbation of hepatitis B in the setting of advanced hepatic fibrosis (two patients in the FTC+CLV group and one patient in the FTC group), and two patients, both in the FTC arm, discontinued due to an episode of posttreatment exacerbation of HBV.
FIG. 1.
CONSORT diagram for study FTCB-204.
Baseline demographic and disease characteristics are summarized in Table 1. The treatment arms were well balanced with respect to all parameters, with no statistically significant differences between arms. The median baseline serum HBV DNA level was 6 log10 copies/ml, affording a median measurable change from baseline to the limit of detection of the Digene HBV Hybrid Capture II assay of 2.3 log10 copies/ml. Only three patients had mutations associated with resistance to FTC at baseline (two patients in the FTC+CLV group and one patient in the FTC group).
TABLE 1.
Baseline demographic and HBV disease characteristics
| Characteristic | FTC+CLV (n = 82) | FTC (n = 81) | Total (n = 163) |
|---|---|---|---|
| Gender [no. (%)] | |||
| Male | 59 (72) | 59 (73) | 118 (72) |
| Female | 23 (28) | 22 (27) | 45 (28) |
| Age (Yr) [mean (±SD)] | 42.0 (±11.1) | 42.2 (±12.2) | 42.1 (±11.6) |
| Ethnic origin [no. (%)] | |||
| Asian | 45 (55) | 41 (51) | 86 (53) |
| Caucasian | 36 (44) | 40 (49) | 76 (47) |
| Hispanic | 1 (1) | 0 (0) | 1 (<1) |
| Wt (kg) [mean (±SD)] | 74.5 (±15.1) | 72.9 (±15.8) | 73.7 (±15.4) |
| Serum HBV DNA level (log10 copies/ml) | |||
| Mean (±SD) | 6.02 (±1.78) | 6.02 (±1.83) | 6.02 (±1.80) |
| Median | 5.95 | 5.96 | 5.96 |
| HBV DNA <4,700 copies/ml [no. (%)] | 8 (10) | 7 (9) | 15 (9) |
| HBeAg status [no. (%)] | |||
| Positive | 44 (54) | 41 (51) | 85 (52) |
| Negative | 38 (46) | 40 (49) | 78 (48) |
| ALT | |||
| Mean IU/liter (±SD) | 75.6 (±77.9) | 72.1 (±72.1) | 73.9 (±74.9) |
| Median IU/liter | 51 | 49 | 51 |
| ALT 0-2× ULN at baseline [no. (%)] | 60 (73) | 60 (74) | 120 (74) |
| ALT >2-5× ULN at baseline [no. (%)] | 16 (20) | 16 (20) | 32 (20) |
| ALT >5× ULN at baseline [no. (%)] | 6 (7) | 5 (6) | 11 (7) |
| Previous FTC treatment in FTCB-301 [no. (%)] | |||
| Treatment naïve | 28 (34) | 28 (35) | 56 (34) |
| Treatment experienced | 54 (66) | 53 (65) | 107 (66) |
Antiviral activity.
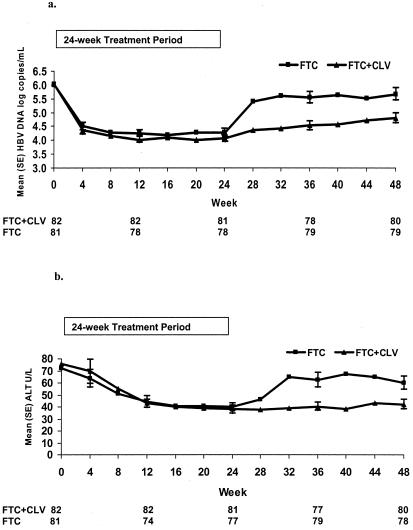
For the intent-to-treat population, at the end of 24 weeks of treatment, no statistically significant differences between the two treatment arms were observed (Table 2). The proportion of patients with serum HBV DNA levels of <4,700 copies/ml was high in both arms: 61/82 (74%) in the FTC+CLV arm and 53/81 (65%) in the FTC monotherapy arm. Using an experimental RT-PCR assay, the proportion of patients with HBV DNA levels of <250 copies/ml was somewhat lower: 46/82 (56%) in the FTC+CLV arm and 37/81 (46%) in the FTC arm (P value was not significant). This lack of a significant difference was also observed in the naïve subgroup analysis and the HBeAg-positive and HBeAg-negative subgroups. Across both assays, the median decrease in serum HBV DNA levels at the end of treatment ranged from 1.8 to 2.3 log10 copies/ml for the FTC+CLV arm and 1.4 to 2.0 log10 copies/ml for the FTC monotherapy arm.
TABLE 2.
Virologic and biochemical response
| Parameterb | No. of patients (%)
|
P valuea | |
|---|---|---|---|
| FTC+CLV (n = 82) | FTC (n = 81) | ||
| Serum HBV DNA level <4,700 copies/ml | |||
| Baseline | 8 (10) | 7 (9) | |
| Wk 24 (end of treatment) | 61 (74) | 53 (65) | 0.114 |
| Wk 48 (24 wks off treatment) | 33 (40) | 19 (23) | 0.025 |
| Sustained virologic response, HBV DNA level <4,700 copies/ml at wk 24 and <105 copies/ml at wk 48 (24 wks off treatment) | 46 (56) | 33 (41) | 0.027 |
| Proportion with normal ALT level | |||
| Baseline | 34 (41) | 31 (38) | 0.585 |
| Wk 24 (end of treatment) | 54 (67) | 54 (66) | 0.968 |
| Wk 48 (24 wks off treatment) | 52 (63) | 34 (42) | 0.002 |
| Serum HBV DNA level <4,700 copies/ml and normal ALT level | |||
| Baseline | 7 (9) | 5 (6) | |
| Wk 24 (end of treatment) | 46 (56) | 43 (53) | 0.554 |
| Wk 48 (24 wks off treatment) | 25 (30) | 11 (14) | 0.007 |
P values for comparisons of the FTC+CLV group with the FTC monotherapy arm controlled for randomization strata (geographic region and previous exposure to FTC) using the Cochran-Mantel-Haenszel test for categorical variables (proportion with HBV DNA levels of <4,700 copies/ml, normal ALT levels, and the composite endpoint).
HBV DNA measured by Digene Hybrid Capture II assay.
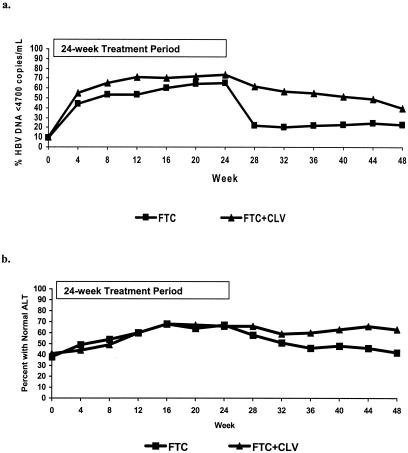
There was prolonged antiviral activity through 24 weeks of treatment-free follow-up (48 weeks of study) in the FTC+CLV arm, resulting in 40% of patients versus 23% (FTC) with serum HBV DNA levels of <4,700 copies/ml (P = 0.025) (Fig. 2a and 3a) and a mean (median) change from baseline of −1.25 (−0.82) log10 copies/ml versus −0.33 (−0.08) log10 copies/ml (P < 0.001). A sustained virologic response, HBV DNA levels of <4,700 copies/ml at week 24 and <105 copies/ml at week 48, was observed in a significantly greater proportion of patients in the FTC+CLV arm than in the FTC monotherapy arm, with 56% and 41%, respectively (P = 0.027) (Table 2). The composite endpoint of undetectable serum HBV DNA and normal ALT levels also significantly favored the FTC+CLV arm, with 30% versus 14%, respectively (P = 0.007). Six months after the end of treatment, serum ALT levels were significantly lower (P < 0.001), and the proportion of patients with normal ALT levels was significantly higher (63% versus 42%, respectively) among patients in the FTC+CLV arm (P = 0.002) (Fig. 2b and 3b).
FIG. 2.
(a) Proportion of patients with HBV DNA levels of <4,700 copies/ml. (b) Proportion of patients with normal ALT levels.
FIG. 3.
(a) Mean serum HBV DNA levels (Digene assay) over time (±standard errors). (b) Mean serum ALT levels over time (±standard errors).
There was no significant difference in serologic responses between treatment arms. Among 85 HBeAg-positive patients, 44 in the FTC+CLV arm and 41 in the FTC arm, a loss of HBeAg was observed at week 24 in seven and four patients, respectively, with seroconversion in all but one patient in each treatment arm (14% for the FTC+CLV group and 7% for the FTC group). After 24 weeks of treatment-free follow-up, 8 and 10 patients had a loss of HBeAg in the combination and monotherapy arms, respectively, with seroconversion in 7 (16%) and 10 (24%) patients, respectively.
Genotypic analysis.
Mutations associated with FTC resistance, rtM204I/V with or without rtL180M and rtV173L, were identified in sera from 14 patients at week 24. Thus, the 24-week incidence of viremia with FTC resistance mutations not found at baseline was 14/162 (9%) overall and was similar in both treatment arms, occurring in six patients (7%) in the FTC+CLV arm and in eight patients (10%) in the FTC arm. With the exception of one patient in the combination arm who was treatment naïve, all patients with treatment-emergent mutations had prior exposure to FTC for 1 year.
At study FTCB-204 baseline, three patients had resistance mutations (two patients in the FTC+CLV arm and one patient in the FTC arm), and all mutations were L180M plus M204V. After 24 weeks of treatment, neither patient in the FTC+CLV arm responded (serum HBV DNA levels declined 0.5 to 0.6 log10 copies/ml; absolute value, >8 log10 copies/ml). The single patient in the FTC arm experienced a decline in the serum HBV DNA level of 3.53 log10 copies/ml, from 7.2 log10 copies/ml to below the LOD (<4,700 copies/ml). Patients with treatment-emergent mutations identified at week 24 had a median decrease in serum HBV DNA levels of 0.84 log10 copies/ml (FTC+CLV) and 0.51 log10 copies/ml (FTC), and none were below the LOD.
Safety.
During the treatment period, the incidence of AEs was similar between FTC+CLV and FTC arms, and the most common AEs were headache (10% and 12%, respectively), influenza (9% in both arms), upper respiratory tract infection (9% and 7%, respectively), fatigue (9% and 4%, respectively), upper abdominal pain (7% and 5%, respectively), nausea (6% and 5%, respectively), arthralgia (6% and 4%, respectively), and pharyngolaryngeal pain (6% and 5%, respectively). No patient discontinued treatment due to an AE. A total of nine patients had a SAE during the treatment phase, three (4%) in the FTC+CLV arm and six (7%) in the FTC arm, all of which resolved without treatment interruption. In the FTC+CLV arm, SAEs included gonococcal arthritis (n = 1) and exacerbation of hepatitis B (n = 2), with one case commencing at baseline, initially worsening, and then improving on treatment. In the FTC arm, SAEs included seizure with fever (n = 1), chest pain of musculoskeletal origin (n = 1), fractured vertebrae (n = 1), nonulcer dyspepsia (n = 1), hyperglycemia associated with diabetes mellitus (n = 1), and elevated ALT levels (n = 1). The incidences of grade 3 or 4 laboratory abnormalities were comparable between arms (Table 3).
TABLE 3.
Incidence of grade 3 or 4 laboratory abnormalities during treatment and treatment-free follow-up
| Parametera | No. of patients (%)
|
P valueb | |
|---|---|---|---|
| FTC+CLV (n = 82) | FTC (n = 81) | ||
| Treatment period, day 0 to wk 24 | |||
| At least 1 grade 3 or 4 laboratory abnormality | 18 (22) | 13 (16) | NS |
| ALT | 6 (7) | 5 (6) | NS |
| AST | 6 (7) | 2 (2) | NS |
| Creatine kinase | 6 (7) | 2 (2) | NS |
| Glucose | 3 (4) | 1 (1) | NS |
| Amylase | 2 (2) | 1 (1) | NS |
| Lipase | 1 (1) | 5 (6) | NS |
| Treatment-free follow-up, wk 24 to 48 | |||
| At least 1 grade 3 or 4 laboratory abnormality | 9 (11) | 22 (28) | 0.009 |
| ALT | 4 (5) | 13 (16) | 0.022 |
| AST | 3 (4) | 10 (13) | 0.046 |
| Creatine kinase | 3 (4) | 4 (5) | NS |
| Glucose | 2 (2) | 2 (3) | NS |
| Amylase | 0 (0) | 5 (6) | 0.028 |
| Lipase | 0 (0) | 3 (4) | NS |
More than one patient.
NS indicates a P level of >0.05.
During the treatment-free follow-up period, 32 patients in the FTC+CLV arm developed AEs (39%), compared to 41 (51%) in the FTC arm. A total of six patients had a SAE, five patients in the FTC monotherapy arm and one patient in the combination arm, and all were posttreatment exacerbations of hepatitis B. The incidence of laboratory abnormalities of at least grade 3 severity was significantly higher in the FTC (22 [28%]) arm than in the combination (9 [11%]) arm during treatment-free follow-up (P = 0.009), with significantly fewer elevations in ALT, AST, and amylase levels in the FTC+CLV arm (Table 3). Higher frequencies of elevations of aminotransferase levels in the FTC arm after the end of treatment corresponded to more frequent posttreatment exacerbations of hepatitis B, which occurred with a higher incidence in the FTC arm (n = 12; 15%) than in the FTC+CLV arm (n = 2; 2%) (P = 0.005) within the confines of the 24-week follow-up period. One patient (FTC arm) developed clinical hepatic decompensation with ascites and coagulopathy in the setting of preexisting cirrhosis that responded to diuretics and lamivudine treatment; all other cases resolved without complications.
DISCUSSION
Study FTCB-204 was a rollover protocol for patients who successfully completed a prior phase 3, 48-week, placebo-controlled study of FTC, which explains the relatively low baseline viral load. Overall, at the end of 24 weeks of treatment, the FTC+CLV arm demonstrated no statistically significant difference in antiviral activity from the FTC monotherapy arm. This was true even after an experimental RT-PCR assay with a lower LOD was employed to evaluate the end-of-treatment response with the intent of increasing the ability to discriminate between treatment regimens in terms of virologic suppression.
As was observed with clevudine monotherapy in prior phase 1/2 clinical studies, (24, 25), there was prolonged antiviral activity following 24 weeks off treatment in the FTC+CLV arm. The coprimary endpoint of suppression of serum HBV DNA levels, <4,700 copies/ml at week 24 and <105 copies/ml at week 48, was achieved in 56% of patients in the FTC+CLV arm and in 41% of patients in the FTC arm (P = 0.027). Furthermore, 40% and 23% of patients, respectively, had serum HBV DNA levels of <4,700 copies/ml. The biochemical response was also significantly greater in the FTC+CLV arm than in the FTC arm. The absence of a clevudine monotherapy arm makes it impossible to assess the contribution of FTC in the combination arm in terms of prolonged virologic suppression 24 weeks off treatment. However, the end-of-treatment response for FTC+CLV therapy was not significantly different from that for FTC monotherapy. This was an unanticipated outcome and suggested that the delayed virologic rebound after the end of treatment could also be explained by an intrinsic effect of CLV rather than an effect of combination treatment, based on phase 1/2 clinical experience with CLV monotherapy (24, 25). The sustained posttreatment virologic suppression observed after clevudine treatment may provide opportunities for new therapeutic strategies in the management of CHB that utilize clevudine alone or in combination with other agents.
The mechanism for sustained suppression of HBV replication following CLV treatment is currently unknown. Although a reduction in covalently closed circular DNA levels has been observed in the woodchuck hepatitis model (26), this has not been examined clinically. Viral reemergence following conventional therapy can be attributed at least in part to the persistence of viral covalently closed circular DNA in the hepatocyte nucleus, which serves as a template for viral transcription and is slowly eliminated during nucleoside treatment (16).
As anticipated, on the basis of prolonged suppression of HBV DNA, there was a lower incidence of posttreatment exacerbation of hepatitis B in the FTC+CLV arm, at least within the confines of the 6-month follow-up period. During treatment, FTC+CLV 10 mg once daily was well tolerated, with a safety profile similar to that of FTC monotherapy.
Acknowledgments
We are indebted to the patients, investigators, and staff at participating medical centers for their dedication and contribution.
This study was conducted and funded by Gilead Sciences Inc., Durham, NC.
Franck Rousseau had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The members of the FTCB-204 Study Group are as follows. Deborah Kargl, Herve Mommeja-Marin, Cary Moxham, and Jane Anderson were members from the Gilead Sciences Study Team. The following people were investigators enrolling in Study FTCB-204: from North America, I. Alam (private practice, Austin, TX), R. Brown, Jr. (Columbia Presbyterian Medical Ctr., New York, NY), S. Chan (New York Hospital at Queens, Flushing, NY), P. Pockros (Scripps Clinic, La Jolla, CA), T. T. Nguyen (private practice, San Diego, CA), S. Sacks (Viridae Clinical Sciences Inc., Vancouver, British Columbia, Canada), M. Shiffman (McGuire VA Medical Center, Richmond, VA), N. Tsai (St. Francis Liver Center, Honolulu, HI), and C. Wang (Harborview Medical Center, Seattle, WA); from Asia, R. Guan (Mount Elizabeth Medical Center, Singapore), K. Liew (Tan Tong Seng Hospital, Singapore), S. G. Lim (National University Hospital Singapore), H. S. Ng (Singapore General Hospital, Singapore), and T. M. Ng (Changi General Hospital, Singapore); and from Europe, P. Husa (University Hospital Brno, Brno, Czech Republic), I. Kotzev (Multifunctional Active Treatment Hospital “St. Marina,” Varna, Bulgaria), Z. Krastev (University Hospital “St. Ivan Rilsky,” Sofia, Bulgaria), G. Mechkov (Vth Polyprofile Hospital for Active Treatment, Sofia, Bulgaria), J. Sperl (Institute of Clinical & Experimental Medicine, Praha, Czech Republic), P. Urbanek (General University Hospital in Prague, Praha, Czech Republic), and M. Volfova (University Hospital Hradec Kralove, Hradec Kralove, Czech Republic).
REFERENCES
- 1.AIDS Clinical Trial Group. 1996. Division of AIDS table for grading severity of adult adverse experiences. Division of AIDS, National Institute of Allergy and Infectious Diseases, Rockville, Md.
- 2.Applied Biosystems. 2003. ABI Prism 3100 genetic analyzers users guide. Applied Biosystems, Foster City, Calif.
- 3.Chappell, B., M. Curtis, A. Snow, Y. Zhu, C. Wakeford, and J. Harris. 2004. A comparative analysis of two hepatitis B virus (HBV) viral load assays: real-time PCR versus hybrid capture, poster abstract 47. Presented at the 17th International Conference on Antiviral Research, Tucson, Ariz., May 2004.
- 4.Chu, C. K., T. Ma, K. Shanmuganathan, C. Wang, Y. Xiang, S. B. Pai, G. Q. Yao, J. P. Sommadossi, and Y. C. Cheng. 1995. Use of 2′-fluoro-methyl-β-l-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob. Agents Chemother. 39:979-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooksley, W. G. 2004. Treatment with interferons (including pegylated interferons) in patients with hepatitis B. Semin. Liver Dis. 24:45-53. [DOI] [PubMed] [Google Scholar]
- 6.Craxi, A., D. Di Bona, and C. Camma. 2003. Interferon alpha for HBeAg positive chronic hepatitis B: systematic review. J. Hepatol. 39(Suppl. 1):99-105. [DOI] [PubMed] [Google Scholar]
- 7.Curtis, M., Y. Zhu, and M. Jackson. 2004. Highly sensitive and accurate real-time PCR assay for hepatitis B viral load quantification, poster abstract 48. Presented at the 17th International Conference on Antiviral Research, Tucson, Ariz., May 2004.
- 8.Dienstag, J. L., E. R. Schiff, T. L. Wright, R. P. Perrillo, H.-W. Hann, Z. Goodman, L. Crowther, L. D. Condreay, M. Woessner, M. Rubin, and N. A. Brown. 1999. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 341:1256-1263. [DOI] [PubMed] [Google Scholar]
- 9.Gish, R. G., N. W. Y. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gish, R. G., H. Trinh, N. Leung, F. K. L. Chan, M. W. Fried, T. L. Wright, C. Wang, J. Anderson, E. Mondou, A. Snow, J. Sorbel, F. Rousseau, and L. Corey. 2005. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection. A two-year study. J. Hepatol. 43:60-66. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T.-T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 12.Jacquard, A. C., M. Nassal, C. Pichoud, S. Ren, U. Schultz, S. Guerret, M. Chevallier, B. Werle, S. Peyrol, C. Jamard, L. T. Rimsky, C. Trepo, and F. Zoulim. 2004. Effect of a combination of clevudine and emtricitabine with adenovirus-mediated delivery of gamma interferon in the woodchuck model of hepatitis B virus infection. Antimicrob. Agents Chemother. 48:2683-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeffe, E. B., D. T. Dieterich, S.-H. B. Han, I. M. Jacobson, P. Martin, E. R. Schiff, H. Tobias, and T. L. Wright. 2004. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin. Gastroenterol. Hepatol. 2:87-106. [DOI] [PubMed] [Google Scholar]
- 14.Korba, B. E. 1996. In vitro evaluation of combination therapies against hepatitis B virus replication. Antivir. Res. 29:49-51. [DOI] [PubMed] [Google Scholar]
- 15.Korba, B. E., C. K. Chu, E. Hill, and P. Furman. 1999. Effectiveness of combination therapies against hepatitis B virus (HBV) replication in vitro, poster abstract 56. Presented at the 12th International Conference on Antiviral Research, Jerusalem, Israel, March 1999.
- 16.Kumar, R., and B. Agrawal. 2004. Novel treatment options for hepatitis B virus infection. Curr. Opin. Investig. Drugs 5:171-178. [PubMed] [Google Scholar]
- 17.Ladner, S. K., T. J. Miller, and R. W. King. 1998. The M539V polymerase variant of human hepatitis B virus demonstrates resistance to 2′-deoxy-3′-thiacytidine and a reduced ability to synthesize viral DNA. Antimicrob. Agents Chemother. 42:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladner, S. K., M. J. Otto, C. S. Barker, K. Zaifert, G. H. Wang, J. T. Guo, C. Seeger, and R. W. King. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 41:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai, C.-L., R.-N. Chien, N. W. Y. Leung, T.-T. Chang, R. Guan, D.-I. Tai, K.-Y. Ng, P.-C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and D. F. Gray. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 20.Lok, A. S., E. J. Heathcote, and J. H. Hoofnagle. 2001. Management of hepatitis B 2000—summary of a workshop. Gastroenterology 120:1828-1853. [DOI] [PubMed] [Google Scholar]
- 21.Mantel, N. 1963. Chi-square tests with one degree of freedom; extensions of the Mantel-Haenszel procedure. Am. Stat. Assoc. J. 58:690-700. [Google Scholar]
- 22.Marcellin, P., T.-T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 23.Marcellin, P., G. K. K. Lau, F. Bonino, P. Farci, S. Hadziyannis, R. Jin, Z.-M. Lu, T. Piratvisuth, G. Germanidis, C. Yurdaydin, M. Diago, S. Gurel, M-Y. Lai, P. Button, and N. Pluck. 2004. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 351:1206-1217. [DOI] [PubMed] [Google Scholar]
- 24.Marcellin, P., N. Leung, H.-W. L. Hann, G. K. K. Lau, C. Trepo, S. Sacks, H. Mommeja-Marin, C. Moxham, A. Snow, F. Rousseau, and S. G. Lim. 2004. A phase II, randomized trial evaluating the safety, pharmacokinetics and antiviral activity of clevudine for 12 weeks in patients with chronic hepatitis B, poster abstract 1129. Presented at the 55th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, Mass., November 2004.
- 25.Marcellin, P., H. Mommeja-Marin, S. L. Sacks, G. K. K. Lau, D. Sereni, J.-P. Bronowicki, B. Conway, C. Trepo, M. R. Blum, B. C. Yoo, E. Mondou, J. Sorbel, A. Snow, F. Rousseau, and H.-S. Lee. 2004. A phase II dose-escalating trial of clevudine in patients with chronic hepatitis B. Hepatology 40:140-148. [DOI] [PubMed] [Google Scholar]
- 26.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-β-L-arabinofuranosyl)uracil)] against woodchuck hepatitis virus and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33:254-266. [DOI] [PubMed] [Google Scholar]
- 27.Seigneres, B., C. Pichoud, P. Martin, P. Furman, C. Trepo, and F. Zoulim. 2002. Inhibitory activity of dioxolane purine analogs on wild-type and lamivudine-resistant mutants of hepadnaviruses. Hepatology 36:710-722. [DOI] [PubMed] [Google Scholar]
- 28.van Elteren, P. H. 1958. On the combination of independent two sample tests of Wilcoxon. Bull. Int. Stat. Inst. 37:351-361. [Google Scholar]
- 29.Zhu, Y., T. Yamamoto, J. Cullen, J. Saputelli, C. E. Aldrich, D. S. Miller, S. Litwin, P. A. Furman, A. R. Jilbert, and W. S. Mason. 2001. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J. Virol. 75:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]