Abstract
The colonization of uropathogenic bacteria on urinary catheters resulting in biofilm formation frequently leads to the infection of surrounding tissue and often requires removal of the catheter. Infections associated with biofilms are difficult to treat since they may be more than 1,000 times more resistant to antibiotics than their planktonic counterparts. We have developed an antibiofilm composition comprising an N-acetyl-d-glucosamine-1-phosphate acetyltransferase (GlmU) inhibitor and protamine sulfate, a cationic polypeptide. The antibiofilm activity of GlmU inhibitors, such as iodoacetamide (IDA), N-ethyl maleimide (NEM), and NEM analogs, including N-phenyl maleimide, N,N′-(1,2-phenylene)dimaleimide (oPDM), and N-(1-pyrenyl)maleimide (PyrM), was tested against that of catheter-associated uropathogens. Both IDA and NEM inhibited biofilm formation in Escherichia coli. All NEM analogs showed antibiofilm activity against clinical isolates of E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus epidermidis, and Enterococcus faecalis. The combination of oPDM with protamine sulfate (PS) enhanced its antibiofilm activity and reduced its effective concentration to as low as 12.5 μM. In addition, we found that the in vitro inhibitory activity of oPDM-plus-PS-coated silicone catheters against P. aeruginosa and S. epidermidis colonization was superior to that of catheters coated with silver hydrogel. Confocal scanning laser microscopy further confirmed that the oPDM-plus-PS-coated silicone catheters were almost free from bacterial colonization. Thus, a broad-spectrum antibiofilm composition comprising a GlmU inhibitor and protamine sulfate shows promise for use in anti-infective coatings for medical devices, including urinary catheters.
Microorganisms can attach to and colonize any biomaterial surface, putting patients at risk for local and systemic infections. More than 900,000 episodes of catheter-associated urinary tract infections occur annually in acute-care hospitals in the United States, accounting for 40% of all nosocomial infections and involving between 10 and 30% of patients with indwelling urinary catheters (30). Catheter-associated urinary tract infection prolongs the hospital stay between an estimated 2.4 and 4.5 days, with resultant increased healthcare costs (15, 16). Recent studies have shown that a wide range of persistent catheter-related infections may be related to the ability of bacteria to form biofilms (6, 28). Treatment of device-related infections with conventional antimicrobial agents frequently fails because microorganisms growing in biofilms are more tolerant or phenotypically resistant to antimicrobial agents than planktonic cells (24). The insensitivity of biofilm bacteria to antibiotics is a function of cell wall composition, surface structure, and phenotypic variation in enzymatic activity (8, 14). It has also been suggested that the negatively charged exopolysaccharide is very effective in protecting bacterial cells from cationic antibiotics by restricting their permeation (2).
In the last decade, several strategies to control biofilm growth on medical devices have been suggested, including using topical antimicrobial ointments, minimizing the length of time of catheterization, using catheters provided with a surgically implanted cuff (12), and coating the catheter lumen with antimicrobial agents (1, 7, 9, 19, 26, 27). Enzymes involved in bacterial cell wall synthesis could provide novel targets for the development of antibiofilm agents. One of those enzymes is N-acetyl-d-glucosamine-1-phosphate acetyltransferase (GlmU), which is involved in the biosynthesis of the activated nucleotide sugar UDP-GlcNAc, an essential precursor of peptidoglycans and lipopolysaccharides in gram-positive and gram-negative bacteria, respectively (25). Furthermore, UDP-GlcNAc is involved in the synthesis of the β-1,6-N-acetyl-d-glucosamine polysaccharide adhesin required for biofilm formation in Escherichia coli and Staphylococcus epidermidis (17). GlmU is a bifunctional enzyme with acetyltransferase and uridyltransferase activities. Its acetyltransferase activity is inactivated in the presence of thiol-specific reagents, such as iodoacetamide and N-substituted maleimides (21, 23). In the recent past, GlmU enzyme inhibitors, which belong to a thiol-specific reagent group, were reported to inactivate bacterial pathogens (11, 31).
There seems to be no published information on the antibiofilm activity of N-substituted maleimides. We determined the antibiofilm activity of GlmU inhibitors, which included iodoacetamide, N-ethylmaleimide, and maleimide analogs against catheter-associated uropathogens. Further, we developed a broad-spectrum antibiofilm composition comprised of a GlmU inhibitor and a cationic polypeptide, protamine sulfate (PS). PS has been shown to enhance the activity of certain antibiotics (26, 29). We also compared the antiadhesion effect of GlmU inhibitor-plus-PS-coated urinary catheters on P. aeruginosa and S. epidermidis with that of commercially available silver hydrogel and nitrofurazone coatings. The inhibitory effect of GlmU inhibitor-plus-PS coating against S. epidermidis colonization on urinary catheters was further confirmed by confocal scanning laser microscopy (CSLM).
MATERIALS AND METHODS
Chemicals.
The antibiofilm compounds used include GlmU inhibitors, such as iodoacetamide (IDA), N-ethylmaleimide (NEM), N,N′-(1,2-phenylene)dimaleimide (oPDM), N-(1-pyrenyl)maleimide (PyrM)m and N-phenyl maleimide (NPM). The reagents were dissolved in 10% dimethyl sulfoxide. PS was dissolved in distilled water. All the chemicals used were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO). The antibiofilm concentrations were determined empirically. The Alexa Fluor 488 wheat germ agglutinin (WGA) was purchased from Molecular Probes, Inc. (Eugene, OR).
Bacteria and culture conditions.
The bacterial cultures used in this study included catheter-associated uropathogenic Escherichia coli P18, Pseudomonas aeruginosa PA01, Staphylococcus epidermidis 1457, Klebsiella pneumoniae P30, Proteus mirabilis 6285, and Enterococcus faecalis 36171. All the strains were maintained at −80°C in 15% glycerol and recovered onto Luria-Bertani (LB) agar or tryptic soy agar (TSA; BD Diagnostic Systems, Sparks, MD). For inoculum preparation, an isolated colony was inoculated into LB broth, tryptic soy broth, or brain heart infusion (BHI) broth and incubated at 37°C for 16 to 18 h.
Biofilm assay.
Biofilms were assayed by crystal violet staining, as described previously (18). The overnight-grown cultures were diluted to 5% in colony-forming antigen medium and grown in 96-well microtiter plates (Corning Inc., New York). Biofilm growth was determined by measuring the absorbance at 630 nm. At least six replicates were conducted for each sample, and each experiment was performed at least three times. The results were calculated as averages and standard deviations from three or more experiments. Statistical analysis was performed with Student's t test. P values of ≤0.001 were considered statistically significant.
Susceptibility studies.
E. coli P18, K. pneumoniae, P. aeruginosa, P. mirabilis, E. faecalis, and S. epidermidis were tested for susceptibility to the oPDM-plus-PS combination using a disk diffusion assay (9). Each culture was spread on the surface of TSA plates. Sterile paper disks (6-mm diameter) were placed on the surface and impregnated with a combination of 50 μg of oPDM and 50 μg of PS. Plates were incubated at 37°C for 24 h. The diameters of zones of inhibition were recorded by subtracting the 6-mm diameter of the disk from each measurement at 24 h.
Catheters.
Uncoated silicon catheters (Tyco Health Care, Toronto, ON, Canada) and nitrofurazone (Release-NF; Rochester Medical Corp, Stewartville, MN)- and silver hydrogel (Bardex IC Lubricath; C. R. Bard, Covington, GA)-coated silicon catheters were obtained in sterile packaging. Silicone catheters were coated with the oPDM-plus-PS combination (10 μg/mm) by Biocompatibles UK Ltd. (Farnham, United Kingdom) (22). The antimicrobial catheters were used prior to their expiration date as indicated by the manufacturers. Catheters were sectioned with a scalpel to obtain 1-cm segments.
Bacterial adhesion assay.
The inoculum was prepared by diluting overnight- grown S. epidermidis and P. aeruginosa cultures to 1:10 in fresh BHI broth and incubated at 37°C for 2 h with shaking at 100 rpm. Catheter segments were immersed in artificial urine medium for 30 min (4). After 30 min, the catheters were removed and placed in 15-ml sterile culture tubes (17 by 100 mm), each containing 10 ml BHI broth. The tubes were inoculated with 100 μl of bacterial inoculum (1 × 107 cells/ml) and incubated in a water bath for 3 h at 37°C with shaking at 100 rpm. After incubation, the catheter segments were washed three times in saline and transferred into a sterile 2-ml tube containing 1 ml saline. Adherent bacteria were removed by sonication for 30 s, followed by vortexing for 1 min. The cells were serially diluted in saline and then plated onto LB agar plates. The plates were incubated at 37°C for 24 h, and colonies were counted.
Confocal microscopy.
Biofilm staining and CSLM were performed as described previously (5). Biofilm grown on silicone catheters was stained with wheat germ agglutinin, which selectively binds to N-acetylglucosamine and gives green fluorescence. The biofilm was observed using an Olympus IX-70 with an argon laser for excitation at 488 nm (green fluorescence). Images were captured and processed by using Fluoview software and Image Pro Plus software.
RESULTS
Antibiofilm activity of GlmU inhibitors against catheter-associated uropathogens.
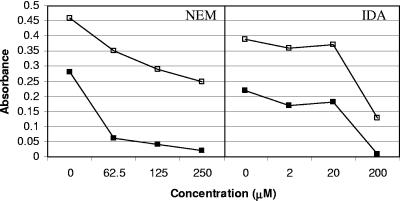
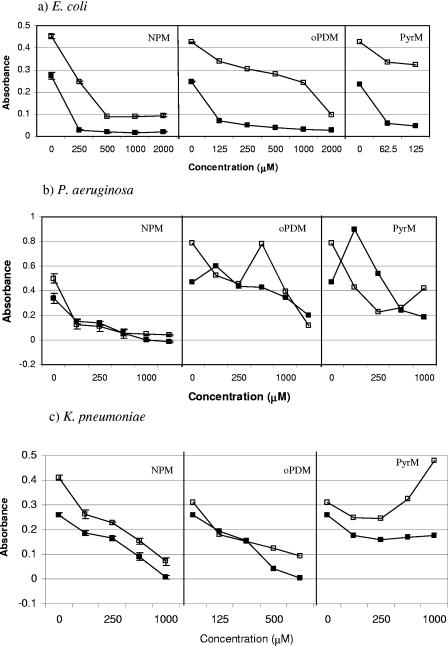
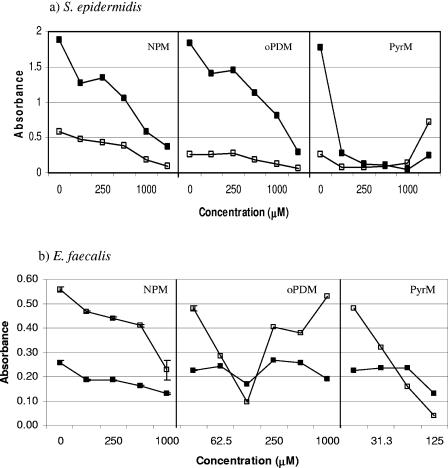
In this study, we tested the antibiofilm activity of GlmU inhibitors, such as NEM and IDA, against E. coli P18 isolated from a colonized urinary catheter using a quantitative biofilm assay method (18). It was observed that while NEM at a concentration of 62.5 μM was effective in reducing the biofilm formation by about 80%, 200 μM of IDA was required to achieve a similar reduction in E. coli P18 biofilm (Fig. 1). After establishing the fact that NEM is effective in reducing E. coli P18 biofilm, we tested its analogs, NPM, PyrM, and oPDM, against catheter-associated E. coli P18, P. aeruginosa, K. pneumoniae, S. epidermidis, and E. faecalis (Fig. 2 and 3). The three analogs reduced biofilm formation in both gram-negative and gram-positive uropathogens to various degrees, with the exception of P. aeruginosa and E. faecalis, which were less susceptible to oPDM and PyrM. Although NPM reduced P. aeruginosa biofilm formation to an undetectable level at 1,000 μM concentration, it had a limited effect on E. faecalis biofilm formation.
FIG. 1.
Effect of GlmU inhibitors on growth (□) and biofilm formation (▪) of E. coli P18 as determined by a microtiter plate assay. Error bars are not visible where the standard deviations are less than the area occupied by a given symbol.
FIG. 2.
Effect of GlmU inhibitors on growth (□) and biofilm formation (▪) of gram-positive catheter-associated pathogens (a) E. coli P18, (b) P. aeruginosa, and (c) K. pneumoniae as determined by a microtiter plate assay. Error bars are not visible where the standard deviations are less than the area occupied by a given symbol.
FIG. 3.
Effect of GlmU inhibitors on growth (□) and biofilm formation (▪) of gram-positive catheter-associated pathogens a) S. epidermidis and b) E. faecalis as determined by a microtiter plate assay. Error bars are not visible where the standard deviations are less than the area occupied by a given symbol.
Antimicrobial activity of oPDM-plus-PS combination against catheter-associated uropathogens.
In order to enhance the antimicrobial activity of NEM analogs and to develop a broad-spectrum antimicrobial composition, oPDM (50 μg/ml) was combined with protamine sulfate (50 μg/ml) and tested against catheter-associated uropathogens using an agar diffusion method (Table 1). The oPDM-plus-PS combination was effective against all the organisms tested, as evident from the zones of inhibition.
TABLE 1.
Susceptibility of catheter-associated uropathogens to combinations of oPDM and PS (50 μg/ml each) using agar diffusiona
| Uropathogen | Zone of inhibition (mm)b |
|---|---|
| E. coli P18 | 9.5 ± 0.71 |
| K. pneumoniae | 10.0 ± 1.41 |
| P. mirabilis | 9.0 ± 0 |
| P. aeruginosa | 9.5 ± 0.71 |
| E. faecalis | 11.5 ± 0.71 |
| S. epidermidis | 10.0 ± 1.41 |
Suspensions of bacteria (106 CFU/ml) were spread on TSA plates. oPDM and PS were used to impregnate paper disks on the agar surface. Plates were incubated at 37°C, and the zones of inhibition were measured the following day.
The values are means of triplicate determinations ± standard deviations.
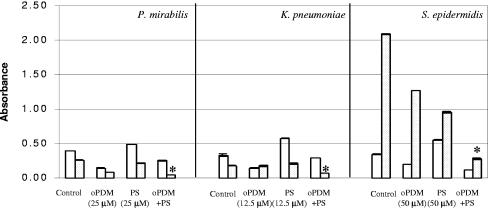
Effect of oPDM and PS alone and in combination on biofilm formation in catheter-associated uropathogens.
The oPDM-plus-PS combination was tested for antibiofilm activity against catheter-associated uropathogens using a standard quantitative biofilm assay method. PS in combination with oPDM showed a significant synergistic inhibitory effect on P. mirabilis, K. pneumoniae, and S. epidermidis biofilms (P < 0.001) (Fig. 4). S. epidermidis biofilm formation was reduced 10-fold when oPDM and PS were used together at a concentration of 50 μM each.
FIG. 4.
Effect of oPDM and PS alone and in combination on growth (□) and biofilm formation (░⃞) of P. mirabilis, S. epidermidis, and K. pneumoniae as determined by a microtiter plate assay. Error bars are not visible where the standard deviations are less than the area occupied by a given symbol. Asterisks indicate a significant difference (P < 0.001) between biofilm formation in the presence of the oPDM-plus-PS combination and that in the presence of the control and oPDM and PS alone.
In vitro adhesion of P. aeruginosa and S. epidermidis to oPDM-plus-PS-coated silicone catheters.
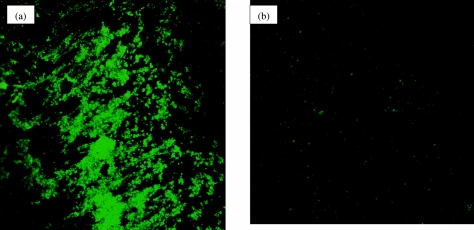
As the main objective of our research program is to develop an effective broad-spectrum antibiofilm catheter coating capable of impeding bacterial colonization in vivo, it was first necessary to document in vitro activity. Specifically, it was necessary to determine the antiadhesion effect of the oPDM-plus-PS-coated catheter. The effectiveness of oPDM-plus-PS coating compared to that of commercially available silver hydrogel and nitrofurazone coatings in inhibiting the adherence of P. aeruginosa and S. epidermidis to catheters was determined using a bacterial adhesion assay. Silicone catheter segments coated with oPDM plus PS showed 40.9% (59.1% inhibition) and 35.6% (64.4% inhibition) adhesion of P. aeruginosa and S. epidermidis, respectively (Table 2). oPDM-plus-PS coating was superior to silver hydrogel coating in inhibiting P. aeruginosa and S. epidermidis adherence to catheters. Furthermore, this combination was more effective than nitrofurazone coating in inhibiting the adherence of P. aeruginosa to catheters. The effect of oPDM-plus-PS coating on S. epidermidis biofilm formation on silicone catheters was also determined using CSLM. The confocal image showed uniform colonization on uncoated catheters, but the catheters coated with oPDM plus PS showed little or no colonization (Fig. 5).
TABLE 2.
Effect of catheter coatings on bacterial adherencea
| Catheter coating | % Adherenceb |
|
|---|---|---|
| P. aeruginosa | S. epidermidis | |
| None | 100 | 100 |
| Nitrofurazone | 78.4 | 1.5 |
| Silver hydrogel | 73.9 | 78 |
| oPDM + PS | 40.9 | 35.6 |
Catheter segments (1-cm length) were exposed to an inoculum of 1 × 107 cells/ml for 3 h at 37°C.
The percent adherence of each organism was calculated relative to that of uncoated segments.
FIG. 5.
Confocal images of S. epidermidis biofilm formation on urinary catheters. a) Uncoated. b) Coated with oPDM plus PS.
DISCUSSION
In this study, we evaluated the antibiofilm activity of GlmU inhibitors against both gram-positive and gram-negative catheter-associated uropathogens. GlmU inhibitors belong to a maleimide class of compounds that are quite reactive against sulfhydryl-containing enzymes (23) and have poor specificity, making them broad-spectrum antimicrobial compounds (11, 31). Although NPM, an analog of NEM, showed antibiofilm activity against all the uropathogens tested (Fig. 2 and 3), oPDM and PyrM were less effective against P. aeruginosa and E. faecalis. This observation is consistent with the higher MIC of maleimide (128 μg/ml) reported for P. aeruginosa (31).
In addition to its antimicrobial properties, protamine sulfate has been shown to enhance the activity of antibiotics (26, 29). As oPDM alone appeared to be less effective against P. aeruginosa and E. faecalis (Fig. 2 and 3), we combined it with PS to increase its antibiofilm activity (Fig. 4). Furthermore, the results of the disk diffusion assay showed the broad-spectrum antimicrobial activity of the oPDM-plus-PS combination (Table 1). The observed synergistic inhibitory effect of oPDM plus PS against P. mirabilis, K. pneumoniae, and S. epidermidis biofilm formation (Fig. 4) may be due to an alteration in membrane permeability and dilation of ionic channels by protamine sulfate, which facilitates the transport of antimicrobial compounds to the cytoplasm (3). Protamine sulfate not only improved the antibiofilm activity of oPDM but also helped to reduce the effective concentrations of GlmU inhibitors to as low as 12.5 μM.
A second major finding of this study was the comparatively poor antimicrobial activity of the silver hydrogel catheter. This catheter failed to inhibit the colonization of P. aeruginosa and S. epidermidis, which are pathogens of great concern in catheterized patients (10, 13). The oPDM-plus-PS coating was more effective than silver hydrogel coating in inhibiting the adherence of P. aeruginosa and S. epidermidis to catheters (Table 2). Furthermore, this combination was superior to nitrofurazone coating in inhibiting P. aeruginosa adherence to catheters (Table 2). This is in agreement with the findings of Johnson et al. (19, 20) that both silver hydrogel and nitrofurazone coatings are not effective against P. aeruginosa. The adhesion assay results were further confirmed by CSLM. The silicone catheters coated with oPDM plus PS showed little or no colonization of S. epidermidis.
We determined the antibiofilm activities of both the active agents and the coated catheters against clinical isolates from patients with indwelling urinary catheters. Therefore, these experiments examined an authentic sample of the typical flora of catheter-associated infections. However, it is acknowledged that the results need to be verified with clinical isolates obtained from different populations, which would increase confidence in the present study's findings. Also, further studies are required to assess the in vivo efficacy of the proposed coating in an animal model of catheter-associated infection.
In summary, we found that the GlmU inhibitors were broadly effective against biofilm formation in catheter-associated uropathogens. To our knowledge, this is the first report on the antibiofilm activity of N-substituted maleimides. Protamine sulfate in combination with a GlmU inhibitor showed synergistic antibiofilm activity against uropathogens. The oPDM-plus-PS-coated catheter was superior to silver hydrogel- and nitrofurazone-coated catheters in preventing the bacterial colonization. It is evident from our findings that the oPDM-plus-PS composition can be used to develop anti-infective coatings for medical devices such as catheters to prevent device-related nosocomial infections and to reduce antibiotic use in the current era of emerging multidrug antibiotic resistance.
Acknowledgments
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for awarding the Industrial Research Fellowship (IRF) to N. Yakandawala and for financial assistance from the National Research Council-Industrial Research Assistance Program (NRC-IRAP), Canada.
REFERENCES
- 1.Ahearn, D. G., D. T. Grace, M. J. Jennings, R. N. Borazjani, K. J. Boles, L. J. Rose, R. B. Simmons, and E. N. Ahanotu. 2000. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infection. Curr. Microbiol. 41:120-125. [DOI] [PubMed] [Google Scholar]
- 2.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation of Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antohi, S., and A. Popescu. 1979. Lethal effect of protamine and histone on competent Bacillus subtilis cells. Mol. Gen. Genet. 170:345-349. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, T., and C. W. Keevil. 1997. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 24:203-206. [DOI] [PubMed] [Google Scholar]
- 5.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Darouiche, R. O., I. I. Raad, S. O. Heard, J. I. Thornby, O. C. Wenker, A. Gabrielli, J. Berg, N. Khardori, H. Hanna, R. Hachem, R. L. Harris, and G. Mayhall. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 9.Domenico, P., L. Baldassarri, P. E. Schoch, K. Kaehler, M. Sasatsu, and B. A. Cunha. 2001. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrob. Agents Chemother. 45:1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filho, V. C., T. Pinhbiro, R. J. Nunes, and R. A. Yunes. 1994. Antibacterial activity of N-phenylmaleimides, N-phenylsuccinimides and related compounds. Structure-activity relationships. Farmaco 49:675-677. [PubMed] [Google Scholar]
- 12.Flowers, R. H., K. J. Schwenzer, R. F. Kopel, M. J. Fish, S. I. Tucker, and B. M. Farr. 1989. Efficacy of an attachable subcutaneous cuff for the prevention of intravascular catheter-related infection. A randomized controlled trial. JAMA 261:878-883. [PubMed] [Google Scholar]
- 13.Furusawa, T., T. Uete, T. Kawada, and A. Okuma. 1986. Resistance to cefsulodin and gentamicin in five areas of Japan between 1980 and 1983. J. Antimicrob. Chemother. 17:755-762. [DOI] [PubMed] [Google Scholar]
- 14.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 15.Givens, C. D., and R. P. Wenzel. 1980. Catheter-associated urinary tract infections in surgical patients: a controlled study of the excess morbidity and costs. J. Urol. 124:646-648. [DOI] [PubMed] [Google Scholar]
- 16.Green, M. S., E. Rubinstein, and P. Amit. 1982. Estimating the effects of nosocomial infections on the length of hospitalization. J. Infect. Dis. 145:667-672. [DOI] [PubMed] [Google Scholar]
- 17.Itoh, Y., X. Wang, B. J. Hinnebusch, J. F. Preston III, and T. Romeo. 2005. Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol. 187:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Bioiflm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, J. R., P. Delavari, and M. Azar. 1999. Activities of a nitrofurazone-containing urinary catheter and a silver hydrogel catheter against multidrug-resistant bacteria characteristic of catheter-associated urinary tract infection. Antimicrob. Agents Chemother. 43:2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, J. R., T. Berggren, and A. J. Conway. 1993. Activity of a nitrofurazone matrix urinary catheter against catheter-associated uropathogens. Antimicrob. Agents Chemother. 37:2033-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mengin-Lecreulx, D., and J. van Heijenoort. 1994. Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J. Bacteriol. 176:5788-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy, E. F., J. R. Lu, J. Brewer, J. Russell, and J. Penfold. 1999. The reduced adsorption of proteins at the phosphoryl choline incorporated polymer-water interface. Langmuir 15:1313-1322. [Google Scholar]
- 23.Pompeo, F., J. van Heijenoort, and D. Mengin-Lecreulx. 1998. Probing the role of cysteine residues in glucosamine-1-phosphate acetyltranferase activity of the bifunctional GlmU protein from Escherichia coli: site-directed mutagenesis and characterization of the mutant enzymes. J. Bacteriol. 180:4799-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Presterl, E., A. J. Grisold, S. Reichmann, A. M. Hirschl, A. Georgopoulos, and W. Graninger. 2005. Viridans streptococci in endocarditis and neutropenic sepsis: biofilm formation and effects of antibiotics. J. Antimicrob. Chemother. 55:45-50. [DOI] [PubMed] [Google Scholar]
- 25.Raetz, C. R. H. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p. 1035-1063. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanki, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 26.Sobho, F., A. E. Khoury, A. C. Zamboni, D. Davidson, and M. W. Mittelman. 1995. Effects of ciprofloxacin and protamine sulfate combinations against catheter-associated Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 39:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stickler, D. J. 1996. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 94:293-305. [Google Scholar]
- 28.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 29.Teichman, J. M. H., V. E. Abraham, P. C. Stein, and C. L. Parsons. 1994. Protamine sulfate and vancomycin are synergistic against Staphylococcus epidermidis prosthesis infection in vivo. J. Urol. 152:213-216. [DOI] [PubMed] [Google Scholar]
- 30.Warren, J. W. 1987. Catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 1:823-854. [PubMed] [Google Scholar]
- 31.Zentz, F., A. Valla, R. L. Guillou, R. Labia, A. G. Mathot, and D. Sirot. 2002. Synthesis and antimicrobial activities of N-substituted imides. Farmaco 57:421-426. [DOI] [PubMed] [Google Scholar]