Abstract
Rats immunosuppressed by the administration of cyclophosphamide and cortisone acetate and then infected with Aspergillus fumigatus were treated with an antifungal drug, EDTA, or a combination of one of the antifungal agents, amphotericin B lipid complex (ABLC; 5 mg/kg of body weight/day for 7 days), and EDTA (30 mg/kg/day for 7 days). The mortality rate was reduced, the duration of survival was increased, fewer A. fumigatus organisms were recovered from the lungs, and less-severe lung lesions were seen histopathologically in the rats receiving the combination treatment than in the rats receiving either an antifungal agent or EDTA alone. Further studies regarding the mechanisms of EDTA and its interactions with ABLC are warranted, and further studies are needed to more fully examine the safety, tolerance, and optimal dosing of EDTA in the treatment of this and other fungal infections.
Invasive aspergillosis remains a major cause of morbidity and death in neutropenic patients with hematologic malignancies, especially in patients undergoing induction chemotherapy and hematopoietic stem cell transplantation (1, 2, 5, 9). Despite recent advances in antifungal therapy, the overall mortality rate in patients with invasive pulmonary aspergillosis remains very high, approaching 90% (3, 6, 9, 12, 17). Current choices of antifungal agents consist of amphotericin B preparations, echinocandins, and triazoles (3, 7, 9, 13). There is, thus, an urgent need for an alternative or additional antifungal therapy that can improve the prospects for patients with this life-threatening infection.
Because EDTA, a chelator agent used as the standard treatment for lead poisoning, has been shown to have antifungal activity in vitro (14), we conjectured that it might serve as an adjunct antifungal therapy. Our hypothesis was that EDTA would serve as a chelator of divalent cations and thereby potentiate the activity of the amphotericin B-based antifungal regimen being administered. To test this hypothesis, we examined the efficacy of EDTA alone and in combination with amphotericin B lipid complex (ABLC) (Abelcet; Elan, San Diego, CA) in an immunosuppressed rat model with invasive pulmonary aspergillosis to determine whether the combination improved the outcome in this rat model.
MATERIALS AND METHODS
Animals and animal care.
Eighty male Sprague-Dawley rats (Charles River Breeding Laboratories, Inc., Wilmington, MA) weighing 120 to 175 g each were used for all experiments. The rats were housed (three per cage) in presterilized, filter-topped cages and had access to a pathogen-free diet and water ad libitum in the biohazard isolation suite at The University of Texas M. D. Anderson Cancer Center. Animals were kept under strict hygienic conditions and allowed free access to rodent laboratory fodder. Animals were checked daily, and mortality was recorded for up to 20 days, after which the surviving rats were sacrificed. Ciprofloxacin was added to the drinking water to prevent bacterial superinfection. Experiments were approved by the Institutional Animal Care and Ethics Committee of the M. D. Anderson Cancer Center, and all animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (11a).
Antifungal agents.
Amphotericin B (AMB) deoxycholate (Fungizone) was purchased from Bristol-Myers Squibb (Princeton, NJ). A 5.0-mg/ml solution was prepared with distilled water immediately prior to its use. ABLC in 5-mg/ml vials was donated by Enzon Pharmaceuticals (Bridgewater, NJ). EDTA (Endrate) was purchased (Abbott Laboratories, North Chicago, IL) in vials of 20 ml at 50 mg/ml. The solution was diluted in distilled water to the desired concentration prior to its use in animals.
Inoculum preparation and inoculation procedure.
Aspergillus fumigatus strain AF293 was used in this infected-animal model. To prepare the inoculum, A. fumigatus was grown on Sabouraud's dextrose agar plates for 1 week at 37°C. Conidia were collected by flooding the plates with 10 ml of sterile normal saline with 0.1% Tween 80. The inocula were scraped off gently with an L-shaped sterile glass (inoculum loop), and then the harvested solutions were filtered through 4- by 4-inch (10- by 10-cm) gauze to eliminate the hyphal branching elements and mycelial fragments. The remaining suspension, consisting mostly of conidia, was concentrated by centrifugation, and the supernatant was removed. Conidia were then suspended to achieve a final concentration of 1 × 109 conidia/ml. The viability of all inocula was confirmed to be ≥95% by hemocytometer counting, followed by manual counting of the conidia in serial suspensions on plates. The MIC at 48 h for this isolate was 0.5 μg/ml for AMB and 1 μg/ml for ABLC, which was determined by using the NCCLS method adapted for molds (11). For inoculation (300 μl) of the fungi, a suspension of 1 × 107 conidia of A. fumigatus was delivered with a micropipette (Corning, Corning, NY) to the nares of the animal. All the inoculation procedures with the immunosuppressed rats were performed with the animal in an induction box under spontaneous ventilation with a 4% isoflurane and oxygen (5 liters/min) gas mixture.
Time-kill curve study.
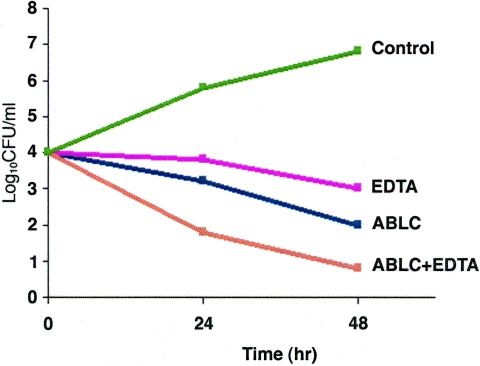
Aliquots of the suspension of A. fumigatus strain AF293 were prepared in 50 ml of RPMI medium at 1 × 104 conidia/ml in the presence of EDTA, ABLC, and ABLC plus EDTA at concentrations ranging from 0.25 μg/ml to 16 μg/ml after 24 h of drug exposure in a shaker at 35°C. Aliquots (0.1 ml) of the conidial suspension were removed and diluted to obtain 10- to 104-fold dilutions and were plated on Sabouraud dextrose agar plates. The plates were incubated at 35°C for 24 h, and the number of CFU/ml was determined. The same procedure was repeated for the remaining aliquots after 48 h of drug exposure. A time-kill curve (see Fig. 3) was constructed by plotting the mean log number of CFU/ml against the time conidia were exposed to the antifungal drug.
FIG. 3.
The time-kill curves show the effects of EDTA and ABLC alone and in combination on the growth of A. fumigatus strain AF293 after 24 or 48 h of drug exposure.
Immunosuppression procedure.
To render the rats neutropenic during the study period, cyclophosphamide (100 mg/kg of body weight; Sigma-Aldrich Chemical Co., St. Louis, MO), reconstituted in sterile distilled water, was administered via intraperitoneal (i.p.) injection (500 μl) on days −5 and −1 (before inoculation) and on days +3 and +7 (after inoculation) of the fungi. In addition, cortisone acetate (100 mg/kg; Sigma) was prepared as a suspension in sterile phosphate-buffered saline with 0.02% Tween 80 and was administered by subcutaneous injection (200 μl) only on day −1 before the inoculation.
To confirm the duration of neutropenia, blood samples obtained by serial tail venophlebotomy (20-μl samples) from three rats in each group were sent for hematologic and chemistry studies on day 0 (baseline, 10 min before inoculation), on day +7 (at the end of therapy), on day +14, and on day +20 (at the end of the study). Absolute neutrophil counts showed that most of the rats tested remained neutropenic, with an absolute neutrophil count of <500 until day 14. However, on day 20, most of the rats showed some immune recovery from neutropenia, with absolute neutrophil counts ranging between 500 and 3,000.
Toxicity determinations.
Serum creatinine and calcium concentrations were assessed in the same three rats from each group to rule out toxicity. For the purposes of this study, an abnormal creatinine or calcium level was indicated by a 25% increase from the baseline value (see data presented in Table 2). During the histopathologic analysis of lung specimens, tissue was also examined for damage attributable to the EDTA.
TABLE 2.
Assessment of drug impact on rat serum calcium and creatinine concentrations
| Assay | Day | Mean concn in serum ± SDa
|
||||
|---|---|---|---|---|---|---|
| Control | AMB | ABLC | EDTA | ABLC + EDTA | ||
| Calciumb | 0 (baseline) | 11.0 ± 0.50 | 10.7 ± 0.47 | 10.7 ± 0.38 | 10.8 ± 0.26 | 11.1 ± 0.31 |
| 7 | 10.9 ± 0.55 | 10.8 ± 0.38 | 10.4 ± 0.70 | 10.6 ± 0.40 | 10.8 ± 0.44 | |
| 20 | 10.0 ± 0.72 | 9.0 ± 0.57 | 9.3 ± 0.76 | 9.4 ± 0.67 | 9.9 ± 0.40 | |
| Creatininec | 0 (baseline) | 0.63 ± 0.058 | 0.57 ± 0.058 | 0.57 ± 0.115 | 0.53 ± 0.058 | 0.60 ± 0.100 |
| 7 | 0.57 ± 0.058 | 0.60 ± 0.100 | 0.57 ± 0.058 | 0.53 ± 0.058 | 0.53 ± 0.058 | |
| 20 | 0.63 ± 0.058 | 0.57 ± 0.058 | 0.57 ± 0.058 | 0.70 ± 0.100 | 0.57 ± 0.058 | |
Changes in serum calcium and creatinine concentration were detected in nine rats from each group using analysis of variance tests.
Calcium concentrations in the AMB group decreased by 15% from baseline to day 20 and by 16% from day 7 to day 20; in the EDTA group, it decreased by 13% from baseline to day 20 and by 11% from day 7 to day 20; and in the combination group ABLC plus EDTA, it decreased by 10% from baseline to day 20.
There was no significant change in creatinine concentration for any group during the study.
Antifungal treatment.
The rats were divided on the basis of their treatment into five groups, each consisting of 16 animals, as follows: group 1, no drug administered (control group); group 2, intravenous (i.v.) injection with 1 mg of AMB alone; group 3, i.v. injection with 5 mg/kg ABLC alone; group 4, intraperitoneal (i.p. injection with 30 mg/kg EDTA alone; and group 5, i.p. injection with 30 mg/kg EDTA and i.v. injection with 5 mg/kg ABLC. Treatments were started 18 h after rats were inoculated with the fungi (described below) and continued for 7 days.
To assess the effect of treatment on the lungs of the animals, four rats from each group were sacrificed on day 7. At sacrifice, anesthetized animals were bled by cardiac puncture and euthanized with carbon dioxide gas. The lung tissues were removed aseptically, weighed, and homogenized in 5 ml of sterile saline for uniform times at the same speed. One milliliter of the lung homogenate was placed onto Sabouraud's dextrose agar to quantify the fungal burden in the lungs in terms of numbers of CFU/g of lung tissue. Another lung tissue sample was sent for histopathologic analysis. To assess the effect of treatment on survival, the remaining 12 rats in each group were followed for a total of 20 days, at which time they were sacrificed.
Analysis of data.
We compared the median numbers of CFU/g of lung tissue among the different treatment groups using the Wilcoxon rank sum test normal approximation with continuity correction. Analyses were conducted for rats that survived 1 week or less and for rats that survived for more than 1 week. Histopathologic grading of the angioinvasive lung lesions was done semiquantitatively, using a scoring system ranging from 0 to 4+, which indicated both the severity of the pulmonary lesions and the abundance of organisms. Fisher's exact test was used to compare the grades among the five groups.
The Kaplan-Meier product limit method was used to examine the effects of treatment on survival. Median survival and the corresponding 95% confidence interval values for survival were calculated for rats in each treatment group. The survival rates for the treatment groups were compared by the log rank test. All tests were two-tailed, and an alpha value of 0.05 was considered to indicate significance.
RESULTS
The mean number of CFU/g of lung tissue was significantly lower in all four treatment groups than in the control group, both for the rats that survived 1 week or less and for the rats that survived for more than 1 week. The results of the postmortem quantification of cultures of A. fumigatus from the lungs after treatment are shown in Table 1.
TABLE 1.
Comparative efficacies of EDTA alone and in combination with amphotericin B preparations in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats
| Group | Total no. of rats | Mean log10 no. of CFU/g of lung (range) (no. of rats)
|
Angioinvasive lung lesion histopathology grade (range) | |
|---|---|---|---|---|
| At 7 days | From rats surviving >7 days | |||
| Control | 16 | 3.65 (2.93-4.26) (12) | 3.46 (3.32-3.83) (4) | 3+ (2+-4+) |
| AMB | 16 | 2.29 (0-3.68) (8) | 0.61 (0-2.3) (8) | 3+ (0+-3+) |
| ABLC | 16 | 1.65 (0-3.46) (7) | 0.67 (0-2.8) (9) | 2+ (2+-4+) |
| EDTA | 16 | 2.27 (0-3.75) (7) | 1.0 (0-3.3) (9) | 3+ (0+-3+) |
| ABLC + EDTA | 16 | 0.45 (0-1.7) (7) | 0.25 (0-1.8) (9) | 0+ (0+-2+) |
Additionally, rats in the ABLC-plus-EDTA treatment group (group 5) that survived for 1 week or less had a significantly lower median number of CFU/g of lung tissue than did the rats in the AMB or EDTA treatment groups (groups 2 and 4). The mean number of CFU/g of lung tissue for the ABLC-plus-EDTA treatment group, however, was not significantly lower than that of the ABLC (group 3) treatment group. None of the other pair-wise comparisons showed a statistically significant difference in the mean numbers of CFU/g of lung tissue.
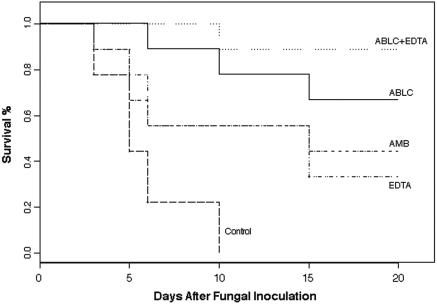
The effect of the study drugs on the survival of rats with pulmonary aspergillosis is shown in Fig. 1. The overall test for equality over strata indicated that the survival curves for the five groups differed significantly (P < 0.0001). That is, all four treatment groups fared significantly better in terms of survival than did the control group. However, survival duration was significantly better in the ABLC-plus-EDTA treatment group rats than in the rats in either the AMB treatment group or the EDTA treatment group. In addition, the survival duration in the ABLC-plus-EDTA group showed a trend toward being significantly longer than that of the ABLC group. The EDTA-treated group was comparable to the AMB-treated group in terms of showing a significantly prolonged survival compared with that of the control group (P ≥ 0.02 and 0.03, respectively).
FIG. 1.
Percentages of survival of immunosuppressed rats infected with A. fumigatus. Treatments were started 18 h after the inoculation and continued for 7 days.
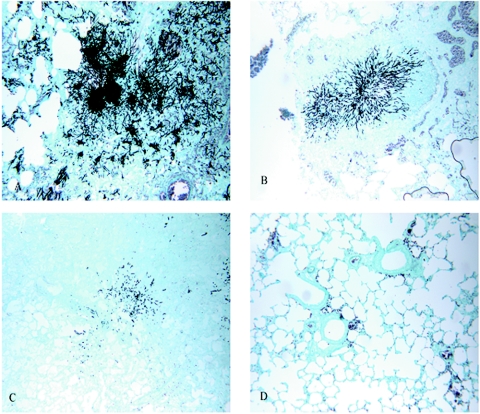
Microscopic examination of sections of lungs from the untreated control rats in group 1 revealed thrombosis of the pulmonary vasculature that correlated with moderate to severe (3 to 4+) necrosis and disseminated granulomatous pneumonia. In addition, lungs from the control animals contained an abundance (3 to 4+) of branching, fungal hyphae that exhibited prominent septation and widespread lung consolidation. In contrast, the lungs from rats treated with AMB, ABLC, or EDTA as a single agent (groups 2 through 4) showed milder thrombosis, necrosis, and pneumonia that were typically graded 2+; similarly, the abundance of organisms in these lungs was graded 2+. However, the lungs from the group 5 rats, which were given ABLC plus EDTA, showed no marked microscopic lesions and no organisms. In addition, the lesions that were present were generally focal and graded as mild (1+), and the fungi that were encountered consisted only of ovoid spores without any hyphal morphology. The lungs from the group given EDTA plus ABLC also showed less consolidation. Silver-stained sections prepared to show the relative abundances of organisms in the infected lungs are shown in Fig. 2.
FIG. 2.
Sections of lungs from immunosuppressed rats infected with invasive A. fumigatus (Grocott-Gomori methenamine-silver nitrate stain; magnification, ×100). (A) Untreated lung tissue from a control infected rat showing severe granulomatous pneumonia and large numbers of branching fungal hyphae; (B) lung tissue from an infected rat treated for 7 days with EDTA alone showing mild consolidation of the parenchyma and accumulation of fungal hyphae; (C) lung tissue from an infected rat treated for 7 days with ABLC alone showing mild pneumonia and an abundance of organisms; (D) lung tissue from an infected rat treated for 7 days with ABLC plus EDTA showing no significant lesions and no organisms.
In addition, a time-kill curve in vitro study was done and demonstrated the fungicidal activity of EDTA and ABLC alone and in combination (Fig. 3). There was a 2-log decrease in the number of CFU with each drug alone compared to the number for the control and a substantial decrease in growth with combination drugs compared to growth with each drug alone. Calcium and creatinine measurement showed no toxicity related to any of the drugs used. Detailed values of calcium levels and creatinine are represented in Table 2. Likewise, no tissue damage was seen in the histopathologic specimens that could be attributed to EDTA toxicity (Fig. 1).
DISCUSSION
In the present study, in which we evaluated a novel treatment for pulmonary aspergillosis consisting of EDTA plus ABLC in a model of severely immunosuppressed rats infected with A. fumigatus, we found that the combination of EDTA with an antifungal agent conferred a considerable survival benefit and thus that it appeared to potentiate ABLC without additional toxicity.
In this invasive-pulmonary-aspergillosis model, the rats were maintained in a neutropenic state with cyclophosphamide and cortisone acetate for at least 2 weeks in order to closely mimic the clinical situation that is typically so difficult to treat. In this lethal invasive-pulmonary-infection model, the majority of untreated rats died by day 6 after inoculation. However, all the rats treated with EDTA, AMB, or ABLC alone showed longer survival times (Fig. 1) and a lower tissue burden of Aspergillus organisms (Table 1) than did the untreated controls. In addition, the combination of EDTA plus ABLC resulted in longer survival than did either agent alone. Similarly, the combination treatment resulted in a reduced tissue fungal burden and minimal angioinvasion, as shown by histopathologic studies (Fig. 2). These findings suggest that the two drugs have an additive effect and that concurrent therapies with EDTA and ABLC may be of value in patients with hematologic malignancies, especially in patients with persistent neutropenia.
In a recent study that included 232 hematologic malignancy patients with invasive aspergillosis treated at our institution with either ABLC or amphotericin B liposome (AmBisome) as single agents, results showed a response rate of only 11% in patients who received 7 days of therapy, and 89% to 97% of the patients died with aspergillosis in 12 weeks (R. Hachem et al., 43rd Ann. Meet. Infect. Dis. Soc. Am., abstr. 743, 2005). Novel antifungal agents such as echinocandins (caspofungin) and azoles (voriconazole) have recently been introduced and approved for the treatment of invasive aspergillosis in high-risk patients. In two studies by Herbrecht and Maertens (6, 8), the response rate of neutropenic patients with invasive aspergillosis to either caspofungin or voriconazole used as a single-agent therapy did not exceed 30% (6, 8). Furthermore, a recent study from our institution showed that the combination of the lipid formulation of amphotericin B and caspofungin is of limited benefit in improving the outcome of invasive aspergillosis in patients with hematologic malignancy (7). Hence, the results of the current animal study could have substantial clinical implications related to the improvement of the response of invasive aspergillosis to amphotericin B lipid formulation through the addition of EDTA as an antifungal-enhancing agent.
The current study is the first study to show the beneficial in vivo activity of EDTA against invasive pulmonary aspergillosis. Our study is also unique in showing the enhanced activity of a conventional antifungal agent such as ABLC in combination with EDTA. In terms of the mechanism of action of EDTA, EDTA likely inhibits aspergillus growth and causes fungal death by competing with aspergillus siderophores for any of the trace iron and calcium ions that are essential to the maintenance of the life cycle of fungi. This inhibitory effect of EDTA was clearly demonstrated in our study by the test animals' prolonged survival, the reduced tissue fungal burden, and the decreased angioinvasion noted in histopathologic preparations seen in all treatment groups but especially in the group receiving both EDTA and ABLC. Wei and Bobek recently demonstrated that EDTA enhanced the antifungal activity in human salivary mucin peptides mainly by chelating the divalent cations (such as Ca+) (18). In another study, Sen et al. showed that EDTA demonstrates the highest antifungal activity against Candida albicans, compared with those of routine antifungal drugs (15).
To verify the safety of EDTA, we randomly checked the serum calcium and creatinine levels at different times during therapy. Our observations of the rats and the data in Table 2 are consistent with an absence of significant direct toxic effects of EDTA. All of the tested animals showed normal levels. Furthermore, no tissue damage was seen in the histopathologic specimens that could be attributed to the EDTA. The safety of EDTA was also assessed with a rat model by Sanchez-Fructuoso et al. (14), who similarly found that EDTA at doses equivalent to those used in clinical practice appeared to slow the progression of the lesions caused by chronic lead poisoning without causing the damage that can occur with chelating agents.
Our infection model was comparable to other animal models that have been used to show the beneficial activity of ABLC, echinocandin, or a triazole in comparisons with untreated control animals (4, 10, 13, 16). However, in our study, we used a novel approach in which we added the chelator EDTA to potentiate the activity of the antifungal agent being administered.
We concluded from our findings that the combination of EDTA and ABLC given once daily for 7 days is the optimal treatment in this animal model. In addition, the combination therapy showed greater antifungal activities than did AMB, EDTA, or ABLC alone. Further studies are needed to more fully examine the safety, tolerance, efficacy, and optimal dosing of EDTA in the treatment of various fungal infections.
REFERENCES
- 1.Andriole, V. T. 1996. Aspergillus infections: problems in diagnosis and treatment. Infect. Agents Dis. 54:47-54. [PubMed] [Google Scholar]
- 2.Andriole, V. T. 1993. Infections with Aspergillus species. Clin. Infect. Dis. 17:S481-S486. [DOI] [PubMed] [Google Scholar]
- 3.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 4.Graybill, J. R., R. Bocanegra, G. M. Gonzalez, and L. K. Najvar. 2003. Combination antifungal therapy of murine aspergillosis: liposomal amphotericin B and micafungin. J. Antimicrob. Chemother. 52:656-662. [DOI] [PubMed] [Google Scholar]
- 5.Groll, A. H., P. M. Shah, M. Mentzel, G. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 6.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, M. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 7.Kontoyiannis, D. P., R. Hachem, R. E. Lewis, G. A. Rivero, H. A. Torres, J. Thornby, R. Champlin, H. Kantarjian, G. P. Bodey, and I. I. Raad. 2003. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 98:292-299. [DOI] [PubMed] [Google Scholar]
- 8.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh for the Caspofungin Salvage Aspergillosis Study Group. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 9.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 10.Martin, M. T., J. Gavalda, P. Lopez, X. Gomis, J. L. Ramirez, D. Rodriguez, O. Len, Q. Jordano, I. Ruiz, M. Rosal, B. Almirante, and A. Pahissa. 2003. Efficacy of high doses of liposomal amphotericin B in the treatment of experimental aspergillosis. J. Antimicrob. Chemother. 52:1032-1034. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard. NCCLS document M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 12.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, J. R. Graybill, et al. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 13.Roberts, J., K. Schock, S. Marino, and V. T. Andriole. 2000. Efficacies of two new antifungal agents, the triazole ravuconazole and the echinocandin LY-303366, in an experimental model of invasive aspergillosis. Antimicrob. Agents Chemother. 44:3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Fructuoso, A. I., J. Blanco, M. Cano, L. Ortega, M. Arroyo, C. Fernandez, D. Prats, and A. Barrientos. 2002. Experimental lead nephropathy: treatment with calcium disodium ethylenediaminetetraacetate. Am. J. Kidney Dis. 40:59-67. [DOI] [PubMed] [Google Scholar]
- 15.Sen, B. H., G. Akdeniz, and A. Denizci. 2000. The effect of ethylenediamine-tetraacetic acid on Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90:651-655. [DOI] [PubMed] [Google Scholar]
- 16.Van Etten, E. W. M., L. E. T. Stearne-Cullen, M. T. Ten Kate, and I. A. J. M. Bakker-Woudenberg. 2000. Efficacy of liposomal amphotericin B with prolonged circulation in blood in treatment of severe pulmonary aspergillosis in leukopenic rats. Antimicrob. Agents Chemother. 44:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petrsen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, E. Anaissie, and J. Lee for the National Institute of Allergy and Infectious Diseases Mycoses Study Group. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]
- 18.Wei, G.-X., and L. A. Bobek. 2005. Human salivary mucin MUC7 12-Mer-l and 12-Mer-d peptides: antifungal activity in saliva, enhancement of activity with protease inhibitor cocktail or EDTA, and cytotoxicity to human cells. Antimicrob. Agents Chemother. 49:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]