Abstract
Tetra-acetamide pyrroloquinazolinediamine (PQD-A4) and bis-ethylcarbamyl pyrroloquinazolinediamine (PQD-BE) are new derivatives of pyrroloquinazolinediamine (PQD) and are being investigated as potential chemotherapeutic agents for the treatment of malaria. Comparative studies to assess the therapeutic indices of PQD-A4, PQD-BE, and PQD were conducted in Plasmodium berghei-infected rats following daily intragastric dosing for three consecutive days. Artesunate (AS), a standard drug for treatment of severe malaria, was used as a comparator. The minimum doses required to clear malaria parasitemia were 156 μmol/kg of body weight for AS and 2.4 μmol/kg for PQD, PQD-4A, and PQD-BE. The maximum tolerated dose (MTD) of AS was 625 μmol/kg, and its therapeutic index was calculated to be 4. The MTDs of PQD-A4, PQD-BE, and PQD were found to be 190, 77, and 24 μmol/kg, respectively, yielding therapeutic indices of 80, 32, and 10, respectively. Although PQD-A4 and PQD-BE are only half as potent as PQD based on their curative effects, the two new derivatives, PQD-4A and PQD-BE, are 8.0-fold and 3.2-fold safer, respectively, than their parent compound when they are dosed for three consecutive days. Oral PQD-A4 and PQD-BE are 44 to 70 times more potent on an mg basis than intravenous AS. As assessed from the therapeutic index over 3 days, PQD-A4, PQD-BE, and PQD administered orally are 20.0, 8.0, and 2.5 times safer than AS given intravenously. The results indicate that PQD-4A is a promising candidate for antimalarial treatment.
Malaria is a life-threatening parasitic infection caused by one of four Plasmodium species. Approximately 40% of the world's population, mostly those living in the world's poorest countries, is at risk of this disease. Each year, there are 300 million to 500 million new Plasmodium infections, resulting in 1.5 million to 2.7 million deaths. Furthermore, malaria is a major threat to travelers to the tropics, and the U.S. military had more casualties from malaria than from combat in every campaign of the 20th century (18). There are no effective malaria vaccines, and the efficacies of antimalarial drugs continue to decrease as a consequence of the emergence of drug-resistant parasites. In addition, the first-line antimalarial drugs, such as quinine, quinidine, and mefloquine (18), are still in use and are reported to have from mild to severe adverse effects (7, 10, 11, 20). Fortunately, many academic centers and organizations, including the U.S. Army, are seeking new antimalarial products (26).
Pyrroloquinazolinediamine (PQD) and its derivatives are reported to possess anticancer, antimicrobial, and antimalarial activities (21). PQD and derivatives of it are very active antimalarial agents in vivo and in vitro (6, 12, 16, 21). Some derivatives were the most potent antimalarials discovered at the Walter Reed Army Institute of Research (WRAIR), with 50% inhibitory concentrations <0.01 ng/ml and excellent activity against Plasmodium falciparum strains resistant to pyrimethamine (unpublished data). However, PQD also exhibited high host toxicity, with a 50% lethal dose in mice of less than 20 mg/kg of body weight when it was administered subcutaneously and causing deaths in Aotus monkeys at doses less than 2 mg/kg (unpublished data). This low therapeutic index of PQD severely limits its potential value as an antimalarial agent. Nevertheless, the high efficacy prompted us to synthesize new, safer derivatives.
To overcome the toxicity and solubility problems of PQD, several derivatives were prepared; and two of these derivatives, tetra-acetamide pyrroloquinazolinediamine (PQD-A4) and bis-ethylcarbamyl pyrroloquinazolinediamine (PQD-BE), were more potent and less toxic than the parent compound, PQD (Fig. 1). This compound not only displayed high in vitro activity against P. falciparum, with a 50% inhibitory concentration of 0.01 ng/ml, but also was highly active against P. berghei in a rodent model, with a 100% curative oral dose between 0.1 and 4 mg/kg. These two derivatives were also highly active in Aotus monkeys (oral curative dose, 1 mg/kg). Furthermore, PQD-A4 and PQD-BE showed no host toxicity at oral doses of 3 mg/kg/day for 3 days in Aotus monkeys and 10 mg/kg/day for 7 days in Rhesus monkeys (13).
FIG. 1.
Chemical structures of PQD-A4, PQD-BE, PQD, and AS.
It has recently been demonstrated that PQD-A4 and PQD-BE are prodrugs of PQD (25). The prodrug approach was developed to overcome the physical and pharmacological shortcomings of various therapeutic classes of agents (22). A prodrug undergoes a predictable metabolic activation before it exhibits its pharmacological effects in a target tissue (25). The derivatives of PQD are targeted prodrugs, in which the goal was an improvement in the therapeutic index. In order to reduce the toxicity of PQD, the analogs were designed to essentially provide a slow release of the parent drug (14). This approach successfully reduced the toxicity of the PQD derivatives in this study, even though the prodrugs themselves did not have improved efficacy.
The therapeutic index (the ratio of the effective dose/toxic dose) is a key criterion for candidate drug selection, and the Food and Drug Administration recommends that therapeutic-index assessments (efficacy and toxicity studies) be carried out with the same animal species, as cross-species scaling is often inaccurate (17). A rat model of malaria which resembles malaria in humans in terms of its symptoms, hyperparasitemia, and lethality (5, 9, 30) has successfully been developed in our laboratory for efficacy and toxicity studies (24, 31).
The rats used in this model were infected with the P. berghei ANKA strain. The result is different from that seen in mice, where untreated infection is always lethal. In this model, the rats sometimes develop a nonlethal infection with occasional self-clearance, as in humans. Parasitemia increased through days 3 to 11, with peak levels reaching 31% in most cases, followed (days 12 to 14) by self-clearance in 80% of the animals (approximately 17.9% mortality) to no parasitemia by day 21. Hyperparasitemia in the rat model (21 to 87%) is the same as that in the mouse (18 to 92%) (1, 28). However, the rat model displays a longer therapeutic time window of 5 to 7 days when effective therapy can be started (24, 31) rather than the 2 to 4 days in the P. berghei mouse model (8, 23). Although P. berghei has the limitation for human-specific complications such as cerebral malaria, rats infected with P. berghei ANKA show some symptoms similar to those in severe malaria in humans, for instance, hemolytic anemia, renal failure, hypoglycemia, hyperlactatemia, and metabolic acidosis, which develop after approximately 14 days of infection (15). One additional advantage to the rat model is that it is much easier to perform multiple studies, such as toxicity, pharmacokinetic, and pharmacodynamic studies, which require multiple blood samplings that cannot be performed with mice.
Aotus or Saimiri monkeys are World Health Organization-recommended primate species for studies of human malaria, and they can both be infected with P. falciparum (3). Healthy and splenectomized animals are susceptible to infection (19); the intact ones are able to keep parasitemia at lower levels for several days as a nonlethal pattern, but they develop complications such as severe anemia. Splenectomized monkeys develop higher levels of parasitemia and die (2, 27). The therapeutic time window for intact or splenectomized monkey models of P. falciparum (4, 29) infection is 0.4 to 3 days, with an average of about 2 days. However, the monkey model is not optimal for severe human malaria research because of the low level of parasitemia (<3%) in intact monkeys, and neither major complications nor immunoreactions are seen in the splenectomized monkeys (3). Although the splenectomized monkey model is often used in severe malaria research, the therapeutic time window is usually too short to treat the animals before they die from hyperparasitemia. Therefore, the rat model is more suitable for some aspects of severe malaria research than mouse or monkey models.
The present study assessed the therapeutic index and efficacy of the PQD derivatives PQD-A4 and PQD-BE in P. berghei ANKA-infected rats, an animal model that has close similarity to human severe malaria in terms of hyperparasitemia, anemia, renal failure, and mortality. The lead compound (PQD) and sodium artesunate (AS) were used as controls in the study.
MATERIALS AND METHODS
Materials and drugs.
The lead pyrroloquinazolinediamine derivative, PQD, was prepared by ChemPacific Inc. (Baltimore, MD). PQD-A4 and PQD-BE (Fig. 1) were synthesized in the Department of Medicinal Chemistry, Walter Reed Army Institute of Research, Silver Spring, MD. The carboxymethyl cellulose-Tween 80-0.9% saline solution was purchased from Abbott Laboratories (Chicago, IL). Sodium citrate, heparin, d-glucose, glycerol, and methanol were purchased from Sigma Chemical Co. Hema 3 stain was obtained from Fisher Scientific Co.
Oral formulation and administration.
Suspensions of PQD-A4, PQD-BE, and PQD were prepared fresh daily in 1% (wt/vol) carboxymethyl cellulose in distilled water by use of a Sonics Vibra Cell sonicator (Sonics & Materials, Inc., Danbury, CT), which was programmed to run with 5-s pulses and 5-s standbys for 8 min. To the suspension, 0.2% (vol/vol) of Tween 80 was added and the suspension was resonicated for another 2 min. The maximum concentration of the three test compounds was 50 mg/ml. The final suspension was checked for the size of the PQD-A4 particles by using a Horiba LA-930 light-scattering particle size and distribution analyzer connected to a personal computer.
Prior to each run for determination of the particle size, the sonicator was rinsed with pure water to remove any excess substances and an autoblank was performed. Occasionally, the accumulation of contaminants from past samples required comprehensive cleaning of the instrument. Particles of 6.4 to 17.8 μm of the three drugs were used in the dose-range, efficacy, and tolerant dose studies. Drugs were administered via gastric gavage. The vehicle (1% carboxymethyl cellulose suspension) was administered at the same volume, duration, and route as the test compounds.
Animals.
The ANKA strain of P. berghei used in this study was adapted from a mouse strain for use in rates by three successive 4-week passages through 7-week-old rats. Parasitized blood from these animals was cryopreserved in a large batch and used to inoculate donor animals for the efficacy and toxicity experiments. Seven-week old P. berghei-infected and uninfected Sprague-Dawley rats (body weight, 186 to 213 g) were randomly assigned into study groups of 6 or 10 animals each. The animal protocol was approved by the IACUC, WRAIR. The research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and it adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (26a).
All animals were quarantined (stabilized) for at least 7 days prior to infection. Rats were housed individually, with food and water supplied ad libitum. The rats were inoculated intraperitoneally with cryopreserved P. berghei-infected rat blood (2 × 107 parasitized erythrocytes/rat in 0.5 ml glucose-citrate solution) obtained from donor rats infected 1 week earlier with cryopreserved parasites. Two pretreatment smears were taken from all animals for parasitemia analysis. Animals with >4% parasitemia were selected for efficacy and maximum tolerated dose (MTD) studies. Eighteen posttreatment smears were obtained from each rat at 0, 3, 5, 8, and 12 h on day 6 and at 0, 3, and 6 h on days 7 and 8. From day 9 until day 28, blood smears were obtained from each animal once daily. Infected rats were used for efficacy, toxicity, and therapeutic-index studies.
Efficacy experiments.
Rats with malaria (without delayed infection) randomized into groups of five to seven were given PQD-A4, PQD-BE, PQD, or AS (drug vehicle and various doses) intragastrically or intravenously (1.0 ml drug solution/kg) on days 6, 7, and 8 postinfection. For high oral doses (95 μmol/kg), 2.0 ml/kg of the appropriate drug dilution (at concentrations of 95 μmol drug base/ml) was administered orally. The composition and preparation of the drug vehicles used are described above. The general experimental design was similar to that of previously published efficacy studies with weanling rats (24). The general health status of the animals was monitored daily. Unless indicated otherwise, parasitemia was determined daily during days 6 through 12 postadministration and every other day or every third day thereafter. The experiments were terminated on day 28 (as by this time surviving rats would have cleared their infections).
MTD-defining experiments.
Since the selection of dosage is one of the most critical issues to the success of the study, a preliminary dosage-defining test was performed for each new candidate drug prior to the definitive stage. This pilot test aimed to assess an appropriate minimum toxic dose and a maximum lethal dose of the selected compound(s) with oral or intravenous administrations in male rats, similar to the efficacy study. Three to five doses likely to encompass the MTD were tested with a small number of animals (four per group). Therefore, about 12 to 20 male rats per drug were included in the test. Death and other toxic signs identified by clinical observation and pathology were noted. The MTD was estimated and adjusted for definitive studies.
Data analysis and therapeutic-index determination.
In the efficacy experiments, the parasite suppression, clearance, malaria cure, parasite clearance time (PCT), duration (days) of clearance, and time to recrudescence were calculated as described previously (24). Parasitemia suppression was deemed to have occurred if the level of parasitemia initially fell but was not cleared by drug treatment. The clearance effect of each drug was determined as the minimum dose that could clear parasites in 100% of animals without causing obvious clinical toxicity (minimum clearance dose [MCD]). Clearance was defined as two negative blood smears, taken 4 to 24 h apart, prior to day 12 postinfection. The detection limit for a negative thin smear was defined as the failure to observe a parasite after examination of 10,000 red blood cells (i.e., 0.01% parasitemia). The curative effect of each drug was determined as the minimum dose that could cure parasites in 100% of animals without causing obvious clinical toxicity (minimum curative dose [MCureD]). The negative thin smears were monitored until day 28 after inoculation.
MTD was defined as the dose that caused clinical toxicity in 100% of the animals but that did not cause death. To evaluate the overall therapeutic index (a numerical estimate of the relationship between the toxic dose of a drug and its therapeutic dose), the MTD was divided by MCD and MCureD. The data were generally found to fit a normal distribution. Means and standard deviations were calculated. Coefficients of variation were calculated as a percentage and were the standard deviation divided by mean value. Statistical analysis was conducted with Microsoft Excel software by using a Student t test for dependent samples to compare the means of paired and unpaired samples between treatment groups.
RESULTS
Treatment of the infected rats with the test compounds was conducted on days 6, 7, and 8 postinoculation. Each study lasted for 28 days, and some prolonged clinical observation lasted for 40 days. Parasitemia suppression, clearance, and curative rates were evaluated during the treatment period in the rat model of malaria. Two hundred sixty-two rats were infected during the present study, with an achieved infection rate (the percentage of animals really infected with any parasitemia on day 5 among all of the rats inoculated) of 98.5% and a delayed infection rate of 1.5%. Parasitemia can usually be found on the day following inoculation by examination of blood smears.
Efficacy and MTD of PQD-A4.
The efficacy of PQD-A4 following 3-day oral administration was evaluated in P. berghei-infected rats (Table 1). A great suppressive effect of PQD-A4 was found in rats with malaria after administration of an oral dose of 0.625 mg/kg. The mean parasitemia of 87.2% was reduced after the first dosing of PQD-A4. When the dose was doubled to 1.25 mg/kg, a minimum clearance effect (MCD) was observed in all rats. During the treatments with 1.25, 2.5, and 5.0 mg/kg, the clearance times were 3.1, 5.5, and 9.8 days, respectively, suggesting that the clearance time is dose dependent. The minimum curative effect (MCureD) of PQD-A4 was found at the dose of 10 mg/kg and resulted in no recrudescence (Table 1). The MTD of PQD-A4 was evaluated over a dose range from 25 to 200 mg/kg; all rats survived up to the dose level of 100 mg/kg, while all rats died at the dose level of 200 mg/kg. As a result, the maximally tolerated dose of PQD-A4 was 100 mg/kg (190 μmol/kg).
TABLE 1.
Mean efficacy parameters for PQD-A4, PQD-BE, PQD, and AS following daily intragastric or intravenous administrations for 3 days in rats infected with P. berghei ANKAa
| Parametera | Result obtained with the following drug at the indicated daily dose (mg/kg [μmol/kg])a:
|
|||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral PQD-A4
|
Oral PQD-BE
|
Oral PQD
|
Intravenous AS
|
|||||||||||||||||||||||||||||||||||
| Control (0) | VC (0) | 0.625 (1.2) | 1.25 (2.4) | 2.5 (4.8) | 5 (9.5) | 10 (19.0) | 20 (38.1) | 100 (190.0) | 200 (381.0) | VC (0) | 0.6 (1.2) | 1.2 (2.4) | 2.4 (4.8) | 4.8 (9.5) | 9.6 (19.0) | 19.2 (38.1) | 38.4 (76.2) | 76.8 (153.0) | Control (0) | VC (0) | 0.43 (1.2) | 0.86 (2.4) | 1.71 (4.8) | 3.42 (9.5) | 8.6 (24.0) | 17.1 (48.0) | 34.2 (95.0) | Control (0) | VC (0) | 2.3 (6.0) | 4.6 (12.0) | 9.2 (23.9) | 18.4 (47.8) | 30.0 (95.6) | 60.0 (156.3) | 240 (625.0) | 480 (1,250) | |
| No. of rats | 4 | 5 | 5 | 5 | 5 | 4 | 7 | 6 | 5 | 4 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 5 | 4 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 5 |
| Parasitemia (%) on day 6 | 4.8 | 5.1 | 5.2 | 4.9 | 6.0 | 4.3 | 6.4 | 4.7 | 4.2 | 5.0 | 5.1 | 5.2 | 4.9 | 5.0 | 4.3 | 6.1 | 4.5 | 4.8 | 5.1 | 5.0 | 5.1 | 6.2 | 4.7 | 5.8 | 5.3 | 4.9 | 5.2 | 5.0 | 3.9 | 2.5 | 3.3 | 7.6 | 4.6 | 4.5 | 5.4 | 6.5 | 6.7 | 3.9 |
| Suppression (%) | − | − | 87.2 | 0 | 77.3 | 0 | 0 | 91.4 | − | − | − | − | − | − | 0 | 0 | − | − | 23.3 | 80.7 | 89.5 | − | − | − | ||||||||||||||
| No. of rats with clearance/total no. | 0/4 | 0/5 | 0/5 | 5/5 | 5/5 | 5/5 | 7/7 | 6/6 | − | − | 0/5 | 0/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | − | 0/5 | 0/5 | 0/5 | 5/5 | 5/5 | 5/5 | 4/4 | − | − | 0/5 | 0/4 | 0/4 | 0/6 | 0/6 | 3/6 | 4/6 | 6/6 | 6/6 | − |
| PCT (h) | − | − | − | 49.8 | 46.8 | 58.5 | 49.5 | 52.4 | − | − | − | − | 59.4 | 69.6 | 43.5 | 48.0 | 40.8 | 36.5 | − | − | − | − | 49.2 | 68.4 | 49.5 | 47.4 | − | − | − | − | − | − | − | 48.0 | 59.5 | 62.6 | 49.0 | 54.0 |
| No. of days to clearance | − | − | − | 3.1 | 5.5 | 9.8 | Cured | Cured | − | − | 2.3 | 4.7 | 11.0 | Cured | Cured | Cured | − | − | − | 4.6 | 9.1 | Cured | Cured | − | − | − | − | − | − | − | 3.5 | 7.8 | − | |||||
| No. of rats in recrudescence/total | − | − | − | 5/5 | 5/5 | 4/4 | 0/7 | 0/6 | − | − | − | − | 5/5 | 5/5 | 2/4 | 0/5 | 0/5 | 0/5 | − | − | − | − | 5/5 | 5/5 | 0/5 | 0/4 | − | − | − | − | − | − | − | 3/2 | 4/4 | 6/6 | 6/6 | − |
| No. of rats cured/total no. | 0/4 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 7/7 | 6/6 | − | − | 0/5 | 0/5 | 0/5 | 0/5 | 2/4 | 5/5 | 5/5 | 5/5 | − | 0/3 | 0/5 | 0/5 | 0/5 | 0/5 | 5/5 | 4/4 | − | − | − | − | − | 0 | 0 | 0 | 0 | 0 | 0 | − |
| Mortality rate (%) | 0 | 20.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 100 | 20 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 |
VC, vehicle control; −, no application. The administration times of the oral and intravenous doses are days 6, 7, and 8 after infection. Recrudescence has been calculated with the first day of oral or intravenous administration as day 0.
Efficacy and MTD of PQD-BE.
The efficacy of PQD-BE following 3-day oral administration was evaluated in P. berghei-infected rats (Table 1). A suppressive effect of PQD-BE was found after administration of an oral dose of 0.6 mg/kg. The mean parasitemia of 77.3% was reduced after the first dosing of PQD-BE. When the dose was doubled to 1.2 mg/kg, an MCD was observed in all animals. At this MCD, the PCT of PQD-BE was 59.4 h, and all animals exhibited a recrudescence of parasitemia. During treatment with 1.2, 2.4, and 4.8 mg/kg, the clearance times were 2.3, 4.7, and 11.0 days, respectively. A MCureD was found for PQD-BE at a dose of 9.6 mg/kg. A similar curative effect was seen in animals treated at the higher dose levels. The MTD of PQD-BE was evaluated over a dose range of 19.2 to 76.8 mg/kg; all rats survived up to the dose level of 38.4 mg/kg, while three of four rats died after administration of the dose level of 76.8 mg/kg.
Efficacy and MTD of PQD.
The efficacy of PQD following 3-day oral administration was also evaluated (Table 1). A great suppressive effect (91.4%) of PQD was found in rats with malaria after administration of an oral dose of 0.43 mg/kg. When the dose was doubled to 0.86 mg/kg, an MCD was observed in all animals. At this MCD, the PCT of PQD was 49.2 h, but all animals exhibited a recrudescence of the parasitemia. The parasitemia-clearing effects of PQD remained following the administration of daily oral doses of 1.71 mg/kg, with a PCT of 68.4 h. An MCureD was found for PQD at a dose of 3.42 mg/kg. The MTD of PQD was evaluated following the administration of three daily intragastric doses. The dose range of PQD was from 3.42 to 34.2 mg/kg; all rats survived up to the dose level of 8.6 mg/kg, while half of the rats died after administration of the dose level of 17.1 mg/kg.
Efficacy and MTD of AS.
The efficacy of AS following 3-day intravenous administration was evaluated in P. berghei-infected rats (Table 1). Infections were suppressed in all rats by AS at 9.2 mg/kg, with a mean suppression of 23.3%. Artesunate exhibited a great suppressive effect (80.7%) at a dose of 18.4 mg/kg. At the same dose level, AS cleared parasitemias in two of six rats. In order to achieve the MCD, the dose had to be increased to 60 mg/kg. At this and higher levels of 120 and 240 mg/kg, administered intravenously once daily for 3 consecutive days, AS cleared the parasitemia; however, the curative effect was not achieved at these high dose levels. The MTD of AS was evaluated following three daily intravenous injections. The dose range of AS was used was from 30 to 480 mg/kg; all rats survived up to the dose level of 240 mg/kg, while four of five rats died after administration of the dose level of 480 mg/kg (Table 1).
Primary therapeutic indices.
Antimalarial potency was measured by determination of the minimum clearance effect, a commonly used efficacy measurement that is particularly appropriate when new agents are compared to AS, which does not cure malaria in rodent models. The MCDs of PQD-A4, PQD-BE, PQD, and AS were 1.25, 1.2, 0.86, and 60 mg/kg, respectively. It is noteworthy that the MCDs of the three PQDs are the same molar dosage level of 2.4 μmol/kg (Table 2). The MTDs of PQD-A4, PQD-BE, PQD, and AS were estimated to be 100, 38.4, 8.6, and 240 mg/kg, respectively. The therapeutic indices, which were obtained by using the MCDs as the effective parameters, were estimated to be 80 for PQD-A4, 32 for PQD-BE, 10 for PQD, and 4 for AS.
TABLE 2.
Therapeutic indices, MCDs, MCureDs, and MTDs of PQD-A4, PQD-BE, PQD, and AS following administration of oral or intravenous doses daily for 3 daysa
| Efficacy parameter | Oral PQD-A4 | Oral PQD-BE | Oral PQD | Intravenous artesunate |
|---|---|---|---|---|
| Antimalarial potency | 46.2 ± 9.6 | 43.8 ± 11.6 | 70.3 ± 5.2 | 1 |
| MCD (μmol/kg [mg/kg]) | 2.4 (1.25) | 2.4 (1.20) | 2.4 (0.86) | 156.2 (60.0) |
| MCureD (μmol/kg [mg/kg]) | 19.0 (10.0) | 19.0 (9.6) | 9.5 (3.42) | None |
| MTD (μmol/kg [mg/kg]) | 190.0 (100.0) | 76.8 (38.4) | 24.0 (8.6) | 625.0 (240.0) |
| Primary therapeutic indexb | 80 | 32 | 10 | 4 |
| Secondary therapeutic indexc | 10 | 4 | 2.5 | None |
Four to seven rats were included in each group.
Primary therapeutic indices were calculated as MTD divided by MCD.
Secondary therapeutic indices were calculated as MTD divided by MCureD.
Secondary therapeutic indices.
A secondary measure of antimalarial potency was determined by use of the dose for the minimum curative effect (MCureD). The MCureDs of PQD-A4, PQD-BE, and PQD were 19 μmol/kg (10 mg/kg), 19 μmol/kg (9.6 mg/kg), and 9.5 μmol/kg (3.42 mg/kg), respectively. It is noteworthy that the doses differed from the MCDs of the three compounds used at an equimolar dose of 2.4 μmol/kg daily for 3 days (Table 2). The MCureD was not found for AS at any dose levels. The MTDs of PQD-A4, PQD-BE, and PQD were estimated to be 100, 38.4, and 8.6 mg/kg, respectively. By using MCureDs as the effective parameters, the therapeutic indices were calculated to be 10 for PQD-A4, 4 for PQD-BE, and 2.5 for PQD. Based on the therapeutic-index data, the three PQDs have the same antimalarial potency based on parasitemia clearance measurement. When the antimalarial potential was measured with the curative effects, the parent compound (PQD) was found to have a twofold higher antimalarial potential than PQD-A4 and PQD-BE. However, PQD-A4 is 8-fold safer than its parent compound, and PQD-BE is 3.2-fold safer than its parent compound (Table 2).
Comparison of PQD-A4 and AS.
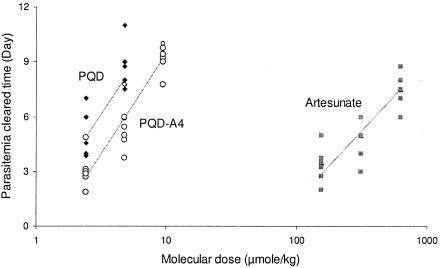
The efficacies of oral PQD-A4 and intravenous AS following 3-day intragastric or intravenous administration were compared by investigating the clearance of parasitemia in P. berghei-infected rats (Tables 1 and 2). The minimum clearance doses were 1.25 mg/kg for PQD-A4, which cleared the parasitemia within 3.1 days posttreatment, and 60 mg/kg for AS, which cleared the parasitemia within 4.2 days posttreatment. PQD-A4 and AS showed parallel responses in terms of their efficacy measurements following the administration of increased or decreased dose levels (Table 1). Therefore, the antimalarial potency of oral PQD-A4 was calculated to be 46.2 times higher than that of AS given by intravenous injection in P. berghei-infected animals when their antimalarial potencies were compared at the three dose levels by a parallel test for the number of days of parasitemia clearance (Fig. 2). PQD-A4 cures the rodents of malaria at a 10-mg/kg dose level, but AS does not. The advantage of the curative effect of oral PQD-A4 for the treatment of malaria is absolutely superior to that of AS intravenous injection.
FIG. 2.
Antimalarial potencies of oral PQD-A4 (46.2-fold) and PQD (70.3-fold) compared with that of AS administered by intravenous injection in P. berghei-infected rats when they were compared by a parallel test with measurement of the number of days of parasitemia clearance (parasitemia cleared days).
DISCUSSION
By appropriately optimizing the age selection, inoculum sizes, and the number of prior passages, an adult rat P. berghei ANKA infection model that provided a high incidence and consistency of infection with low rates of delayed infection was developed (24, 31). This model of severe malaria in rats was used for the pharmacological comparison of three candidate antimalarial agents (PQD-A4, PQD-BE, and PQD) and a positive control agent, AS.
We used two parameters to compare the efficacies of PQD-A4, PQD-BE, and PQD with that of the positive control, AS: (i) the dose of drug that resulted in minimum clearance of the parasite from all animals with no toxicity (MCD) and (ii) the dose of drug that resulted in a minimum curative treatment (MCureD). We also made note of recrudescence rates and times but did not consider these parameters to be critical for comparison of the efficacies of PQD-A4, PQD-BE, and PQD, given the stated clinical indications for their use.
The effects of PQD-A4, PQD-BE, and PQD on P. berghei malaria were not similar to those observed clinically with artemisinin derivatives such as AS; that is, the PQD class of agents could cure the rodents of malaria at 9.5 to 19.0 μmol/kg without recrudescence; but AS does not have such a curative effect even when it is used at a very high dose level of 625 μmol/kg, which is the maximum tolerated dose in the rat model. The 100% MCDs of PQD-A4, PQD-BE, and PQD were all 2.4 μmol/kg, suggesting that similar antimalarial potency was found for the three candidates with the MCD endpoint. The 100% MCureDs of PQD-A4, PQD-BE, and PQD were all within the range from 9.5 to 19.0 μmol/kg, indicating that the antimalarial potency of PQD is two times higher than those of PQD-A4 and PQD-BE at the MCureD measurement. However, the two derivatives showed a greater safety margin than the parent compound, PQD.
In conclusion, PQD-A4 and PQD-BE have the same efficacy as PQD, based on a comparison of the parasitemia clearance dose measurements, and have only half the antimalarial potency of PQD if the comparison is based on a curative dose level. However, PQD-A4 and PQD-BE can both be classified as non-narrow-window agents, with therapeutic indices of 80 and 32, respectively, and as much safer drug candidates (8-fold and 3.2-fold, respectively) than their parent compound, PQD. In addition, the three compounds PQD-A4, PQD-BE, and PQD are 20.0, 8.0, and 2.5 times safer, respectively, than AS based on a comparison of therapeutic indices.
Acknowledgments
This study was supported by the U.S. Army Research and Materiel Command.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the U.S. Department of Defense.
REFERENCES
- 1.Andersen, S. L., A. Ager, P. McGreevy, B. G. Schuster, D. Wesche, R. Kuschner, C. Ohrt, W. Ellis, R. Rossan, and J. Berman. 1995. Activity of azithromycin as a blood schizonticide against rodent and human plasmodia in vivo. Am. J. Trop. Med. Hyg. 52:159-161. [DOI] [PubMed] [Google Scholar]
- 2.Burghaus, P. A., B. T. Wellde, T. Hall, R. L. Richards, A. F. Egan, E. M. Riley, W. R. Ballou, and A. A. Holder. 1996. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect. Immun. 64:3614-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho, L. J., S. G. Oliveira, F. A. Alves, M. C. Brigido, J. A. Muniz, and C. T. Daniel-Ribeiro. 2000. Aotus infulatus monkey is susceptible to Plasmodium falciparum infection and may constitute an alternative experimental model for malaria. Mem. Inst. Oswaldo Cruz 95:363-365. [DOI] [PubMed] [Google Scholar]
- 4.Collins, W. E., J. C. Skinner, J. R. Broderson, B. B. Richardson, N. S. Ma, and P. S. Stanfill. 1991. Infection of Aotus vociferans monkeys with different strains of Plasmodium falciparum. J. Parasitol. 77:562-567. [PubMed] [Google Scholar]
- 5.Dascombe, M. J., and J. Y. Sidara. 1997. Ketanserin effects on thermoregulation in malarial and normal rats. Ann. N.Y. Acad. Sci. 813:392-397. [DOI] [PubMed] [Google Scholar]
- 6.Davoll, J., A. M. Johnson, H. J. Davies, O. D. Bird, J. Clarke, and E. F. Elslager. 1972. Folate antagonists. 2. 2,4-Diamino-6-((aralkyl and (heterocyclic)methyl)amino)quinazolines, a novel class of antimetabolites of interest in drug-resistant malaria and Chagas' disease. J. Med. Chem. 15:812-826. [DOI] [PubMed] [Google Scholar]
- 7.Deglin, S. M., J. M. Deglin, and E. K. Chung. 1977. Drug-induced cardiovascular diseases. Drugs 14:29-40. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez, J. N., J. E. Charris, G. Lobo, N. G. de Dominguez, M. M. Moreno, F. Rigginoe, E. Sanchez, J. Olson, and P. J. Rosenthal. 2001. Synthesis of quinolinyl chalcones and evaluation of their antimalarial activity. Eur. J. Med. Chem. 36:555-560. [DOI] [PubMed] [Google Scholar]
- 9.Dow, G. S., J. A. Reynoldson, and R. C. A. Thompson. 1999. Plasmodium berghei: a new rat model for assessment of blood schizonticidal activity. Exp. Parasitol. 93:92-94. [DOI] [PubMed] [Google Scholar]
- 10.Dow, G. S., T. H. Hudson, M. Vahey, and M. L. Koenig. 2003. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malaria J. 2:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dow, G. S., M. L. Koenig, L. Wolf, L. Gerena, M. Lopez-Sanchez, T. H. Hudson, and A. K. Bhattacharjee. 2004. The antimalarial potential of 4-quinolinecarbinolamines may be limited due to neurotoxicity and cross-resistance in mefloquine-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 48:2624-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elslager, E. F., O. D. Bird, J. Clarke, S. C. Perricone, and D. F. Worth. 1972. Folate antagonists. 9. 2,4-Diamino-6-((aralkyl)alkylamino)quinazolines, a potent class of antimetabolites with prodigious antimalarial effects. J. Med. Chem. 15:1138-1146. [DOI] [PubMed] [Google Scholar]
- 13.Guan, J., Q. Zhang, M. O'Neil, N. Obaldia III, A. Ager, L. Gerena, and A. J. Lin. 2005. Antimalarial activity of new pyrrolo[3,2-f]Quinazoline-1,3-Diamine derivatives. Antimicrob. Agents Chemother. 49:4928-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han, H. K., and G. L. Amidon. 2000. Targeted prodrug design to optimize drug delivery. AAPS Pharm. Sci. 2:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway, P. A., S. Krishna, and N. J. White. 1991. Plasmodium berghei: lactic acidosis and hypoglycaemia in a rodent model of severe malaria; effects of glucose, quinine, and dichloroacetate. Exp. Parasitol. 72:123-133. [DOI] [PubMed] [Google Scholar]
- 16.Hua, H., M. Cheng, X. Li, and Y. Pei. 2002. A new pyrroloquinazoline alkaloid from Linaria vulgaris. Chem. Pharm. Bull. (Tokyo) 50:1393-1394. [DOI] [PubMed] [Google Scholar]
- 17.International Conference on Harmonisation. 1994. Harmonised tripartite guidance. Guidance for industry: detection of toxicity to reproduction for medicinal products. [Online.] http://www.fda.gov/cder/guidance/s5a.pdf.
- 18.Johns Hopkins Research Institute. 2004. Malaria. A grand challenge. Proceedings of a Johns Hopkins Research Institute Meeting. 25-26 March 2004, Baltimore, Maryland, USA. Int. J. Parasitol. 34:1413-1554. [DOI] [PubMed] [Google Scholar]
- 19.Jones, T. R., D. F. Stroncek, A. S. Gozalo, N. Obaldia III, E. M. Andersen, C. Lucas, D. L. Narum, A. J. Magill, B. K. Sim, and S. L. Hoffman. 2002. Anemia in parasite- and recombinant protein-immunized Aotus monkeys infected with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 66:672-679. [DOI] [PubMed] [Google Scholar]
- 20.Kim, S. Y., and N. L. Benowitz. 1990. Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide. Drug Safety 5:393-420. [DOI] [PubMed] [Google Scholar]
- 21.Ledig, K. W. 3October1978. 7-(Substituted)-7H-pyrrolo[3,2,-f]quinazoline-1,3-diamines. U.S. patent 4,118,561.
- 22.Lee, H. J., J. S. Cooperwood, Z. You, and D. H. Ko. 2002. Prodrug and antedrug: two diametrical approaches in designing safer drugs. Arch. Pharm. Res. 25:111-136. [DOI] [PubMed] [Google Scholar]
- 23.Li, C., E. Seixas, and J. Langhorne. 2001. Rodent malaria: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Med. Microbiol. Immunol. 189:115-126. [DOI] [PubMed] [Google Scholar]
- 24.Li, Q. G., Y. Z. Si, P. Lee, E. Wong, L. H. Xie, D. E. Kyle, and G. S. Dow. 2003. Efficacy comparison of intravenous artelinate and artesunate in Plasmodium berghei-infected Sprague-Dawley rats. Parasitology 126:283-291. [DOI] [PubMed] [Google Scholar]
- 25.Li, Q. G., M. P. Kozar, L. H. Xie, T. Shearer, Y. Z. Si, L. Anova, J. Zhang, A. J. Lin, W. K. Milhous, and D. R. Skillman. 2004. Potential antimalarial agents, pyrroloquinazoline analogs, for blood or prophylactic stage malarias. Am. J. Trop. Med. Hyg. 71:90. [Google Scholar]
- 26.Medicines for Malaria Venture. 2005. Curing malaria together. [Online.] http://www.mmv.org/pages/content_frame.asp?center_page=page1_0004_1.htm.
- 26a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Research Council, Washington, D.C.
- 27.Neimark, H., A. Barnaud, P. Gounon, J. C. Michel, and H. Contamin. 2002. The putative Haemobartonella that influences Plasmodium falciparum parasitaemia in squirrel monkeys is a haemotrophic mycoplasma. Microbes Infect. 4:693-698. [DOI] [PubMed] [Google Scholar]
- 28.Puri, S. K., and N. Singh. 2000. Azithromycin: antimalarial profile against blood- and sporozoite-induced infections in mice and monkeys. Exp. Parasitol. 94:8-14. [DOI] [PubMed] [Google Scholar]
- 29.Pye, D., C. M. O'Brien, P. Franchina, C. Monger, and R. F. Anders. 1994. Plasmodium falciparum infection of splenectomized and intact Guyanan Saimiri monkeys. J. Parasitol. 80:558-562. [PubMed] [Google Scholar]
- 30.Scheller, L. F., K. C. Stump, and A. F. Azad. 1995. Plasmodium berghei: production and quantitation of hepatic stages derived from irradiated sporozoites in rats and mice. J. Parasitol. 81:58-62. [PubMed] [Google Scholar]
- 31.Xie, L. H., T. O. Johnson, P. J. Weina, Y. Z. Si, A. Haeberle, R. Upadhyay, E. Wang, and Q. G. Li. 2005. Risk assessment and therapeutic indices of artesunate and artelinate in P. berghei infected and uninfected rats. J. Int. Toxicol. 24:251-264. [DOI] [PubMed] [Google Scholar]