Abstract
The nonsteroidal anti-inflammatory drugs have been shown to support cytoprotection of cells by shifting cells toward a quiescent state (G0/G1). Extracellular signal-regulated kinase (ERK) is required for cells to pass from G1 phase into S phase, and macrolide antibiotics can inhibit ERK1/2 phosphorylation. However, previous reports suggest that macrolide antibiotics do not affect cell growth in bronchial epithelial cells. Therefore, we studied normal human bronchial epithelial (NHBE) cells to determine whether clarithromycin (CAM) suppresses ERK, delays bronchial epithelial cells from progressing to S phase, and delays cell growth. Exposure to CAM at 10 μg/ml daily over 4 days irreversibly decreased the cell proliferation with and without growth supplements (P < 0.0001). CAM also inhibited ERK1/2 phosphorylation over the first 90 min of exposure (P < 0.05 for 30 min, P < 0.0001 for 60 min, and P < 0.01 for 90 min) and decreased the ratio of phosphorylated ERK1/2 (pERK1/2) to total ERK1/2 (tERK1/2) (P < 0.0001). Incubation with CAM for 48 h increased the proportion of cells in G1 phase (means ± standard deviations) from 63.5% ± 0.9% to 79.1% ± 1.4% (P < 0.0001), decreased that in S phase from 19.8% ± 1.2% to 10.0% ± 2.1% (P < 0.01), and decreased that in G2/M phase from 16.7% ± 0.4% to 11.0% ± 0.8% (P < 0.001). In contrast, the ratio of pMEK1/2 to tMEK1/2 was not altered after exposure to CAM. These results suggest that macrolide antibiotics can delay the progression of NHBE cells from G1 phase to S phase and can slow cell growth, probably through the suppression of ERK1/2.
The extracellular signal-regulated protein kinase (ERK) pathway, one of the mitogen-activated protein kinase (MAPK) pathways, consisting of Raf, MAP kinase/ERK kinase 1/2 (MEK1/2), and ERK1/2, is involved predominantly in the control of cell proliferation, migration, and differentiation (6, 31). ERK pathway activation has been shown to be essential for cells to pass from G1 phase into S phase (23), and the inhibition of this pathway results in G1 arrest in a variety of cell types (15, 20).
Macrolide antibiotics, such as clarithromycin (CAM), erythromycin, roxithromycin, and azithromycin (AZM), have been used to treat patients with cystic fibrosis or diffuse panbronchiolitis by virtue of their immunomodulatory properties, including the suppression of hyperimmunity and inflammation without overt immunosuppression (18). Several studies of the relationship between macrolide antibiotics and ERK have been conducted. For example, AZM inhibits the quorum-sensing signal molecule N-(3-oxododecanoyl)homoserine lactone-induced expression of MUC5AC, the major gel-forming mucin protein expressed in the airway, probably through the inhibition of ERK phosphorylation in the NCI-H292 epithelial cell line in vitro (5), and CAM therapy decreases ERK1/2 phosphorylation in the infected lung in vivo (7). Those papers suggest that ERK is constitutively activated in human bronchial cells, that ERK can be further stimulated by inflammatory mediators (22), and that this activation can be inhibited by macrolide antibiotics (5, 17).
In contrast, other studies have shown that exposure to CAM has no effect on the proliferation or viability of BET-1A cells (1) and that erythromycin did not change the distribution of the cell cycle phase of HT-29 cells (2). However, these results were not obtained from normal primary human cells but from either cancer cells or cells transformed by virus. Several oncogenes can constitutively activate ERK, and this uncontrolled activation of ERK can also lead to malignant transformation (12, 29). In nonsmall lung cancer cells, ERK1/2 is activated and associated with advanced tumors (26). This uncontrolled activation of ERK could explain the conflicting data.
Stress, including oxygen radicals and inflammation, can decrease cell proliferation by retarding cell cycle progression. Cells are prevented from entering S phase and remain in a dormant stage to protect themselves from damage (19). Studies of cell kinetics revealed that cells in G2/M phase are more sensitive to stress than the cells in G0/G1 phase (13). The nonsteroidal anti-inflammatory drugs can shift cells toward a quiescent stage, G0/G1, affording cytoprotection as inflammation is the most damaging to growing cells (11). Therefore, we hypothesized that macrolide antibiotics might suppress ERK, preventing cells from entering S phase, and thus retard cell growth. In this experiment, using normal human bronchial epithelial (NHBE) cells in culture, we evaluated the time-dependent effects of CAM on cell growth and examined cell cycle changes in the pattern of G1, S, and G2/M with CAM exposure. We also determined the upstream cell signaling intermediates responsible for this macrolide activity.
MATERIALS AND METHODS
Reagents.
CAM was donated by Abbott Labs, Abbott Park, IL. Dimethyl sulfoxide (DMSO), anti-β-actin, and anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP) were purchased from Sigma Chemical Co. Ltd., St. Louis, MO, and phospho- and non-phospho-specific anti-p42/44 MAPK, anti-MEK1/2, and anti-c-Raf as well as anti-rabbit IgG HRP antibodies were purchased from Cell Signaling Technology (Beverly, MA).
NHBE cell culture.
NHBE cells (Cambrex Bio Science, Walkersville, MD) were plated at 3,500 cells/cm2 in culture dishes containing bronchial epithelial cell growth medium, supplemented with 52 μg/ml bovine pituitary extract, 0.5 μg/ml hydrocortisone, 0.5 pg/ml human recombinant epidermal growth factor, 0.5 μg/ml epinephrine, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid, and 6.5 ng/ml triiodothyronine (as growth supplements) without antibiotics, and cultured at 37°C in a 5% CO2 incubator (17, 28). Endotoxin-free medium was used (<0.005 endotoxin units/ml). We used second-passage cells for all experiments. NHBE cells were routinely cultured in 25-cm2 tissue culture flasks coated with type 1 rat tail collagen (Sigma). The medium was changed at day 1 and subsequently every 48 h. The cells were grown to confluence for 6 days.
Cultures without antibiotics were then transferred to six-well or 35-mm dishes or 25-cm2 flasks for each experiment. In order for cells to be confluent, they were seeded at 0.2 × 105, 2 × 105, or 0.35 × 105 cells/dish or flask depending on the day of harvesting, which was determined by the duration of exposure to CAM. We evaluated phospho-MAP kinase (pMAPK) by Western blot analysis and cell cycle analysis at the time of cell confluence rather than normalizing the relative number of cells, because cell maturation could potentially affect cell signaling and cell cycle distribution (the proportion of cells in each phase of the cell cycle at a given time) and, at confluence, all cells are at similar growth stages. The medium was changed every 24 h. As growth factors can stimulate cell signaling (30), cells were grown in supplement-free bronchial epithelial cell growth medium for 24 h before CAM exposure for Western blot analysis and cell cycle analysis. Cells were also cultured for 30, 60, and 90 min following stimulation for Western blot analysis.
CAM exposure (cell stimulation and inhibition).
CAM was dissolved in DMSO at a final concentration of 3 mg/ml. It has been reported that there is no difference in cell cycle distribution when cells are exposed to 0.5% DMSO or no DMSO (9). Solutions were stored at −20°C. The cells were exposed to CAM (10 μg/ml) for 24 h once daily. Ten micrograms of CAM per ml is the mean therapeutic concentration reported in lung epithelial lining fluid (14, 21). It has also been reported that CAM has immunomodulatory properties at 10 μg/ml, but not at 1 μg/ml (16), and inhibits tumor necrosis factor alpha-stimulated interleukin 8 (IL-8) release from BET-1A cells (1). CAM was added to cultures at 30, 60, and 90 min before they were harvested for Western blot analysis and 48 h before they were harvested for cell cycle analysis.
Cell growth.
The cell number was determined using a hemocytometer, and cell viability was assessed by trypan blue dye exclusion. Cell morphology was evaluated using a phase-contrast microscope (Olympus, Tokyo, Japan).
DNA histogram analysis by flow cytometry.
Cells (1 × 106) were harvested and washed twice with phosphate-buffered saline; cellular DNA was stained with 50 μg/ml propidium iodide, 37 μg/ml RNase, and 0.6% Nonidet P-40 in a 3.6 mM citrate buffer (pH 7.4), and 30,000 cells were analyzed for DNA content (10, 27). DNA cell cycle was measured on a FACStar Plus flow cytometer (BD Biosciences, Mansfield, MA). We generated DNA histograms after gating on cellular populations and removing aggregates by doublet discrimination. The DNA histogram was analyzed with Modfit (Modfit Verity Software House, Topsham, ME), and the percentage of cells in each fraction of the cell cycle (G1, S, and G2/M phases) was calculated as described previously (10). Cells located in the sub-G1 area were considered apoptotic cells (3).
Measurement of pMAPK.
Analysis of pMAPK was performed according to the manufacturer's instructions (17). Following stimulation, the cells were washed twice with 2 ml cold phosphate-buffered saline. After the supernatants were completely aspirated, the cells were lysed on ice in a modified radio immunoprecipitation buffer (1% NP-40, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris, pH 7.5, 5 mM sodium pyrophosphate, 1 mM NaVO4, 5 mM NaF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 0.1 mM phenylmethylsulfonyl fluoride) for 15 min and then scraped from the dishes and collected into a tube. DNA was sheared by passing the lysate though a 27-gauge needle, and insoluble material was removed by centrifugation at 20,000 × g for 15 min at 4°C. The protein concentration of the resulting supernatant was quantified by a detergent-compatible protein assay (Bio-Rad, Hercules, CA), and the lysate was stored at −70°C until used. The Bio-Rad detergent-compatible protein assay is a modified Lowry assay that works in the presence of 1% ionic or nonionic detergent. Equal amounts of protein extracts were loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis mini gel and transferred to a nitrocellulose membrane (Bio-Rad) by electroblotting overnight. Membranes were rinsed with distilled water, incubated for 1 h at room temperature in Tris-buffered saline (0.8% NaCl and 20 mM Tris, pH 7.6) with 0.1% Tween 20 (TBS-T), with 5% nonfat dry milk to block nonspecific interactions, rinsed twice, and washed three times for 10 min with TBS-T. After being washed, membranes were exposed overnight to 1 μg/ml of the primary antibody p44/42 MAPK (Thr202/Tyr204), pMEK1/2 (Ser217/221), or p-c-Raf (Ser338) rabbit polyclonal IgG (Cell Signaling Technology Inc.) at 4°C in TBS-T with 5% milk. The blots were then washed and incubated at room temperature for 2 h with the anti-rabbit IgG HRP secondary antibody. Subsequently, the membranes were washed again, and antibody binding was detected using LumiGLO chemiluminescent substrate peroxide (Cell Signaling Technology Inc., Beverly, MA).
Membranes were stripped with a stripping buffer (100 mM β-mercaptoethanol, 2% sodium dodecyl sulfate, 62.5 mM Tris-HCl, pH 6.7) for 30 min at 60°C. The blots were washed twice with TBS-T and reprobed with anti-p44/42 MAPK, anti-MEK1/2, or anti-c-Raf antibody, followed by anti-rabbit IgG HRP secondary antibody. Blots were stripped again, and equivalent protein loading was confirmed by Western blotting using anti-human β-actin antibody, followed by anti-mouse IgG HRP secondary antibody, and detected by enhanced chemiluminescence (Amersham, Piscataway, NJ). Western blot images were scanned and analyzed using NIH Image J software (http://rsb.info.nih.gov/ij/; accessed June 2005).
Statistical methods.
Results are expressed as the mean values ± standard deviations or standard errors of the means (SEMs) as appropriate. Statistical analysis of data was performed using the StatView 5 statistics package (SAS Institute, Cary, NC). Where appropriate, data were analyzed by an unpaired t test or a Mann-Whitney U test. Parametric testing was conducted after it was confirmed that raw data were normally distributed. Conventionally, a P value of <0.05 was considered statistically significant.
RESULTS
Effects of CAM on cell growth.
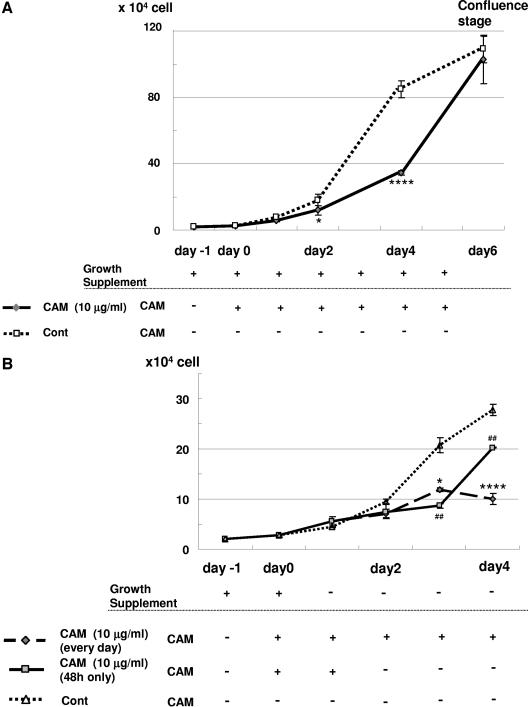
To evaluate the effect of CAM on cell growth with growth supplements, NHBE cells were seeded at 0.2 × 105 cells/35-mm dish on day −1, 10 μg/ml CAM was added on day 0, and medium with growth supplements was changed daily as shown in Fig. 1 A. Compared with the control, CAM significantly decreased the cell proliferation of NHBE cells at day 2 (P < 0.05) and at day 4 (P < 0.0001) (Fig. 1). The slope of the control cell growth curve from day 2 to day 4 [(33.4 ± 0.7) × 104 cells/day] was almost the same as that of the growth curve with CAM from day 4 to day 6 [(34.3 ± 6.8) × 104 cells/day]. The control cells reached confluence on the 35-mm dish at day 6. Cell viability was greater than 90% in all samples, and DMSO at the concentrations used had no significant effect on cell count or cell viability at day 4 compared with that of the control (data not shown).
FIG. 1.
(A) CAM (10 μg/ml) inhibits NHBE cell proliferation. NHBE cells were seeded onto 35-mm dishes at a density of 0.2 × 105 on day −1. CAM (10 μg/ml) was added on day 0 (diamonds, solid line), or cells were cultured with growth-supplemented medium only (squares, dotted line). Cells were counted on days 0, 1, 2, 4, and 6 using a hemocytometer, and a cell viability of more than 90% was confirmed by trypan blue dye exclusion. The growth supplements used were 52 μg/ml bovine pituitary extract, 0.5 μg/ml hydrocortisone, 0.5 pg/ml human recombinant epidermal growth factor, 0.5 μg/ml epinephrine, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid, and 6.5 ng/ml triiodothyronine. These experiments were repeated four times. Data are shown as means ± standard deviations. *, P < 0.05; ****, P < 0.0001 compared with the control (Cont). (B) CAM (10 μg/ml) inhibits NHBE cell proliferation in the absence of growth supplements. NHBE cells were seeded onto 35-mm dishes at a density of 0.2 × 105 on day −1, and CAM (10 μg/ml) was added on the following day (day 0) (diamonds, wide dotted line). CAM was added from day 0 to day 2 but withdrawn on day 2 (squares, solid line), or NHBE cells were cultured with medium only (control) (triangle, dotted line). Cells were counted on days 0, 1, 2, 3, and 4 using a hemocytometer. These experiments were repeated four times. Data are shown as means ± SEMs. *, P < 0.05; ##, P < 0.01; ****, P < 0.0001 compared with the control.
In the absence of growth supplements, CAM significantly decreased the proliferation of NHBE cells at day 3 (P < 0.05) and day 4 (P < 0.0001) (Fig. 1B). Fig. 1B also shows that daily CAM exposure for 48 h delayed cell proliferation only for the next 24 h (until day 3) compared with the control. However, the slope of the cell growth curve from day 3 to day 4 [(11.6 ± 0.3) × 104 cells/day] was almost the same as that of the control from day 2 to day 3 [(11.3 ± 1.0) × 104 cells/day].
Effect of CAM on threonine and tyrosine phosphorylation of ERK1/2.
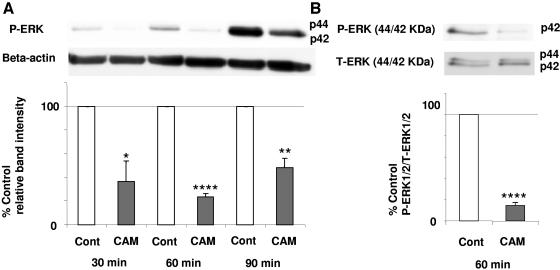
ERK1/2 is involved in the control of cell proliferation (6, 31) and is required for cells to pass from G1 into S phase (23). Therefore, we measured the effect of CAM (10 μg/ml) on the phosphorylation of ERK1/2. Growth factors were withdrawn from the culture medium at 24 h, and NHBE cells were then exposed to CAM for 30, 60, and 90 min. The phosphorylation of ERK was measured by Western blotting. There was a 64% decrease in phosphorylation of ERK (pERK) at 30 min (P < 0.05), a 76% decrease at 60 min (P < 0.0001), and a 52% decrease at 90 min (P < 0.01) compared with that of the control (Fig. 2A). There was also a decrease in the ratio of pERK1/2 to total ERK1/2 (tERK1/2) at 60 min (P < 0.0001) (Fig. 2B).
FIG. 2.
Effects of CAM (10 μg/ml) on pERK. Growth factors were withdrawn from the culture medium at 24 h before CAM exposure. NHBE cells were exposed to CAM for 30, 60, and 90 min. ERK phosphorylation was measured by Western blotting. The baseline pERK1/2 level or the ratio of pERK to tERK was set at 100%. These experiments were repeated three times, and results are shown as means ± SEMs. (A) pERK expression decreased in CAM-exposed cells compared with that of controls at 30, 60, and 90 min. (B) There was a decrease in the ratio of pERK1/2 to tERK1/2 at 60 min. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 compared with the control (Cont).
Effect of CAM on cell cycle.
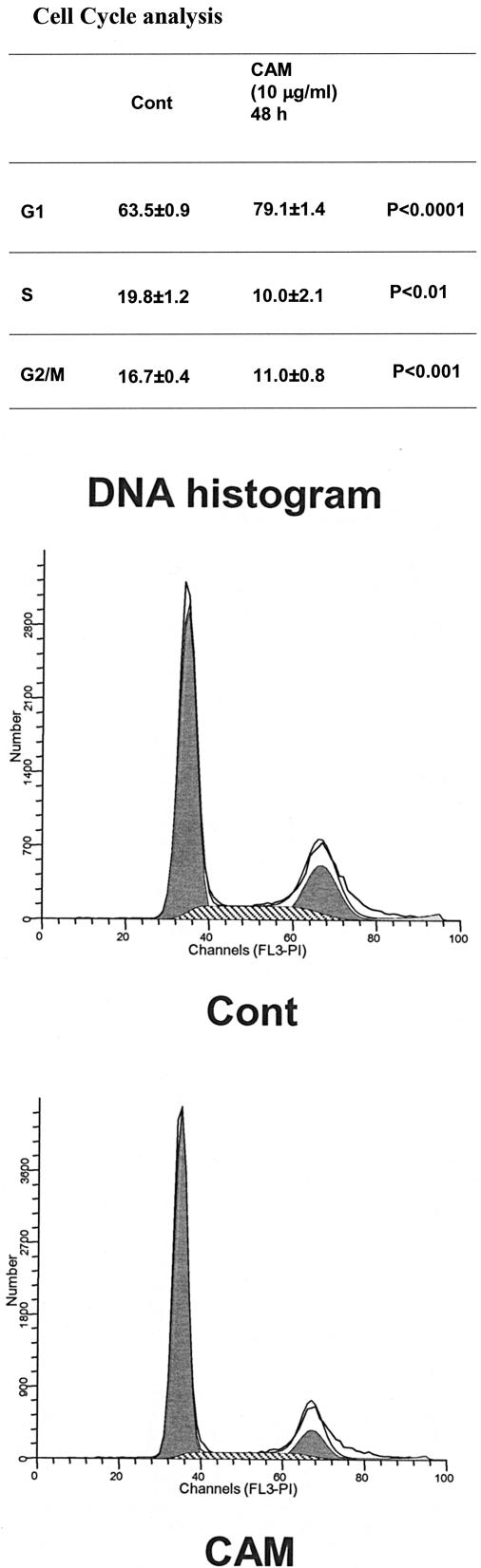
Next, we determined the proportion of cells at different phases of the cell cycle after exposure to CAM for 48 h. CAM (10 μg/ml) significantly increased the proportion of cells in G1 phase from 63.5% ± 0.9% to 79.1% ± 1.4% (P < 0.0001), decreased the proportion of cells in S phase from 19.8% ± 1.2% to 10.0% ± 2.1% (P < 0.01), and decreased the proportion of cells in G2/M phase from 16.7% ± 0.4% to 11.0% ± 0.8% (P < 0.001) (Fig. 3), implying that CAM prevents the cells from entering S phase and shifts cells toward G1 phase. There were very few cells located in the sub-G1 area in both DNA histograms (apoptotic fraction).
FIG. 3.
CAM at 10 μg/ml increases the proportion of NHBE cells in G1 phase (P < 0.0001) and decreases the proportions in S phase (P < 0.01) and G2/M phase (P < 0.001). Cells were seeded in 25-cm2 flasks and cultured in the absence (top graph) or presence (bottom graph) of CAM. Growth factors were withdrawn from the culture medium 24 h before CAM exposure. At 48 h, flow cytometric analysis was performed. The area under the white curve is the raw data. The filled area is the curve fit for the G1, S, and G2/M phases of the cell cycle. The tall peak represents cells in G1, and the crosshatched area represents cells in S phase from the DNA histogram. These experiments were repeated three times, and data are shown as means ± SEMs. Cont, control.
Effect of CAM on MEK1/2 and c-Raf phosphorylation.
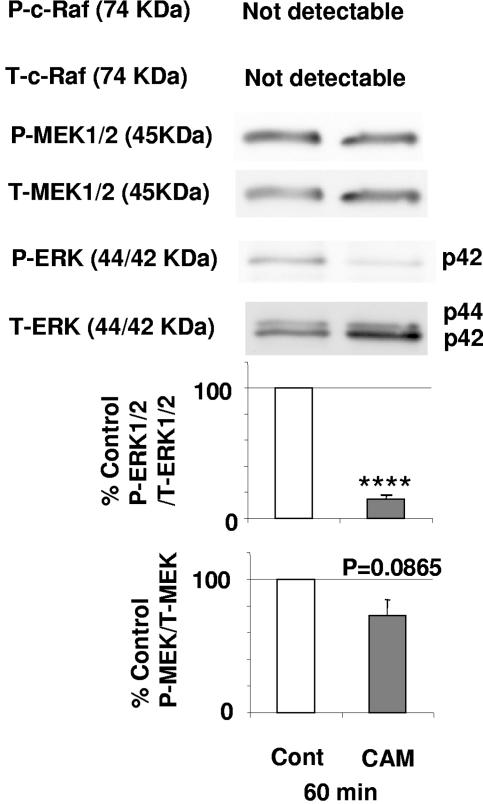
To determine whether CAM directly inhibits ERK1/2 or acts on upstream components of the ERK/MEK pathway, we examined its effect on the phosphorylation of MEK1/2 and c-Raf (31). As shown in Fig. 4, MEK1/2 was not decreased at 60 min after exposure to CAM (P = 0.09) compared with the control, and c-Raf was not detectable. There was an 86% decrease in the ratio of pERK1/2 to tERK1/2 at 60 min (P < 0.0001), and there was a 27% decrease in the ratio of pMEK to tMEK at the same time point.
FIG. 4.
CAM at 10 μg/ml has no effect on pMEK or p-c-Raf. Baseline ratios of pERK to tERK and pMEK1/2 to tMEK1/2 were set at 100%. These experiments were repeated three times, and data are shown as means ± SEMs. ****, P < 0.0001 compared with the control.
DISCUSSION
Although macrolide antibiotics can decrease ERK phosphorylation (5), which is required for cellular mitogenesis, data from some cell lines suggested that these drugs do not affect cell cycle phase distribution (2), proliferation, or viability (1). However, it is possible that macrolides could modulate proliferation of human bronchial epithelial cells depending on the magnitude of ERK activation. We report here that CAM at a physiologic concentration significantly retarded the proliferation of NHBE cells. The slope of the upward, rapid-growth portion of the growth curve of control cells from day 2 to day 4 was similar to that of CAM-exposed cells from day 4 to day 6, indicating that CAM does not kill these cells but merely delays their proliferation, possibly by competing with growth factors. We have also shown that daily CAM exposure from day 0 to day 2 delayed cell proliferation for 24 h compared with the control, but the cells then proliferated after day 3 at the same rate as that of the control. This time lag of 24 h between the time of CAM withdrawal and the onset of cell proliferation suggests that CAM can reversibly impede the progress of cells from G1 into active cell division (23).
It is generally accepted that ERK plays an important role in the control of cell proliferation (6, 30, 31). A biphasic activation of ERK at G1 is correlated with its ability to enter S phase (23), and the inhibition of MEK/ERK results in the decrease of cell proliferation via G1/S arrest in the cell cycle (8, 15). The MEK/ERK cascade is thus responsible, in part, for the regulation of G1/S progression (31). Our data showed that exposure to CAM decreased pERK over 90 min compared with the control. Furthermore, CAM significantly increased the proportion of cells in G1 and decreased those in S and G2/M, indicating an inhibition of cell proliferation of NHBE cells by impeding progression of cells from G1 phase of the cell cycle.
ERK is constitutively activated in human bronchial cells under normal conditions (22). Our data showing that the band intensity of pERK for control cells increased from 30 min up to 90 min were highly reproducible. Perhaps the change from medium with growth factors to medium lacking growth factors may paradoxically trigger phosphorylation, as suggested by others (4, 30). It may be ironic that we deliberately removed growth factors to try to prevent kinase activation.
AZM inhibits N-(3-oxododecanoyl)homoserine lactone-induced MUC5AC production in NCI-H292 epithelial cells (5) and N-formyl-methionyl-leucyl-phenylalanine-induced neutrophil migration (25) by inhibiting ERK phosphorylation. We have also demonstrated that CAM initially decreases and subsequently increases IL-8 secretion through the inhibition and activation of ERK, respectively (17). ERK activation is mediated by MEK1, which in turn is phosphorylated by c-Raf. The only known substrate of MEK1 is ERK1/2, and therefore, MEK1/2 is an important regulator of ERK signal transduction (6, 30, 31). We have shown that MEK1/2 and c-Raf were not blocked by CAM. These data suggest that CAM inhibits ERK rather than MEK/ERK, which is upstream. c-Raf was not detectable after a 60-min exposure to CAM. However, Raf-1 kinase activity peaks in kidney cells at 5 min (24); thus, Raf expression may have been missed when evaluated at 60 min in our experiments.
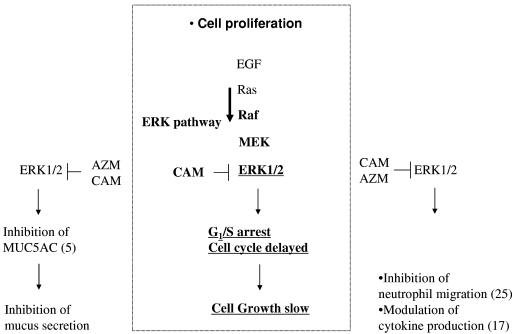
The immunomodulatory properties of macrolide antibiotics are thought to include the suppression of hyperimmunity and inflammation without overt immunosuppression (16, 18). It has also been proposed that macrolide antibiotics reduce mucin production (5) and modulate IL-8 secretion (17) as well as neutrophil migration (25) by interfering with ERK signal transduction. Our data suggest that macrolides can reversibly retard cell proliferation, probably through the inhibition of ERK. This inhibition of ERK could play an important role in macrolide immunomodulation, as proposed in Fig. 5.
FIG. 5.
Relationship between macrolide antibiotics and ERK1/2. The diagram shows possible mechanisms of the macrolide effect on cell proliferation (center) by inhibition of ERK1/2. Numbers in parentheses represent references.
Acknowledgments
We thank Lauren Vannoy for editorial assistance and acknowledge helpful discussions with Chang-Sik Park and Samir A. Shah.
This study was funded in part by a grant from the U.S. Cystic Fibrosis Foundation. M.S. was a postdoctoral research fellow of the Japan Defense Agency.
REFERENCES
- 1.Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaida, K. Matsushima, and H. Tomoike. 2000. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 2.Chen, S. Z., M. Jiang, and Y. S. Zhen. 2005. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother. Pharmacol. 56:212-220. [DOI] [PubMed] [Google Scholar]
- 3.Compton, M. M., J. S. Haskill, and J. A. Cidlowski. 1988. Analysis of glucocorticoid actions on rat thymocyte deoxyribonucleic acid by fluorescence-activated flow cytometry. Endocrinology 122:2158-2164. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty, M. K., J. Muller, D. A. Ritt, M. Zhou, X. Z. Zhou, T. D. Copeland, T. P. Conrads, T. D. Veenstra, K. P. Lu, and D. K. Morrison. 2005. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17:215-224. [DOI] [PubMed] [Google Scholar]
- 5.Imamura, Y., K. Yanagihara, Y. Mizuta, M. Seki, H. Ohno, Y. Higashiyama, Y. Miyazaki, K. Tsukamoto, Y. Hirakata, K. Tomono, J. Kadota, and S. Kohno. 2004. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob. Agents Chemother. 48:3457-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko, Y., K. Yanagihara, M. Seki, M. Kuroki, Y. Miyazaki, Y. Hirakata, H. Mukae, K. Tomono, J. Kadota, and S. Kohno. 2003. Clarithromycin inhibits overproduction of muc5ac core protein in murine model of diffuse panbronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L847-L853. [DOI] [PubMed] [Google Scholar]
- 8.Kim, D. H., H. K. Na, T. Y. Oh, W. B. Kim, and Y. J. Surh. 2004. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem. Pharmacol. 68:1081-1087. [DOI] [PubMed] [Google Scholar]
- 9.Kute, T. E., and Y. Quadri. 1991. Measurement of proliferation nuclear and membrane markers in tumor cells by flow cytometry. J. Histochem. Cytochem. 39:1125-1130. [DOI] [PubMed] [Google Scholar]
- 10.Kute, T. E., Z. M. Shao, N. K. Sugg, R. T. Long, G. B. Russell, and L. D. Case. 1992. Cathepsin D as a prognostic indicator for node-negative breast cancer patients using both immunoassays and enzymatic assays. Cancer Res. 52:5198-5203. [PubMed] [Google Scholar]
- 11.Lee, T. K., and I. Stupans. 2002. Radioprotection: the non-steroidal anti-inflammatory drugs (NSAIDs) and prostaglandins. J. Pharm. Pharmacol. 54:1435-1445. [DOI] [PubMed] [Google Scholar]
- 12.Mishima, K., K. Inoue, and Y. Hayashi. 2002. Overexpression of extracellular-signal regulated kinases on oral squamous cell carcinoma. Oral Oncol. 38:468-474. [DOI] [PubMed] [Google Scholar]
- 13.Morris, G. M. 1996. Review article: effects of radiation on the cell proliferation kinetics of epithelial tissues-therapeutic implications. Br. J. Radiol. 69:795-803. [DOI] [PubMed] [Google Scholar]
- 14.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roovers, K., and R. K. Assoian. 2000. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays 22:818-826. [DOI] [PubMed] [Google Scholar]
- 16.Rubin, B. K., and J. Tamaoki. 2004. Antibiotics as anti-inflammatory and immunomodulatory agents. Birkhauser Verlag, Basel, Switzerland.
- 17.Shinkai, M., G. H. Foster, and B. K. Rubin. 2006. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L75-L85. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai, M., and B. K. Rubin. 2005. Macrolides and airway inflammation in children. Paediatr. Respir. Rev. 6:227-235. [DOI] [PubMed] [Google Scholar]
- 19.Shinkai, M., N. Shinomiya, S. Kanoh, K. Motoyoshi, and H. Kobayashi. 2004. Oxygen stress effects on proliferation rates and heat shock proteins in lymphocytes. Aviat. Space Environ. Med. 75:109-113. [PubMed] [Google Scholar]
- 20.Squires, M. S., P. M. Nixon, and S. J. Cook. 2002. Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem. J. 366:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suwa, T., H. Urano, and H. Watanabe. 1988. Pharmacokinetic study of TE-031 (8). Absorption and excretion following oral administration of healthy volunteers. Chemotherapy (Tokyo) 36:75-85. (In Japanese.) [Google Scholar]
- 22.Takami, K., N. Takuwa, H. Okazaki, M. Kobayashi, T. Ohtoshi, S. Kawasaki, M. Dohi, K. Yamamoto, T. Nakamura, M. Tanaka, K. Nakahara, Y. Takuwa, and H. Takizawa. 2002. Interferon-gamma inhibits hepatocyte growth factor-stimulated cell proliferation of human bronchial epithelial cells: upregulation of p27(kip1) cyclin-dependent kinase inhibitor. Am. J. Respir. Cell Mol. Biol. 26:231-238. [DOI] [PubMed] [Google Scholar]
- 23.Tamemoto, H., T. Kadowaki, K. Tobe, K. Ueki, T. Izumi, Y. Chatani, M. Kohno, M. Kasuga, Y. Yazaki, and Y. Akanuma. 1992. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J. Biol. Chem. 267:20293-20297. [PubMed] [Google Scholar]
- 24.Terada, Y., K. Tomita, M. K. Homma, H. Nonoguchi, T. Yang, T. Yamada, Y. Yuasa, E. G. Krebs, S. Sasaki, and F. Marumo. 1994. Sequential activation of Raf-1 kinase, mitogen-activated protein (MAP) kinase kinase, MAP kinase, and S6 kinase by hyperosmolality in renal cells. J. Biol. Chem. 269:31296-31301. [PubMed] [Google Scholar]
- 25.Tsai, W. C., M. L. Rodriguez, K. S. Young, J. C. Deng, V. J. Thannickal, K. Tateda, M. B. Hershenson, and T. J. Standiford. 2004. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 170:1331-1339. [DOI] [PubMed] [Google Scholar]
- 26.Vicent, S., J. M. Lopez-Picazo, G. Toledo, M. D. Lozano, W. Torre, C. Garcia-Corchon, C. Quero, J. C. Soria, S. Martin-Algarra, R. G. Manzano, and L. M. Montuenga. 2004. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br. J. Cancer 90:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vindelov, L. L. 1977. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch. B 24:227-242. [PubMed] [Google Scholar]
- 28.Wagner, D. D., J. B. Olmsted, and V. J. Marder. 1982. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J. Cell Biol. 95:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb, C. P., L. Van Aelst, M. H. Wigler, and G. F. Woude. 1998. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc. Natl. Acad. Sci. USA 95:8773-8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widmann, C., S. Gibson, M. B. Jarpe, and G. L. Johnson. 1999. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79:143-180. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, W., and H. T. Liu. 2002. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12:9-18. [DOI] [PubMed] [Google Scholar]