Abstract
A ca. 150-kbp Vibrio cholerae O1 biotype El Tor plasmid includes blaCTX-M-2 and a variant of aac(6′)-Ib within InV117, an orf513-bearing class 1 integron. InV117 is linked to a tnp1696 module in which IRl carries an insertion of IS4321R. The complete structure could be a potential mobile element.
A Vibrio cholerae O1 biotype El Tor isolate from Argentina harbors a conjugative ca. 150-kbp plasmid, named pAS1, which includes blaCTX-M-2 and genes coding for resistance to non-β-lactam antibiotics, including amikacin (12). The blaCTX-M-2 gene, commonly found in clinical isolates from South America, is most often found in orf513-bearing integrons such as InS21, In35, or In116 (1, 2, 5, 14). Our analysis of pAS1 indicated that the resistance genes are included in an orf513-bearing integron, InV117, which is linked to a transposition module.
V. cholerae O1 El Tor M1516 (Ogawa serotype), isolated in 1993 during the course of the second Argentinean cholera season, has been described previously (12). Escherichia coli M3099 was obtained by transfer of pAS1 from V. cholerae O1 El Tor M1516 to E. coli ER1793 (New England Biolabs, Beverly, Mass.) by conjugation (12). Transformation of E. coli cells was performed as described previously, and bacteria were cultured in Lennox Luria broth (15). PCR mapping was carried out using the appropriate primers as described before (7). Amplicons obtained by PCR for sequencing were either sequenced directly or sequenced after cloning into pGEM-T Easy (Promega, Madison, Wis.). DNA sequencing was performed on an ABI PRISM 3100 sequencer (Applied Biosystems, Foster City, CA) using the BigDye terminator method. Both strands of the whole DNA fragment described here were sequenced. Amino acid sequence analysis was performed using the CLUSTALW program (16). The N terminus of AAC(6′)-Ib was determined as described before (4), but the Edman degradation was carried out at the LANAIS-PRO facility (University of Buenos Aires).
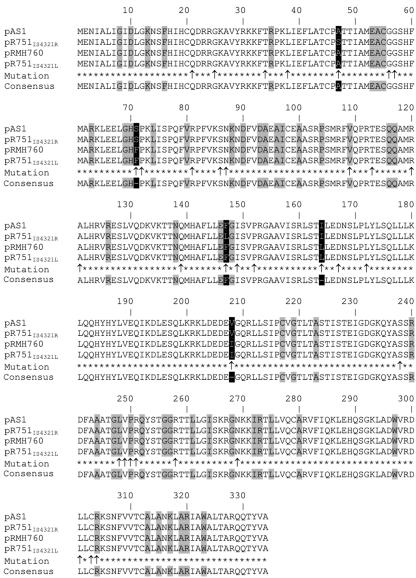
Sequencing and analysis of a 15,723-bp pAS1 DNA fragment confirmed the identity of the CTX-M-type β-lactamase blaCTX-M-2 gene and a copy of aac(6′)-Ib. PCR-mapping experiments using DNA from V. cholerae M1516 confirmed that no rearrangements occurred during the transfer of pAS1 from V. cholerae M1516 to E. coli ER1793 (not shown). The aac(6′)-Ib gene, present as a gene cassette with the usual attC locus (18), was cloned and expressed in E. coli from its natural promoter. The N terminus of the AAC(6′)-Ib protein was LRSSKTKLGITKY, different from those coded for by other variants of the gene (Fig. 1). AAC(6′)-Ib variants are the most prevalent AAC(6′) type I aminoglycoside acetyltransferase among various gram-negative microorganisms (13, 18, 19). A factor contributing to this predominance may be a high flexibility in the structural requirements at the N terminus (see Fig. 1) (3).
FIG. 1.
Amino acid sequence alignment of AAC(6′)-Ib variants. Alignments were generated using the CLUSTALW program (16). The accession numbers for each sequence are shown to the left. The amino acids identified by Edman degradation in the pAS1 variant are boxed. Vc, V. cholerae.
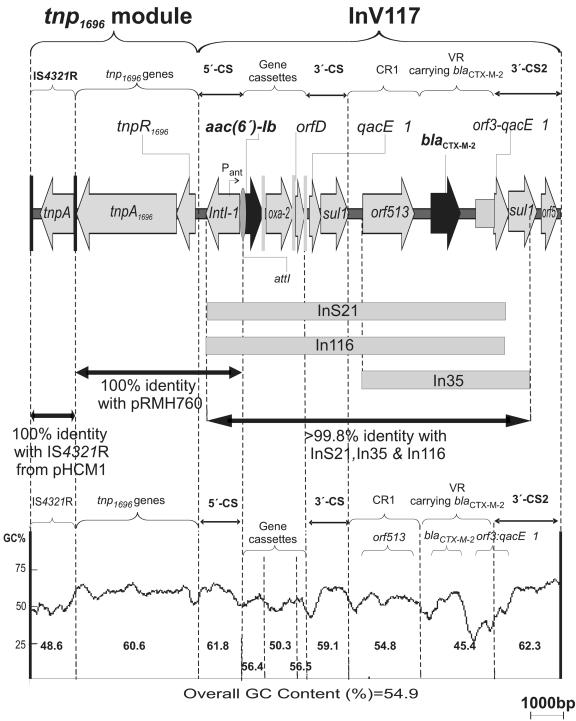
Analysis of the nucleotide sequence of the genetic environment of blaCTX-M-2 and aac(6′)-Ib showed the presence of an orf513-bearing class 1 integron, named InV117, highly related to InS21, In35, and In116 (2, 5, 6, 14). Alignments carried out between InV117 and the sequenced regions of In35, InS21, and In116 (Fig. 2) showed 99.98%, 99.88%, and 99.86% identity, indicating a common origin. The differences found between the InV117 sequence and each of the other three integrons are due to single-nucleotide substitutions. A detailed diagram of the InV117 structure, a G+C content plot, and some of its characteristics are shown in Fig. 2.
FIG. 2.
Genetic structure of InV117 and the adjacent region. The diagram shows the genes and other elements present in the 15,723-bp pAS1 DNA fragment sequenced. The arrows indicate genes and their direction of transcription. Black and gray vertical bars represent inverted repeats and attC loci. The gray oval represents attI. Relevant regions are grouped and named: CR1, common region 1 (10); VR, variable region. Thick arrows with double arrowheads indicate the degree of homology with the specified element. Gray bars show the sequenced portions of integrons InS21, In116, and In35; >99.8% identity refers to identity between the sequenced portion of each integron and the cognate region in InV117. The GC content plot was generated using a window size of 300 bp, except for gene cassettes where the window size used was 50. Numbers between dotted lines indicate the GC content for each region.
InV117 is associated with a potentially mobile element. Upstream of the integron, there is a module that includes transposition-related genes and shares homology with the tnp1696 module, a genetic structure found adjacent to In34 in pRMH760 (9) (Fig. 2). The tnp1696 module includes a copy of the Tn1696 tnpA and tnpR genes with the insertion of an IS4321-like element within IRl (9). IS4321pAS1 and the tnp1696 module showed different percents GC, suggesting different origins (Fig. 2). While the tnpApAS1 and tnpRpAS1 genes were identical to tnpApRMH760 and tnpRpRMH760, the sequence of IS4321pAS1 shares higher homology with another IS4321 variant, IS4321pHCM1, as compared to the homology shared with IS4321pRMH760 (100% and 97% identity, respectively) (Fig. 2) (8, 9, 11). The finding of an IS4321 inserted within a Tn1696 inverted repeat is in keeping with the previous observation that IS4321 and IS5075, both members of the IS1111 family, target terminal inverted repeats (TIRs) of transposons belonging to the Tn501/Tn21 family (11). Two nonidentical copies of IS4321 (IS4321L and IS4321R) are located at the ends of the composite transposon Tn4321, found in the R751 plasmid (17). IS4321pAS1 and IS4321pHCM1 share higher identity (99%) with IS4321R than with IS4321L (96%). On the other hand, IS4321pRMH760 is more closely related to IS4321L than to IS4321R. Therefore, it was of interest that in the pAS1 structure, the sequence of the TIR of tnp1696 is interrupted by an IS4321R copy, while so far all cases described showed an insertion of an IS4321L or an incomplete IS4321R within the TIR (11). Although the insertion of IS4321 within the TIR would most probably inactivate the transposition capacity of Tn1696, the ability of IS4321 to reconstitute the target TIR by precise excision (11) should reverse this inactivation. A comparison of the nucleotide sequences of the transposase genes encoded by IS4321pAS1, IS4321pRMH760, and IS4321pR751 showed several differences. IS4321pAS1 and IS4321pRMH760 showed 97.1% identity at the nucleotide level. Although 33 out of 38 different nucleotides found between both insertion sequences were located within the tnpA gene, most of them did not result in an amino acid change (Fig. 3). High degrees of conservation were also observed when comparing the nucleotide sequences of IS4321pAS1 and IS4321RpR751 (99.69% identity) or IS4321pAS1 and IS4321LpR751 (97.01% identity). Furthermore, the few amino acid substitutions found among the different transposase protein versions did not occur at positions highly conserved among this family of transposases (Fig. 3). Our observations suggest that the IS4321pAS1 transposase is well conserved, and it is most probably functional. Therefore, the TIR could be regenerated by precise excision of IS4321pAS1. An attractive theory has recently been proposed: Tn21 family transposases could recognize the IS4321 ends, and as a result, when IS4321 inserts into one end of a Tn21 family transposon, it may promote the transposition of this element along with the IS4321 (17). If this is the case, the whole structure, including the tnp1696 module and InV117, could potentially be able to transpose.
FIG. 3.
Amino acid sequence alignment of TnpA variants. The amino acid sequences encoded by the IS4321RpAS1, IS4321RpR751, IS4321LpRMH760, and IS4321LpR751 tnpA genes were aligned using the CLUSTALW program (16). Differences with respect to pAS1 are marked in white letters on a black background. Conserved residues among IS1111 family transposases are highlighted in gray (11). Arrows indicate positions with different codons.
Nucleotide sequence accession number.
The nucleotide sequence data for pAS1 reported in this work have been submitted to GenBank under accession no. DQ310703.
Acknowledgments
We thank Marta Bravo and María Jimena Ortega for the DNA nucleotide sequencing and Susana Linskens for Edman N-terminal sequencing.
This work was supported by National Institutes of Health grant 1R15AI47115-02 to M.E.T.; PICT 8266, Agencia Nacional de Promoción Científica y Tecnológica, Argentina, to A.Z.; and X-811, UBACyT Programación Científica from the Universidad de Buenos Aires, to A.Z. A.J.C.S.B. was supported by Fundación Ciencias Exactas y Naturales and the University of Buenos Aires.
REFERENCES
- 1.Arduino, S. M., M. Catalano, B. E. Orman, P. H. Roy, and D. Centron. 2003. Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob. Agents Chemother. 47:3945-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arduino, S. M., P. H. Roy, G. A. Jacoby, B. E. Orman, S. A. Pineiro, and D. Centron. 2002. blaCTX-M-2 is located in an unusual class 1 integron (In35) which includes Orf513. Antimicrob. Agents Chemother. 46:2303-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casin, I., F. Bordon, P. Bertin, A. Coutrot, I. Podglajen, R. Brasseur, and E. Collatz. 1998. Aminoglycoside 6′-N-acetyltransferase variants of the Ib type with altered substrate profile in clinical isolates of Enterobacter cloacae and Citrobacter freundii. Antimicrob. Agents Chemother. 42:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dery, K. J., B. Søballe, M. S. L. Witherspoon, D. Bui, R. Koch, D. J. Sherratt, and M. E. Tolmasky. 2003. The aminoglycoside 6′-N-acetyltransferase type Ib encoded by Tn1331 is evenly distributed within the cell's cytoplasm. Antimicrob. Agents Chemother. 47:2897-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Conza, J., J. A. Ayala, P. Power, M. Mollerach, and G. Gutkind. 2002. Novel class 1 integron (InS21) carrying blaCTX-M-2 in Salmonella enterica serovar Infantis. Antimicrob. Agents Chemother. 46:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckert, C., V. Gautier, and G. Arlet. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 57:14-23. [DOI] [PubMed] [Google Scholar]
- 7.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 9.Partridge, S. R., and R. M. Hall. 2004. Complex multiple antibiotic and mercury resistance region derived from the r-det of NR1 (R100). Antimicrob. Agents Chemother. 48:4250-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge, S. R., and R. M. Hall. 2003. In34, a complex In5 family class 1 integron containing orf513 and dfrA10. Antimicrob. Agents Chemother. 47:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partridge, S. R., and R. M. Hall. 2003. The IS1111 family members IS4321 and IS5075 have subterminal inverted repeats and target the terminal inverted repeats of Tn21 family transposons. J. Bacteriol. 185:6371-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petroni, A., A. Corso, R. Melano, M. L. Cacace, A. M. Bru, A. Rossi, and M. Galas. 2002. Plasmidic extended-spectrum β-lactamases in Vibrio cholerae O1 El Tor isolates in Argentina. Antimicrob. Agents Chemother. 46:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourreza, A., M. Witherspoon, J. Fox, J. Newmark, D. Bui, and M. E. Tolmasky. 2005. Mutagenesis analysis of a conserved region involved in acetyl coenzyme A binding in the aminoglycoside 6′-N-acetyltransferase type Ib encoded by plasmid pJHCMW1. Antimicrob. Agents Chemother. 49:2979-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power, P., M. Galleni, J. Di Conza, J. A. Ayala, and G. Gutkind. 2005. Description of In116, the first blaCTX-M-2-containing complex class 1 integron found in Morganella morganii isolates from Buenos Aires, Argentina. J. Antimicrob. Chemother. 55:461-465. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Thompson, J., D. Higgins, and T. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorsted, P. B., D. P. Macartney, P. Akhtar, A. S. Haines, N. Ali, P. Davidson, T. Stafford, M. J. Pocklington, W. Pansegrau, B. M. Wilkins, E. Lanka, and C. M. Thomas. 1998. Complete sequence of the IncPbeta plasmid R751: implications for evolution and organisation of the IncP backbone. J. Mol. Biol. 282:969-990. [DOI] [PubMed] [Google Scholar]
- 18.Tolmasky, M. E. 2000. Bacterial resistance to aminoglycosides and beta-lactams: the Tn1331 transposon paradigm. Front. Biosci. 5:D20-D29. [DOI] [PubMed] [Google Scholar]
- 19.Vakulenko, S. B., and S. Mobashery. 2003. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 16:430-450. [DOI] [PMC free article] [PubMed] [Google Scholar]