Abstract
The effects of ciprofloxacin on mitochondrial DNA (mtDNA) content, oxygen consumption, mitochondrial membrane potential, cellular ATP formation, and capacitative Ca2+ entry into Jurkat cells were investigated. In cells incubated for several days with 25 μg/ml ciprofloxacin, a 60% reduction of mtDNA content, inhibition of the respiratory chain, and a significant decrease in mitochondrial membrane potential were observed. These changes led to a decrease in the calcium buffering capacity of mitochondria which, in turn, resulted in a gradual inhibition of the capacitative Ca2+ entry. On days 4, 7, and 11 of incubation with ciprofloxacin, the initial rates of Ca2+ entry were reduced by 33%, 50%, and 50%, respectively. Ciprofloxacin caused a transient decrease in the cellular capability for ATP formation. In cells incubated for 15 min with glucose, pyruvate, and glutamine as exogenous fuel, ciprofloxacin reduced ATP content by 16% and 35% on days 4 and 7, respectively, of incubation with the drug. However, on day 11 of incubation with ciprofloxacin, a recovery of cellular ATP formation was observed. In conclusion, long-term exposure of Jurkat cells to ciprofloxacin at a concentration of 25 μg/ml seriously affects cellular energy metabolism and calcium homeostasis.
Mitochondria play a crucial role in ATP synthesis in all aerobic animal cells. ATP produced by oxidative phosphorylation covers nearly 90% of cellular energy demands. Apart from ATP synthesis, mitochondria are the site of numerous metabolic processes and are involved in the regulation of cellular calcium signals. To fulfill these functions, mitochondria must generate a high electrochemical proton gradient (ΔΨ) across the inner membrane. Their de-energization due to inhibition of the respiratory chain or to uncoupling of oxidative phosphorylation may result in cellular energy deficiency and impairment of ΔΨ-dependent processes, among them intracellular calcium signaling (11, 15, 26, 27). In metazoa mitochondrial DNA (mtDNA) typically encodes 13 proteins involved in oxidative phosphorylation; two of them are elements of the Fo subunit of mitochondrial ATPase, while 11 proteins are components of the respiratory chain complexes I, III, and IV. In addition, mtDNA contains 22 genes encoding tRNAs and two genes for rRNA. Therefore, perturbations in mtDNA content and/or processing may cause severe impairments in the cellular energy metabolism.
Ciprofloxacin is a 4-fluoroquinolone antibiotic commonly used in therapy of many microbial infections. Its antibacterial activity is based on the inhibition of the bacterial enzyme DNA gyrase. Unfortunately, this compound also inhibits mammalian topoisomerase II, especially its mitochondrial isoform. This side effect results in improper mtDNA replication and therefore causes mtDNA fragmentation and a gradual decrease in mtDNA content (4, 14). A prolonged exposure of murine leukemia cells to ciprofloxacin applied at concentrations ranging from 20 to 80 μg/ml results in a gradual depletion of mtDNA with a concomitant reduction of the respiration rate and stimulation of glycolysis (14).
Disturbance of the mitochondrial energy metabolism has been proposed to explain the cytotoxic effect of ciprofloxacin. The sensitivities of various cell lines to ciprofloxacin may differ significantly (13, 14). Some of them, for example, bladder cancer cells, exposed to low concentrations of this compound may undergo irreversible changes leading to apoptotic death (2, 3). Ciprofloxacin applied at a concentration of 80 μg/ml was found to both stimulate and inhibit transcription of many genes in human lymphocytes (6).
There is a growing body of evidence that ciprofloxacin, especially at higher concentrations, in the range from 50 μg/ml to 400 μg/ml, interferes with the cell cycle at the S/G2 checkpoint and commits cells to the apoptotic pathway. It also induces apoptosis in activated Jurkat cells (12). Recently, numerous reports have been published concerning the antitumor activity of ciprofloxacin based on stimulation of apoptosis in various malignant cells. This makes ciprofloxacin a candidate anticancer drug (3, 10).
Capacitative calcium entry, the most common pathway of Ca2+ influx in electrically nonexcitable cells, is preceded by calcium release from the lumen of the endoplasmic reticulum (ER). In other words, opening of the plasma membrane Ca2+ channels, the so-called store-operated calcium channels (SOCs), depends on the filling state of intracellular calcium stores (17, 19, 20). In many electrically nonexcitable cell types, including Jurkat cells, energized mitochondria are necessary for intensive calcium influx through SOCs to occur. It is well established that respiring, well-coupled mitochondria buffer the excess of calcium accumulated in the vicinity of calcium channels and thereby limit local Ca2+ concentration. This reduces the feedback inhibition of calcium channels and promotes their open state (7, 8, 11, 15, 16).
The main purpose of this study was to correlate the effects of long-term exposure to a relatively low concentration (25 μg/ml) of ciprofloxacin on the mtDNA content and mitochondrial energy state (expressed as the respiration rate and ΔΨ) with those on capacitative calcium influx and viability of human lymphoidal cells (Jurkat). We show that ciprofloxacin induces a decrease in both the mitochondrial respiration rate and ΔΨ in parallel with a reduction of the mtDNA content. More importantly, the changes in the mitochondrial energy metabolism correlate with decreased calcium entry into these cells. Because the capacitative mode of Ca2+ influx is the major route of calcium entry into lymphocytes, it seems possible that ciprofloxacin-evoked inhibition of cell proliferation may, at least partially, result from aberrant calcium signals.
MATERIALS AND METHODS
Chemicals.
Fura-2AM (fura-2 acetoxymethyl ester) and JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) were from Molecular Probes (Eugene, OR). Agarose, ethidium bromide, gel loading solution, glucose-6-phosphate dehydrogenase, hexokinase, ionomycin, NADP+, oligomycin, pyruvate and thapsigargin were purchased from Sigma Chemical Co. (St. Louis, MO). Ciprofloxacin was from ICN Biomedicals Inc. (Aurora, OH). [α-32P]dCTP, the Megaprime DNA labeling system, and salmon sperm DNA were from Amersham Biosciences (United Kingdom). Pronase was purchased from Roche Diagnostics GmbH (Mannheim, Germany). The Gene Ruler 1-kb DNA ladder and HindIII were from Fermentas MBI (Hanover, MD). Gel blot paper GB 003 and nylon transfer membrane Nytran SuPerCharge were from Schleicher and Schuell GmbH (Dassel, Germany). The QIAquick gel extraction kit and Taq PCR core kit (250) were from QIAGEN GmbH (Hilden, Germany). Other chemicals were of analytical grade.
Cell culture.
Human lymphoblastoid T cells (Jurkat) were grown in RPMI 1640 medium supplemented with 2 mM glutamine (Gibco BRL), 50 μM uridine, 10% fetal bovine serum (Gibco BRL), penicillin (100 U/ml), and streptomycin (50 μg/ml) in a humidified atmosphere of 5% CO2-95% air at 37°C. In cases indicated in the figures, the culture medium was supplemented with ciprofloxacin (25 μg/ml). The cells were seeded every 2 days, and all experiments were conducted 1 day after seeding.
Cytosolic Ca2+ concentration ([Ca2]c) measurement.
The buffered saline solution (BSS) consisted of 132 mM NaCl, 5 mM KCl, 25 mM HEPES, 1 mM MgCl2, 0.5 mM NaH2PO4, 1 mM pyruvate, and 5 mM glucose. The pH was adjusted to 7.2 at 30°C. As indicated in Fig. 6, 0.12 μM oligomycin or 100 nM thapsigargin (both dissolved in dimethyl sulfoxide) was added.
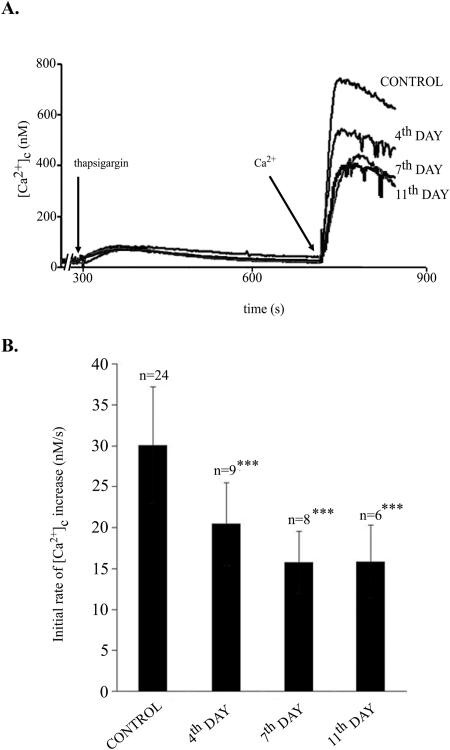
FIG. 6.
Effect of ciprofloxacin on Ca2+ entry into Jurkat cells. Cells suspended in nominally Ca2+-free BSS were preincubated with oligomycin (0.1 mg/ml). After depletion of intracellular calcium stores by the addition of 0.1 μM thapsigargin, the cell suspension was supplemented with 3 mM CaCl2. The rate of Ca2+ entry was estimated as a tangent of the initial part of the curve. (A) A typical experiment. (B) Data collected from several experiments, as indicated. ***, P < 0.001.
[Ca2+]c was measured with the fluorescent probe fura-2AM (9). The cells were loaded with this probe by incubation in the culture medium supplemented with 1 μM fura-2AM at 37°C for 15 min. After being washed by centrifugation in BSS containing 0.1 mM CaCl2, the cells were suspended in nominally calcium-free BSS with 0.05 mM EGTA and used for experiments. As indicated in Fig. 6, the mixture was supplemented with an appropriate amount of CaCl2. The fluorescence was measured at 30°C in a Shimadzu RF5000 fluorimeter set in the ratio mode at wavelengths of 340 nm/380 nm for excitation and 510 nm for emission. The time resolution of the measurements was 1 s. The fluorescence signal was calibrated for the same sample of Jurkat cells in each run using externally added 3 mM CaCl2 and 3 μM ionomycin and 0.003% digitonin.
Cellular ATP content and oxygen uptake.
ATP was measured spectrofluorimetrically in neutralized cellular acidic extracts as an increase in NADPH fluorescence (340-nm and 465-nm excitation and emission wavelengths, respectively) using a standard enzymatic assay with hexokinase and glucose-6-phosphate dehydrogenase (25). Mitochondrial respiration was measured in digitonin-permeabilized cells with a Clark-type oxygen electrode (YSI, Yellow Springs, OH) equipped with a homemade electronic device that enabled recording of the first derivative of the O2 concentration trace (equivalent to the O2 uptake rate, d[O2]/dt) (24).
Mitochondrial membrane potential (ΔΨ).
The electric potential of the inner mitochondrial membrane was measured in situ using flow cytometry in intact cells stained with the JC-1 fluorescent probe (5). The cells (5 × 106) were suspended in 2 ml of the culture medium supplemented with 2 μl of JC-1 (stock 2 mg/ml in dimethyl sulfoxide). The cells were incubated for 10 min at 37°C and washed twice with BSS containing 1 mM pyruvate and 5 mM glucose. Finally, the cells were suspended in BSS and, as indicated in Fig. 3, treated with 0.12 μM oligomycin 15 min prior to ΔΨ measurements. JC-1 fluorescence was measured using a flow cytometer (Becton Dickinson) (excitation with an argon laser at 488 nm and emission wavelength of 530/590 nm) in 104 cells.
FIG. 3.
Effect of ciprofloxacin on mitochondrial membrane potential (ΔΨ) in Jurkat cells. Cells were grown in the presence of ciprofloxacin (25 μg/ml) for 4, 7, or 11 days. ΔΨ was measured in intact cells by flow cytometry with a JC-1 probe. The cells were suspended in PBS supplemented with 1 mM pyruvate and 5 mM glucose. (A) Control cells. The shift of cellular population caused by the addition of valinomycin reflects a complete dissipation of ΔΨ. Oligomycin did not affect ΔΨ in control cells. (B) Ciprofloxacin-treated cells. Exposure to ciprofloxacin led to a gradual decrease in ΔΨ (left-hand column). The addition of oligomycin to the cell suspension shortly before the measurement resulted in a further decrease in ΔΨ (right-hand column). Numbers in each panel express the proportion of cells with dissipated ΔΨ. Axes FL1-H and FL2-H correspond to the intensity levels of green and red fluorescence, respectively, of the JC-1 probe. The figure presents results of one typical experiment out of six.
ΔΨ generated due to oxidation of succinate as a sole substrate was measured in digitonin-permeabilized cells and evaluated from the uptake of safranine O (1). This was determined from fluorescence quenching monitored at the wavelengths of 495 nm and 586 nm for excitation and emission, respectively, with a Shimadzu RF 5000 spectrofluorimeter.
Isolation of cellular DNA.
Cells (12 × 107) were collected by centrifugation at 200 × g for 5 min at room temperature. The supernatant was discarded, and the pellet was rinsed with phosphate-buffered saline (PBS) and centrifuged as above. Then, the pellet was suspended in 7.5 ml of a solution containing 75 mM NaCl and 0.25 mM EDTA, pH 8.0. After the addition of pronase (1 mg/ml) and sodium dodecyl sulfate (1%), the suspension was incubated for 16 h at 37°C. Incubation was terminated by the addition of 3 ml of 6 M NaCl (1.7 M final concentration). This suspension was centrifuged at 18,000 × g at 18°C. The supernatant was collected and centrifuged again under the same conditions. The final supernatant was mixed with a double volume of ice-cold ethanol (96%) and incubated at −20°C for 30 min. After centrifugation (18,000 × g for 10 min at 4°C) the supernatant was discarded, and the pellet was suspended in 1 ml of 70% cold ethanol, incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 18,000 × g. This step was performed in duplicate. The final pellet was air dried. The dry DNA pellet was dissolved in 100 μl of 10 mM Tris-HCl and 1 mM EDTA, pH 7.4, and the DNA concentration was determined spectrophotometrically (21).
Preparation of DNA probes.
For determination of the proportion of mtDNA to total isolated DNA, two 32P-labeled DNA probes homologous to HindIII-digested DNA fragments were prepared. One of them (3,013 bp) was specific for a 5,477-bp mtDNA fragment, and the second one (1,185 bp) was for a nucleotide sequence of about 9,500 bp within the nuclear gene encoding 18S rRNA. Purified probes obtained by PCR were labeled with [α-32P]dCTP using a Megaprime DNA labeling system (Amersham Biosciences).
Gel electrophoresis, Southern hybridization, and autoradiography.
Isolated total cellular DNA (10 μg per sample) was incubated with HindIII endonuclease (7 U per μg of DNA; Fermentas) for 18 h at 37°C in R+ buffer (10 mM Tris-HCl, pH 8.5, 10 mM MgCl2, 100 mM KCl, 0.1 mg/ml bovine serum albumin) in a total volume of 20 μl. The digestion was terminated by the addition of 2 μl of sample buffer containing 200 mM EDTA and 40% Ficoll 400 (wt/vol).
Digested DNA was subjected to electrophoresis in 0.7% horizontal agarose gel (20 cm long) in 0.5× TBE buffer (45 mM Tris-borate, 2 mM EDTA). Following the electrophoresis at 30 V for 56 h, the gel was stained with ethidium bromide, and DNA was visualized in UV light at 254 nm. DNA was transferred onto a nitrocellulose filter (NYTRAN SuPerCharge; Schleicher and Schuell) and hybridized to previously prepared 32P-labeled DNA probes (21).
Expression of results.
Calculated values are means ± standard errors for the number of independent experiments indicated in the figure legends. The statistical evaluation of differences was calculated by Student's t test.
RESULTS
Jurkat cells growing in the presence of ciprofloxacin at a concentration of 25 μg/ml proliferate slowly. Increases in cell culture densities observed between days 1 to 3 and 8 to 10 of treatment by this compound were reduced by 37% and 67%, respectively, in comparison with those observed in the same periods in the control cell culture. In the presence of ciprofloxacin at a concentration of 12.5 μg/ml, no effects were observed at least until the 21st day of the treatment, while doses in the range between 50 μg/ml and 100 μg/ml induced cell death (both apoptotic and necrotic, as recognized by increased staining with trypan blue and the appearance of a sub-G1 fraction detected by flow cytometry in propidium iodide-stained cells) (data not shown). This precluded long-term experiments with such high concentrations of the drug. Because the general aim of the study was to investigate the correlation between (expected) reduction of mtDNA, cellular energy metabolism, and calcium homeostasis, it was necessary to maintain cell culture for several cell cycles without induction of cell death. The highest concentration of ciprofloxacin not causing cell death, i.e., 25 μg/ml, was chosen as the most appropriate one for long-term experiments. Cells exposed to ciprofloxacin at this concentration for 4, 7, or 11 days did not exhibit any features of either apoptosis (no sub-G1 fraction, no annexin V-phycoerythrin staining, and no activation of caspase-3) or necrosis (no trypan blue staining exceeding that found in control cells and no staining with 7-aminoactinomycin D dye). Therefore, it was concluded that the reduced cell densities observed in the presence of ciprofloxacin at 25 μg/ml reflected a lowered rate of cell proliferation.
Effect of ciprofloxacin on mtDNA content.
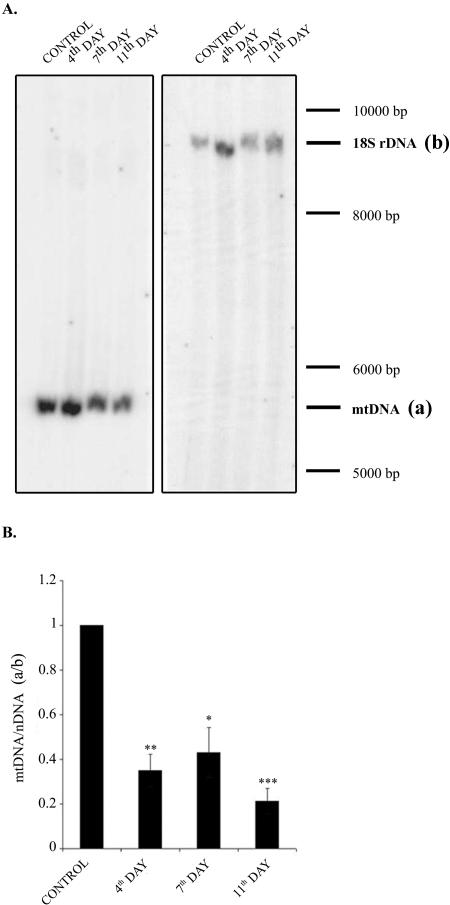
Figure 1A (a typical Southern blot) and B (collective data from three experiments, reflecting changes in mtDNA content in relation to nuclear DNA) show that exposure of Jurkat cells to ciprofloxacin for several days resulted in a substantial reduction in mtDNA content. The levels of mtDNA found on days 4, 7, and 11 of ciprofloxacin treatment were similar and amounted to around 30% of the control value. Presumably, this ciprofloxacin-induced reduction in mtDNA content was secondary to the inhibition of mitochondrial topoisomerase, which in turn caused disturbances in mtDNA replication/maintenance. This effect, also described by other authors (13), was the basis for our further studies. The decrease in mtDNA level was expected to perturb the mitochondrial energy state expressed by a lowered mitochondrial respiration rate and reduced ΔΨ and ATP formation.
FIG. 1.
Effect of ciprofloxacin on mtDNA content. Cells were grown in the absence (control) or in the presence of ciprofloxacin (25 μg/ml) for 4, 7, or 11 days. (A) Southern blots with visualized mtDNA fragment (a, left blot) and nuclear DNA fragment (b, right blot) used as a reference. (B) Collected data from three independent experiments expressed as ratios between the intensity of the radioactivity of bands a and b shown in panel A. The a/b value for control cells is taken as 1. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effect of ciprofloxacin on mitochondrial respiration rate and ΔΨ.
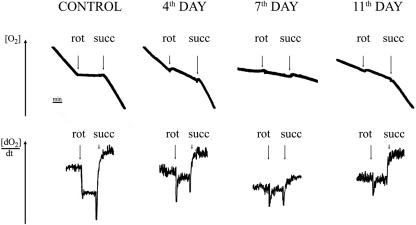
As shown in Fig. 2, treatment of Jurkat cells with ciprofloxacin reduced the rate of oxygen consumption (measured polarographically with a Clark electrode) by digitonin-permeabilized cells incubated in the presence of malate plus glutamate. The inhibition of respiration started to be visible after 7 days of incubation with ciprofloxacin. A similar pattern was also observed with succinate (in the presence of rotenone), a respiratory substrate entering the respiratory chain via complex II. Interestingly, in cells treated with ciprofloxacin for 11 days, the rate of oxygen consumption with succinate plus rotenone (but not with malate plus glutamate) returned to the control value, which suggests that Jurkat cells may partially adapt to ciprofloxacin. In other words, oxidation of succinate (occurring via complexes II, III, and IV) becomes efficient again whereas oxidation of malate plus glutamate (occurring via complexes I, III, and IV) is blocked. This observation indicates a progressive and permanent inhibition of respiratory complex I, strongly limiting the electron flow through the respiratory chain, with the activities of complexes III and IV being apparently impaired to a lower extent, allowing respiration from succinate. Ciprofloxacin added directly to the assay using cells grown in the absence of the drug did not inhibit oxygen consumption (data not shown).
FIG. 2.
Effect of ciprofloxacin on oxygen consumption by permeabilized cells. Cells were grown in the presence of ciprofloxacin (25 μg/ml) for 4, 7, or 11 days. Oxygen consumption was measured in digitonin-permeabilized cells suspended in BSS supplemented with 5 mM malate and 5 mM glutamate. At times indicated by arrows, rotenone (rot) or 5 mM succinate (succ) was added. The traces show results of one typical experiment out of six.
Preincubation of cells with ciprofloxacin for 4, 7, or 11 days led to a reduction of ΔΨ as measured by flow cytometry in intact cells stained with the JC-1 fluorescent probe and incubated in the presence of 1 mM pyruvate and 5 mM glucose. This effect was clearly visible 7 days after the drug was added to the culture medium (Fig. 3B, left column). Moreover, addition of 0.12 μM oligomycin 2 min before measurements to inhibit mitochondrial ATPase and thereby prevent ΔΨ formation at the expense of hydrolyzed ATP resulted in a decrease of ΔΨ in cells exposed to ciprofloxacin for 4 days and enhanced the effect of ciprofloxacin in cells incubated with the drug for longer periods (Fig. 3B, right column). Oligomycin did not affect ΔΨ in control cells (Fig. 3A). Because the cells were incubated in a glucose-containing medium, the effect of oligomycin strongly suggests that Jurkat cells with impaired respiratory function were able to restore ΔΨ (at least to some extent) at the expense of ATP synthesized glycolytically. When the mitochondrial ATPase was inhibited (e.g., by oligomycin), this route of ΔΨ generation was blocked.
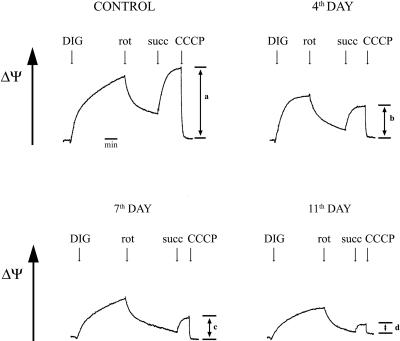
Figure 4 shows the inhibitory effect of ciprofloxacin on ΔΨ measured with safranine O as a fluorescent probe in digitonin-permeabilized cells incubated with succinate (plus rotenone) as a sole substrate. It is noteworthy that the recovery of respiration with succinate observed in cells growing for 11 days with ciprofloxacin (Fig. 2) was not accompanied by a parallel recovery of ΔΨ (Fig. 4).
FIG. 4.
Effect of ciprofloxacin on mitochondrial membrane potential (ΔΨ) in permeabilized cells incubated with succinate. Cells were grown in the presence of ciprofloxacin (25 μg/ml) for 4, 7, or 11 days. ΔΨ was measured with 5 μM safranine O in digitonin (DIG)-permeabilized cells suspended in BSS and incubated in the presence of 5 mM glutamate and 5 mM malate. After inhibition of mitochondrial respiration by 1 μM rotenone (rot), 5 mM succinate (succ) was added. Bars a, b, c, and d show changes in fluorescence after complete mitochondrial uncoupling by carbonyl cyanide m-chlorophenylhydrazone (CCCP), corresponding to increases in ΔΨ due to respiration with succinate as a sole substrate. The figure presents results of one typical experiment out of three.
Effect of ciprofloxacin on cellular ATP synthesis.
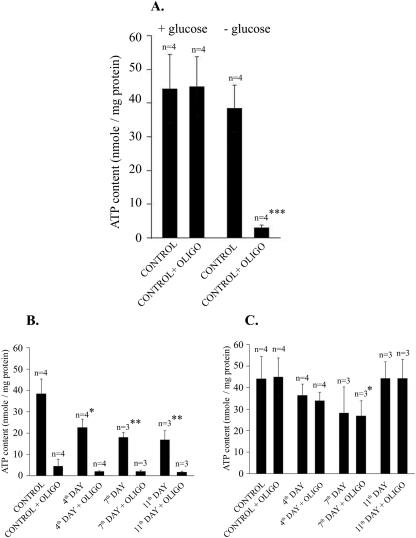
Figure 5 shows the effects of glucose and oligomycin on ATP formation in control and ciprofloxacin-treated cells. Cellular ATP content was measured in extracts prepared from the cells incubated for 15 min in BSS in the presence of various supplements. In control cells incubated in the presence of 1 mM pyruvate, 5 mM glutamine, and 5 mM glucose, the addition of oligomycin had no effect on cellular ATP content, while in glucose-deprived cells it decreased the ATP level by 80% (Fig. 5A). This indicates that Jurkat cells are able to synthesize ATP efficiently both in the oxidative and the glycolytic modes and that the inability to form ATP in mitochondria may be fully overcome by glycolytic phosphorylation. In ciprofloxacin-pretreated cells incubated for 15 min in the presence of 1 mM pyruvate and 5 mM glutamine but without glucose, the cellular content of ATP was significantly lower than in controls (Fig. 5B). It reached its minimum after 7 days of continuous exposure of the cells to ciprofloxacin. As shown in Fig. 5A, the addition of oligomycin to the incubation medium 15 min before cell extraction caused an almost complete depletion of ATP (Fig. 5B). A similar ciprofloxacin-evoked decrease in ATP formation was observed in cells incubated in the presence of glucose. However, under such conditions oligomycin did not affect ATP levels (Fig. 5C). Taken together, the presence of ciprofloxacin in the culture medium resulted in a progressive decrease in both oxidative and glycolytic ATP formation in Jurkat cells. Interestingly, the latter effect was substantially reversed after 11 days of incubation with ciprofloxacin (Fig. 5C), which may suggest adaptation of the cells to ciprofloxacin.
FIG. 5.
Effect of ciprofloxacin and oligomycin on cellular ATP formation. Cells were incubated for 15 min in PBS containing 2 mM glutamine and 1 mM pyruvate, with or without 5 mM glucose and with or without oligomycin (oligo; 1 mg/ml). Then, incubation was terminated, and ATP content in neutralized acidic cellular extracts was determined. (A) Effect of oligomycin in control cells with or without glucose. (B) Effect of oligomycin in the absence of glucose in cells pretreated with ciprofloxacin for 4, 7, or 11 days. (C) Effect of oligomycin in cells pretreated with ciprofloxacin for 4, 7, or 11 days in the presence of 5 mM glucose. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effect of ciprofloxacin on the rate of calcium influx.
Depletion of intracellular calcium stores in the ER due to selective inhibition of Ca2+-ATPase (pumping calcium ions from the cytosol to the lumen of the ER) by thapsigargin (23) results in the opening of SOCs in the plasma membrane and activation of calcium flux into the cell. As shown in Fig. 6A, addition of thapsigargin to cells suspended in calcium-free BSS resulted in a transient increase of [Ca2+]c (Ca2+ flows into the cytosol from the ER), followed by a slow recovery to its basal value. The latter phase reflected calcium efflux from the cell due to the activity of plasma membrane Ca2+-ATPase. Subsequent addition of CaCl2 to the cell suspension caused an immediate increase in [Ca2+]c due to Ca2+ influx through the SOC. The tangent fitted to the initial upstroke represents the initial rate of Ca2+ influx. Figure 6A also shows that exposure of the cells to ciprofloxacin decreased the initial rate of calcium entry in a time-dependent manner. Figure 6B presents data collected from several experiments. It shows that the inhibitory effect of ciprofloxacin on the initial rate of calcium influx was progressive and needed several days to reach a constant value. The time course of these changes was parallel to the ciprofloxacin-evoked decrease in ΔΨ (Fig. 3).
DISCUSSION
Data presented in this paper clearly demonstrate that ciprofloxacin at a concentration of 25 μg/ml causes a significant reduction in the mtDNA content in Jurkat cells. This is accompanied by a gradual decrease in the activity of the respiratory chain and a reduction of the mitochondrial membrane potential (Fig. 2 and 3). Moreover, the observation that oligomycin can further decrease ΔΨ in ciprofloxacin-treated cells indicates that ΔΨ may be sustained at the expense of glycolytically generated ATP. It seems that impairment of respiratory chain-related generation of ΔΨ precedes the inhibition of ATPase (which catalyzes the ATP-dependent restoration of ΔΨ). This in turn suggests a longer lifetime of FoF1-ATPase (encoded partially by mtDNA) in comparison to mitochondrion-encoded respiratory chain subunits. Alternatively, it may indicate that the total activity of mitochondrial ATPase significantly exceeds the maximal activity of the respiratory chain.
An incomplete collapse of ΔΨ as observed in intact cells through day 7 after ciprofloxacin was added to the culture medium and its only partial sensitivity to oligomycin indicate that a large portion of mitochondria are still energetically efficient. In addition, despite the attenuation of mitochondrial ATP synthesis triggered by ciprofloxacin, the availability of ATP is not limiting for the generation of ΔΨ, since ΔΨ may be even further abolished by oligomycin. These facts as well as a full recovery of the cellular ability to restore intracellular ATP content in the presence of glucose after 11 days of cell exposure to ciprofloxacin are most likely due to upregulation of glycolysis and explain why 25 μg/ml ciprofloxacin does not cause cellular necrosis. This is additionally supported by a low proportion of ciprofloxacin-treated cells stained with trypan blue, which was not significantly higher than that observed in control cells (data not shown).
The recovery of respiration in the presence of succinate (plus rotenone) as a sole substrate (Fig. 2) (but not with NADH-delivering substrates) indicates that complexes III and IV are not responsible for the almost complete inhibition of oxygen consumption observed in the presence of glutamate plus malate after 11 days of incubation with ciprofloxacin. On the other hand, no recovery of ΔΨ under such conditions may result from either the uncoupling of oxidative phosphorylation due to an enhanced proton leak through the inner mitochondrial membrane (i.e., through impaired FoF1-ATPase or directly across the lipid bilayer) or from the affected vectorial proton transport by complexes III and IV (despite uninhibited electron flow through the respiratory chain). The latter possibility resembles the so called “slip” phenomenon described previously (18). In that case, ciprofloxacin (most probably acting via partial depletion and/or damage of mtDNA and therefore downregulation of certain elements of the respiratory chain or components of FoF1-ATPase) could alter the stoichiometry of the mitochondrial proton pump(s) in respiratory complexes III and/or IV. Such an effect has been described for bupivacaine-treated mitochondria (22). Oxygen consumption in the presence of malate and glutamate is permanently inhibited most probably because of a serious functional damage of complex I. Interestingly, rotenone exhibits only a partial inhibitory effect on oxygen consumption in cells grown in a ciprofloxacin-containing medium, incubated in the presence of malate plus glutamate, while in the control cells it fully blocks cellular respiration (Fig. 2). In addition, the inhibitory effect of rotenone disappears gradually during the course of ciprofloxacin treatment. This finding indicates that cells exposed to ciprofloxacin may intensively utilize substrates entering the respiratory chain downstream complex I. Most likely, the endogenous levels of such substrates are increased in cells exposed to ciprofloxacin. Taken together, the decrease in mtDNA content observed in Jurkat cells treated with ciprofloxacin (Fig. 1) leads to the complete inactivation of complex I, while the activities of complexes III and IV, although reduced, are still sufficient for electron transport from complex II (succinate dehydrogenase) and from other NAD+-independent substrates. However, it must be emphasized that mitochondrial Ca2+ uptake and therefore the rate of Ca2+ entry into the cell through SOCs directly depend on the ΔΨ but not on the rate of respiration per se (see below). Thus, the decrease in the mitochondrial potential is sufficient to permanently inhibit Ca2+ influx. Explanation of the mechanism responsible for the restoration of oxygen consumption without concomitant energization of the mitochondrial membrane in ciprofloxacin-treated cells incubated with succinate as a sole substrate, although tempting, is beyond the scope of this paper.
The full recovery of the cellular ability to synthesize ATP in the presence of glucose (Fig. 5) as well as the increase in the respiration rate in the presence of succinate observed on day 11 of the experiment (Fig. 2), preceded by a significant reduction of both parameters detected on day 7, may indicate adaptation of Jurkat cells to ciprofloxacin. On the other hand, the persistently abolished ΔΨ (Fig. 3 and 4), irreversibly inhibited respiration in the presence of NAD+-utilizing substrates (Fig. 2), and progressive inhibition of capacitative calcium entry (Fig. 6) point to partial adaptation to ciprofloxacin rather than to selection of ciprofloxacin-resistant cells.
The transient inhibition of glycolytic ATP synthesis on day 7 of incubation with ciprofloxacin indicates that this antibiotic not only acts in an mtDNA-dependent manner but also influences metabolic processes independent of mitochondrial gene expression.
Ca2+ entry into the mitochondrial matrix is driven by ΔΨ. Because the mitochondrial Ca2+ uniporter has a relatively low affinity for calcium ions, only mitochondria exposed to high [Ca2+]c (which happens in the neighborhood of Ca2+ channels) are able to accumulate calcium efficiently. As has been documented by several authors (7, 11, 15, 16), mitochondria located in close vicinity to SOCs accumulate Ca2+ and therefore protect these channels against feedback inhibition. A decrease in ΔΨ prevents mitochondrial Ca2+ uptake and consequently may lead to an inhibition of Ca2+ entry into the cell. The effect of ciprofloxacin on calcium influx requires several days to occur, which coincides with a decrease in ΔΨ. Thus, impairment in the mitochondrial energy state explains the ciprofloxacin-evoked inhibition of capacitative calcium entry into Jurkat cells. These effects may seriously affect calcium signaling and cause improper regulation of many calcium-dependent cellular processes, including cell division, gene expression, and exocytosis. Presumably, the decreased rate of proliferation observed in cells grown in the presence of ciprofloxacin is connected with a depression of capacitative calcium entry. It also seems possible that prolonged treatment with this antibiotic may influence the response of lymphocytes to antigens. However, these conjectures need to be confirmed. Therefore, understanding the precise mechanisms of ciprofloxacin action in human cells seems to be very important.
It has been suggested that ciprofloxacin induces apoptosis in some cell lines. Therefore, this compound could be considered as a potential anticancer drug. However, for such a purpose ciprofloxacin has to be applied at much higher concentrations, and its effects require only a few hours to develop. Such ciprofloxacin-induced cell death is attributed to stimulation of the mitochondrial permeability transition pore and to activation of the mitochondrion-dependent apoptotic pathway (2). The reduction of mtDNA content due to an inhibition of mitochondrial topoisomerase needs several cell cycles to occur. Therefore, the short-term proapoptotic effects of ciprofloxacin cannot be attributed to any long-term decrease in mtDNA content. The data presented in this paper concern the side effects of antimicrobial therapy rather than those related to antitumor treatment and might be important when long-term administration of 4-fluoroquinolones is undertaken.
Acknowledgments
We thank Lech Wojtczak for critical reading of the manuscript.
This work was partially supported by the State Committee for Scientific Research under grant 3 P04A 041 23.
REFERENCES
- 1.Åkerman, K. E. O., and M. K. F. Wikström. 1976. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 68:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Aranha, O., L. Zhu, S. Alhasan, D. P. Wood, Jr., T. H. Kuo, and F. H. Sarkar. 2002. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cells. J. Urol. 167:1288-1294. [PubMed] [Google Scholar]
- 3.Aranha, O., D. P. Wood, Jr., and F. H. Sarkar. 2000. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in human transitional cell carcinoma of the bladder cell line. Clin. Cancer Res. 6:891-900. [PubMed] [Google Scholar]
- 4.Castora, F. J., F. F. Vissering, and M. V. Simpson. 1983. The effects of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria. Biochim. Biophys. Acta 740:417-427. [DOI] [PubMed] [Google Scholar]
- 5.Cossarizza, A., M. Baccarini Contri, G. Kalashnikova, and C. Franceschi. 1993. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 197:40-45. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson, E., A. Forsgren, and K. Riesbeck. 2003. Several gene programs are induced in ciprofloxacin-treated human lymphocytes as revealed by microarray analysis. J. Leukoc. Biol. 74:456-463. [DOI] [PubMed] [Google Scholar]
- 7.Gilabert, J. A., D. Bakowski, and A. B. Parekh. 2001. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store operated calcium influx. EMBO J. 20:2672-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilabert, J. A., and A. B. Parekh. 2000. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I. EMBO J. 19:6401-6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 10.Herold, C., M. Ocker, M. Ganslmayer, H. Gerauer, E. G. Hahn, and D. Schuppan. 2002. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 86:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoth, M., C. M. Fanger, and R. S. Lewis. 1997. Mitochondrial regulation of store-operated calcium signalling in T lymphocytes. J. Cell Biol. 137:633-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun, Y.-T., H.-J. Kim, M.-J. Song, J.-H. Lim, D.-G. Lee, K.-J. Han, S.-M. Choi, J.-H. Yoo, W.-S. Shin, and J.-H. Choi. 2003. In vitro effects of ciprofloxacin and roxithromycin on apoptosis of Jurkat T lymphocytes. Antimicrob. Agents Chemother. 47:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence, J. W., D. C. Claire, V. Weissig, and T. C. Rowe. 1996. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol. Pharm. 55:1178-1188. [PubMed] [Google Scholar]
- 14.Lawrence, J. W., S. Darkin-Rattray, F. Xie, A. H. Neims, and T. C. Rowe. 1993. 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells. J. Cell. Biochem. 51:165-174. [DOI] [PubMed] [Google Scholar]
- 15.Makowska, A., K. Zablocki, and J. Duszyński. 2000. The role of mitochondria in the regulation of calcium influx into Jurkat cells. Eur. J. Biochem. 267:877-884. [DOI] [PubMed] [Google Scholar]
- 16.Malli, R., M. Frieden, K. Osibow, and W. F. Graier. 2003. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 278:10807-10815. [DOI] [PubMed] [Google Scholar]
- 17.Parekh, A. B., and R. Penner. 1997. Store depletion and calcium influx. Physiol. Rev. 77:901-929. [DOI] [PubMed] [Google Scholar]
- 18.Pietrobon, D., G. F. Azzone, and D. Walz. 1981. Effect of funiculosin and antimycin on the redox-driven H+-pumps in mitochondria: on the nature of “leaks.” Eur. J. Biochem. 117:389-394. [DOI] [PubMed] [Google Scholar]
- 19.Putney, J. W., Jr. 1986. A model for receptor-regulated calcium entry. Cell Calcium 7:1-12. [DOI] [PubMed] [Google Scholar]
- 20.Putney, J. W., Jr. 1990. Capacitative calcium entry revisited. Cell Calcium 11:611-624. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Sztark, F., R. Ouhabi, P. Dabadie, and J. P. Mazat. 1997. Effects of the local anaesthetic bupivacaine on mitochondrial energy metabolism: change from uncoupling to decoupling depending on the respiratory state. Biochem. Mol. Biol. Int. 43:997-1003. [DOI] [PubMed] [Google Scholar]
- 23.Thastrup, O., A. P. Dawson, O. Scharff, B. Foder, P. J. Cullen, B. K. Drobak, P. J. Bjerrum, S. B. Christensen, and M. R. Hanley. 1989. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions 27:17-23. [DOI] [PubMed] [Google Scholar]
- 24.Wasilewski, M., M. R. Więckowski, D. Dymkowska, and L. Wojtczak. 2004. Effects of N-acylethanolamines on mitochondrial energetics and permeability transition. Biochim. Biophys. Acta 1657:151-163. [DOI] [PubMed] [Google Scholar]
- 25.Williamson, J. R., and B. N. Corkey. 1969. Assays of intermediates of the citric acid cycle and related compounds by fluorometric enzyme methods. Methods Enzymol. 13:434-513. [Google Scholar]
- 26.Zablocki, K., A. Makowska, and J. Duszyński. 2003. pH-dependent effect of mitochondria on calcium influx into Jurkat cells: a novel mechanism of cell protection against calcium entry during energy stress. Cell Calcium 33:91-99. [DOI] [PubMed] [Google Scholar]
- 27.Zablocki, K., J. Szczepanowska, and J. Duszyński. 2005. Extracellular pH modifies mitochondrial control of capacitative calcium entry in Jurkat cells. J. Biol. Chem. 280:3516-3521. [DOI] [PubMed] [Google Scholar]