Abstract
We investigated the efficacy of LL-37, the C-terminal part of the only cathelicidin in humans identified to date (termed human cationic antimicrobial protein), in three experimental rat models of gram-negative sepsis. Adult male Wistar rats (i) were given an intraperitoneal injection of 1 mg Escherichia coli 0111:B4 LPS, (ii) were given 2 × 1010 CFU of Escherichia coli ATCC 25922, or (iii) had intra-abdominal sepsis induced via cecal ligation and puncture. For each model, all animals were randomized to receive intravenously isotonic sodium chloride solution, 1-mg/kg LL-37, 1-mg/kg polymyxin B, 20-mg/kg imipenem, or 60-mg/kg piperacillin. Lethality; growth of bacteria in blood, peritoneum, spleen, liver, and mesenteric lymph nodes; and endotoxin and tumor necrosis factor alpha (TNF-α) concentrations in plasma were evaluated. All compounds reduced lethality compared to levels in controls. Endotoxin and TNF-α plasma levels were significantly higher in conventional antibiotic-treated rats than in LL-37- and polymyxin B-treated animals. All drugs tested significantly reduced bacterial growth compared to saline treatment. No statistically significant differences between LL-37 and polymyxin B were noted for antimicrobial and antiendotoxin activities. LL-37 and imipenem proved to be the most effective treatments in reducing all variables measured. Due to its multifunctional properties, LL-37 may become an important future consideration for the treatment of sepsis.
Gram-negative sepsis is a common complex clinical syndrome that results from a harmful host response to infection, in which foreign bacteria and lipopolysaccharide (LPS) are potent activators of different immune cells, including monocytes and macrophages (3, 5, 36). Sepsis ranks as the leading cause of death in intensive care units, and its incidence is increasing steadily. Despite better supportive care, mortality rates have not changed much over recent decades (2, 10, 12). LPSs are important components of the outer membrane of gram-negative bacteria and have a pivotal role as initiators of septic shock. They activate the host effector cells through stimulation of receptors on their surface and induce the production of macrophage-derived cytokines (1, 36). Host cells recognize specific microbial components of invading bacteria through pattern recognition receptors such as Toll-like receptors that mediate immune responses by releasing cytokines that recruit inflammatory cells, enhance bacterial clearance, and activate adaptive immune cells to generate pathogen-specific antibodies (1, 3, 25, 33, 34, 37). Upon activation, Toll-like receptors trigger mast cell release of tumor necrosis factor alpha (TNF-α), which aids in recruiting neutrophils to the site of infection and killing of bacteria by cleaving cell wall proteins or destroying virulence factors (16, 18, 23, 32).
Current treatments of gram-negative sepsis in critically ill patients are based on prompt administration of adequate antimicrobial agents, source control directed at removal of the nidus of infection whenever possible, and support of organ dysfunction (22, 24, 31, 35).
Antimicrobial peptides play an important role in innate immunity by acting as effector molecules in host defenses against pathogens (8, 19, 20). The dominating targets are bacterial membranes, and the killing reaction must be faster than the growth rate of the bacteria. Some antibacterial peptides are clearly multifunctional, and an attempt to predict this property from the hydrophobicity of all amino acid side chains has been previously presented (4, 38). Moreover, several cationic peptides bind to the negatively charged residues of LPS of the outer membrane by electrostatic interactions involving the negatively charged phosphoryl groups and by hydrophobic interactions involving the acyl chains of lipid A, thus determining the key mechanistic step in the killing of gram-negative organisms (17, 19, 20). Various families of antimicrobial peptides have been identified, including the cathelicidins (38, 39). They are characterized by conserved propeptide sequences and comprise a family of antimicrobial peptides that have been identified in epithelial tissues and some myeloid cells of humans and animals. LL-37 is the C-terminal part of the only cathelicidin identified to date in humans, termed human cationic antimicrobial protein, which is expressed mainly by neutrophils and epithelial cells. In addition to killing a broad spectrum of microorganisms, LL-37 was demonstrated to display various cellular activities related to inflammation, including cytotoxicity to host cells, chemotaxis, epithelial cell activation, angiogenesis, and epithelial wound repair (6, 7, 27, 28).
The present experimental study was designed to investigate the potential therapeutic role of LL-37 in two rat models of Escherichia coli infection and in a model of cecal ligation and puncture (CLP).
MATERIALS AND METHODS
Reagents.
9-Fluorenylmethoxy carbonyl (Fmoc)-amino acids and Fmoc-Ser(tBu)-PEG-PS resin (0.17 meq/g) were purchased from Applied Biosystems (Foster City), and ChemImpex (Wood Dale, Illinois). All other reagents and solvents were of synthesis grade.
Drugs.
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was chemically synthesized on a Milligen 9050 automated synthesizer (Applied Biosystems, Foster City, Calif.) by Fmoc chemistry. The peptide concentration was determined by measuring absorbance at 257 nm (extinction coefficient, 780.4 [195.1 cm−1 M−1 for each Phe residue]).
Polymyxin B (Sigma-Aldrich S.r.l., Milan, Italy), piperacillin (Wyeth Lederle, Aprilia, Italy), and imipenem (Merck, Sharp, & Dohme, Milan, Italy) powders were dissolved in accordance with manufacturers' recommendations. Solutions were made fresh on the day of assay.
Organisms.
The control strain of Escherichia coli ATCC 25922 was used. E. coli serotype 0111:B4 LPS (Sigma-Aldrich S.r.l., Milan, Italy) was prepared in sterile saline, aliquoted, and stored at −80°C for short periods.
To obtain information about the in vitro susceptible profile of LL-37, we tested the gram-negative and gram-positive species more frequently isolated from the CLP control group. Therefore, 10 E. coli isolates and 10 Enterococcus faecalis isolates were tested. The strains were obtained from different animals. Identification was performed according to standard procedures and confirmed by means of the API-20NE and API-Strep systems (bioMérieux Italia, Italy). E. coli ATCC 25922 and E. faecalis ATCC 29212 were used as quality control strains.
Cytokine and nitric oxide (NO) production by murine macrophage RAW 264.7 cells.
Cells were incubated with LPS from E. coli O111:B4 at 37°C in the absence or presence of the drugs under study. Aliquots of cell supernatants were removed after 6 and 24 h of incubation, and the amounts of TNF-α and of NO were determined by enzyme-linked immunosorbent assay (ELISA; Euroclone Life Sciences, Milan, Italy) and the Griess reagent (Molecular Probes, Eugene, OR), respectively (13).
Susceptibility testing.
MICs were assayed according to the procedures outlined by the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) (26). Polypropylene 96-well plates (Sigma-Aldrich) were incubated for 18 h at 37°C in air. The MIC was taken as the lowest drug concentration at which observable growth was inhibited.
To study in vitro killing effects of the compounds, defined as a 3-log10 reduction in vital organisms, aliquots of exponentially growing bacteria (E. coli ATCC 25922) were resuspended in fresh Mueller-Hinton broth at approximately 107 cells/ml and exposed to each agent at 4× MIC for 0, 5, 10, 15, 20, 25, 30, 40, 50, and 60 min at 37°C. After these exposures, samples were serially diluted in 10 mM of sodium HEPES buffer (pH 7.2) to minimize the carryover effect and plated onto Mueller-Hinton agar plates to obtain viable colonies.
Animals.
Adult male Wistar rats weighting 200 to 300 g each were used. All animals were housed in individual cages under constant temperature (22°C) and humidity with a 12-h light/12-h dark cycle and had access to chow and water as much as desired throughout the study. The study was approved by the animal research ethics committee of the I.N.R.C.A.-I.R.R.C.S., Università Politecnica delle Marche, Ancona, Italy.
Experimental design.
Septic shock was induced by three different experimental methods: (i) by intraperitoneal administration of E. coli serotype 0111:B4 LPS, (ii) by intraperitoneal injection of 2 × 1010 CFU of E. coli ATCC 25922, and (iii) by cecal ligation and puncture.
Model 1.
Five groups, each containing 15 animals, were anesthetized by intramuscular injection of ketamine (50 mg/kg of body weight) and injected intraperitoneally with 1 mg E. coli serotype 0111:B4 LPS in a total volume of 500 μl of sterile saline. Immediately after injection, the animals received one intravenous dose of isotonic sodium chloride solution (control group C0), 1-mg/kg LL-37, 1-mg/kg polymyxin B, 20-mg/kg imipenem, or 60-mg/kg piperacillin.
Model 2.
E. coli ATCC 25922 was grown in brain heart infusion broth. When bacteria were in the log phase of growth, the suspension was centrifuged at 1,000 × g for 15 min, the supernatant was discarded, and the bacteria were resuspended and diluted into sterile saline. All animals (five groups, each group containing 15 animals) were anesthetized as described above. The abdomen of each animal was shaved and prepared with iodine. The rats received 1 ml saline intraperitoneally containing 2 × 1010 CFU of E. coli ATCC 25922. Immediately after bacterial challenge, animals received intravenously isotonic sodium chloride solution (control group C1), 1-mg/kg LL-37, 1-mg/kg polymyxin B, 20-mg/kg imipenem, or 60-mg/kg piperacillin.
Model 3.
All animals (eight groups, each containing 15 animals) were anesthetized as described previously. The abdomen of each animal was shaved and prepared with iodine. Through a midline laparotomy, the cecum was filled with feces by milking the stools back from the descending colon and then ligated just below the ileocaecal valve with a 3-0 silk ligature. The antimesenteric cecal surface was punctured twice with a 23-gauge needle below the ligature, the bowel was placed back into the peritoneal cavity, and the abdomen was closed in two layers. The operative procedure was done under aseptic conditions. The drugs were given immediately after the surgical procedure. The animals received intravenously isotonic sodium chloride solution (control group C2), 1-mg/kg LL-37, 1-mg/kg of polymyxin B, 20-mg/kg of imipenem, or 60-mg/kg of piperacillin. For model 3, the experiment was also performed with administration of the drugs 360 min after the surgical procedure to better investigate the clinical situation where there is an interval between the onset of sepsis and the initiation of therapy.
All animals were monitored for the subsequent 72 h. For each animal model, toxicity was evaluated on the basis of the presence of any drug-related adverse effects, i.e., local signs of inflammation, anorexia, weight loss, vomiting, diarrhea, fever, and behavioral alterations. In particular, to evaluate the physiologic effects of LL-37, leukocyte counts, serum creatinine levels, rectal temperature, pulse, blood pressure, breathing rate, and oxygenation were monitored for a supplementary peptide-treated group without infection or LPS.
Evaluation of treatment.
On the basis of the type of experiment, the rate of lethality; the quantities of bacteria in blood, peritoneum, spleen, liver, and mesenteric lymph nodes (MLNs); the rate of positive blood cultures, and plasma endotoxin, interleukin-6 (IL-6), and TNF-α levels were evaluated at the end of the study.
To detect the presence of bacterial colonization (models 2 and 3), the following samples for bacterial analysis were taken under sterile conditions: blood, spleen, peritoneum, MLNs, and liver.
Blood samples for aerobic and anaerobic cultures were obtained 72 h after the administration of drugs and cultured aerobically and anaerobically. The positive blood cultures were plated out on appropriate agar plate, and species were identified by standard bacteriological techniques. The quantitative bacterial counts from dead animals were performed immediately after death. In the surviving animals, the counts were performed at 72 h postinjection. The surviving animals were killed with chloroform.
For organ colony counts (CFU per gram), the MLN complex, spleen, and liver were removed, weighed, and homogenized in a 10× (vol/wt) quantity of phosphate-buffered saline (8.1 mM phosphate [pH 7]) by using a Stomacher 80. Samples were serially diluted, and a 0.1-ml volume of each dilution was duplicate spread onto blood agar plates for enumeration of developed colonies. For the isolation of anaerobes, specimens were inoculated onto Columbia blood agar plates enriched with hemin and menadione and incubated in an anaerobic chamber in 80% N2-10% H2-10% CO2. The limit of detection was <1 log10 CFU/ml. The plates were incubated both in air and under anaerobic conditions at 35°C for 48 h.
In addition, to perform quantitative bacterial evaluation of the intra-abdominal fluid from each animal, 10 ml of sterile saline was injected intraperitoneally, and samples of the peritoneal lavage fluid were serially diluted. For enumeration of developed colonies (CFU per milliliter), duplicate samples were plated and incubated as described above.
Biochemical assays.
For determination of endotoxin, IL-6, and TNF-α levels in plasma (all models), 0.2-ml blood samples were collected from a tail vein 0, 2, 6, 12, 24, and 48 h after injection (of LPS or bacteria) or surgical procedure into a sterile syringe and transferred to tubes containing EDTA tripotassium salt.
Endotoxin concentrations were measured by the commercially available Limulus amebocyte lysate test (E-TOXATE; Sigma-Aldrich). Endotoxin standards (0, 0.015, 0.03, 0.06, 0.125, 0.25, and 0.5 endotoxin units/ml) were tested in each run, and the concentration of endotoxin in the text samples was calculated by comparison with the standard curve.
TNF-α and IL-6 levels were measured by a solid-phase sandwich ELISA. The lower limit of sensitivity for TNF-α by this assay was 0.05 ng/ml. The detection limit was 12 pg/ml. The assays were performed in duplicate.
Statistical analysis.
Mortality rates and qualitative results for blood cultures between groups were compared by use of Fisher's exact test (the significance level was fixed at 0.05). Mean TNF-α and IL-6 values and quantitative evaluations of the bacteria in intra-abdominal fluid cultures were presented as the mean ± standard deviation (SD) of the mean; statistical comparisons between groups were made by analysis of variance (the significance level was fixed at 0.05). Due to the presence of several values below the lower limit of sensitivity, plasma endotoxin levels were compared between groups by the Kruskal-Wallis nonparametric test, adjusted for ties. The post hoc comparisons were performed by the Bonferroni method for both analysis of variance and the Kruskal-Wallis test; for each comparison, the significance level was fixed at 0.005, obtained by dividing the familywise level (i.e., the number of possible pairwise comparisons) by 10. Each comparison group contained 15 rats.
RESULTS
In vitro antimicrobial activity.
LL-37 showed MICs of 4.0 and 8.0 mg/liter against the E. coli and E. faecalis control strains, respectively. These results were confirmed by the experiments performed with the isolates. All E. coli strains were inhibited by LL-37 at concentrations of 2 to 8 mg/liter. Against E. faecalis isolates, LL-37 exhibited a MIC ranging from 4 to 16 mg/liter. The control agents showed MICs ranging from 4 to 16 mg/liter and 0.5 to 4 mg/liter against E. faecalis and E. coli strains, respectively. In vitro time-killing evaluations showed a potent activity of LL-37 against E. coli within a 10-min exposure. Killing by polymyxin B, imipenem, and piperacillin was complete after 15 to 20 min, respectively (data not shown).
Cytokine and NO production.
The effect of LL-37, polymyxin B, imipenem, and piperacillin on LPS-mediated cellular responses was evaluated by examining the ability of these compounds to inhibit LPS-induced production of TNF-α and NO by RAW 264.7 cells. All drugs were assayed at concentrations comparable to and threefold higher than their MICs. Neither compound alone caused release of these proinflammatory factors at these concentrations (not shown).
The levels of TNF-α into cell-free medium, as assessed by ELISA, were 1,468- to 3,165-fold increased over the control after stimulation with 100-ng/ml LPS and were decreased by 74% and 80%, respectively, in the presence of 8- and 24-mg/liter LL-37, corresponding to 2 to 5 μM peptide (Fig. 1A). All other compounds, even at high doses, were far less effective than LL-37. Compared with LL-37, on a molar basis, imipenem and piperacillin were used at 20- to 30-fold-higher concentrations. At these doses, both antibiotics exhibited a 28 to 35% decrease in LPS-mediated TNF-α release. This moderate inhibition is not readily explained, as neither compound has been reported to inhibit the proinflammatory effects induced by LPS.
FIG. 1.
Effect of LL-37, polymyxin B (POL-B), imipenem (IMP), and piperacillin (PIP) on LPS-induced production of TNF-α (Α) and nitric oxide (B). RAW 264.7 cells were stimulated with 100-ng/ml LPS in the absence or presence of the indicated amounts of each drug. TNF-α (Α) and NO (B) were assayed, respectively, in the cell-free medium of cells incubated for 6 and 24 h. The concentrations of TNF-α and NΟ in untreated cells were 61.49 ± 7.58 pg/ml and 6.15 ± 1.87 μM, respectively. Data are presented as the means of at least three experiments ± SD. Statistical analysis was performed according to Student's t test by comparing samples treated with each drug with control cells stimulated with LPS only. A calculated P value of <0.05 (asterisk) was considered significant.
The amount of NO in the cell-free medium of LPS-stimulated cells was assessed by measuring the accumulation of the stable NO metabolite nitrite with the Griess reagent. A 4.6- to 9.0-fold NO increase was detected in the presence of LPS compared to untreated cells. The production of NO was decreased by 45% and 90% when cells were incubated with LPS in the presence of LL-37 (8 mg/liter and 24 mg/liter) (Fig. 1B). We observed a 56% inhibition with 16-mg/liter polymyxin B or 50-mg/liter imipenem and no effect with up to 150-mg/liter piperacillin (data not shown). Overall, our data indicate that LL-37 is most effective in neutralizing the proinflammatory activity of LPS.
Model 1: intraperitoneal administration of LPS.
In the control group, plasma peak levels of endotoxin, IL-6, and TNF-α were observed 6 h after intravenous administration of 1.0 mg E. coli serotype 0111:B4 LPS. In contrast, intravenous LL-37 and polymyxin B treatments resulted in a marked decrease (P < 0.05) of the cytokine levels and virtually undetectable levels of endotoxin in the plasma of peptide-treated groups, compared to those of the control group (C0). There were significant differences in plasma endotoxin, TNF-α, and IL-6 levels between peptide-treated groups compared to the imipenem- or piperacillin-treated groups, while no significant differences were observed between imipenem- or piperacillin-treated and untreated groups (Table 1).
TABLE 1.
Endotoxin and TNF-α plasma levels in a rat model 6 h after intraperitoneal administration of 1.0 mg E. coli serotype 0111:B4 LPS
| Treatmenta (dose) | Levels of:
|
||
|---|---|---|---|
| Endotoxin (EU/ml)b | IL-6 (pg/ml)b | TNF-α (ng/ml)b | |
| Control group C0 | 27.77 ± 4.02 | 602.34 ± 26.43 | 134.80 ± 16.33 |
| LL-37 (1 mg/kg) | ≤0.015 ± 0.0c | 176.21 ± 11.23c | 0.22 ± 0.01c |
| POL-B (1 mg/kg) | ≤0.015 ± 0.0c | 169.23 ± 10.30c | 0.21 ± 0.01c |
| IMP (20 mg/kg) | 24.69 ± 2.88 | 593.32 ± 26.21 | 121.32 ± 14.9 |
| PIP (120 mg/kg) | 33.49 ± 4.69 | 599.27 ± 25.82 | 131.12 ± 17.0 |
POL-B, polymyxin B; IMP, imipenem; PIP, piperacillin.
Mean ± SD. EU, endotoxin units.
P < 0.05 (familywise significance level) versus the control group C0 and the imipenem- and piperacillin-treated groups.
Model 2: intraperitoneal injection of 2 × 1010 CFU E. coli ATCC 25922.
All animals were monitored for 72 h. The rate of lethality in control group C1 was 100% within 48 h. All antibiotic treatments led to decreased mortality (P < 0.05). In particular, the lowest lethality rate (26.6%) was observed for the group treated with imipenem or LL-37. Bacteriological evaluation of C1 showed 100% positive blood cultures and a high level of bacterial colonization with the number of CFU per gram not lower than 106 for all organs (Table 2). The peritoneum and the MLN complex appeared more involved than other studied anatomical districts by bacterial colonization. All regimens were significantly superior to controls at reducing blood, spleen, peritoneum, liver, and MLN complex bacterial burdens (P < 0.001), whereas imipenem was the most effective (Table 2).
TABLE 2.
Efficacy of LL-37, polymyxin B, piperacillin, and imipenem in a rat model of peritonitis induced by intraperitoneal injection of E. coli
| Treatmenta (mg/kg) | % Lethalityb | Bacterial count (CFU/ml)c in:
|
||||
|---|---|---|---|---|---|---|
| Blood | Peritoneum | Spleen | Liver | MLNs | ||
| No treatment (control group C1) | 100 | 6.1 × 106 ± 2.1 × 106 | 8.0 × 108 ± 2.1 × 108 | 4.9 × 107 ± 0.2 × 107 | 3.6 × 107 ± 2.1 × 107 | 1.4 × 108 ± 8.3 × 107 |
| LL-37 (1) | 26.6 | 7.9 × 104 ± 1.7 × 104d | 7.7 × 106 ± 1.6 × 106d | 6.9 × 105 ± 1.7 × 105d | 7.3 × 105 ± 2.3 × 105d | 4.9 × 106 ± 1.1 × 106d |
| POL-B (1) | 33.3 | 4.2 × 103 ± 1.5 × 103d | 3.1 × 105 ± 1.0 × 105d | 5.4 × 104 ± 2.0 × 104d | 6.4 × 104 ± 2.1 × 104d | 7.0 × 105 ± 2.4 × 105d |
| IMP (20) | 26.6 | 2.2 × 101 ± 0.4 × 101d,e | 3.7 × 103 ± 0.9 × 103d,e | 2.8 × 102 ± 0.8 × 102d,e | 3.0 × 102 ± 0.4 × 102d,e | 2.6 × 103 ± 0.3 × 103d,e |
| PIP (120) | 40.0 | 3.1 × 103 ± 0.3 × 103d | 1.8 × 105 ± 8.2 × 104d | 1.6 × 104 ± 8.8 × 103d | 2.2 × 104 ± 0.2 × 104d | 1.1 × 105 ± 9.9 × 104d |
POL-B, polymyxin B; IMP, imipenem; PIP, piperacillin.
Lethality was monitored for 72 h following the challenge.
Mean ± SD.
P < 0.05 (familywise significance level) versus the control group C1.
P < 0.05 (familywise significance level) versus LL-37-, PIP-, and POL-B-treated groups.
Significant increase in plasma endotoxin, TNF-α, and IL-6 concentrations with mean peak levels achieved at 6 h postinjection were observed in controls and beta-lactam-treated animals. In contrast, LL-37 and polymyxin B produced significant reductions in plasma cytokine and endotoxin levels. No significant differences were observed between LL-37 and polymyxin B (Fig. 2).
FIG. 2.
Plasma levels of IL-6 (top), endotoxin (middle), and TNF-α (bottom) after bacterial challenge. For all three parameters, LL-37 and polymyxin B levels were significantly different (P < 0.05) compared to imipenem and piperacillin levels after 2 h.
Model 3: cecal ligation and puncture.
The rate of lethality in control group C2 was 100% within 48 h. All antibiotic treatments led to decreased rates of mortality (P < 0.05). Specifically, at 72 h, lethality rates of 33.3% were observed for all treated groups, with the exception of piperacillin-treated groups, which showed a 40.0% lethality rate (Table 3). Gram-negative and gram-positive bacteria were simultaneously isolated from >90% of the rats. The microorganisms isolated were primarily members of the Enterobacteriaceae, including E. coli, Klebsiella spp., Enterobacter spp., and gram-positive cocci (especially enterococci). The anaerobic isolates most frequently seen were Bacteroides spp. Imipenem showed the highest level of antimicrobial activity. LL-37 activity was comparable to that of polymyxin B and piperacillin.
TABLE 3.
Efficacy of LL-37, polymyxin B, piperacillin, and imipenem in a rat model of cecal ligation and puncture-induced peritonitis
| Treatmenta (mg/kg) | % Lethalityb | Bacterial count (CFU/ml)c in:
|
||||
|---|---|---|---|---|---|---|
| Blood | Peritoneum | Spleen | Liver | MLNs | ||
| No treatment (control group C1) | 100 | 5.0 × 107 ± 2.2 × 107 | 8.3 × 109 ± 2.4 × 109 | 5.1 × 108 ± 0.3 × 108 | 4.7 × 108 ± 2.3 × 108 | 2.7 × 109 ± 0.4 × 109 |
| LL-37 (1) | 33.3 | 8.4 × 105 ± 2.0 × 105d | 8.0 × 107 ± 2.4 × 107d | 7.2 × 106 ± 1.9 × 106d | 7.7 × 106 ± 2.3 × 106d | 5.4 × 107 ± 1.6 × 107d |
| POL-B (1) | 33.3 | 4.8 × 104 ± 1.9 × 104d | 4.1 × 106 ± 1.6 × 106d | 5.9 × 105 ± 2.1 × 105d | 6.9 × 105 ± 2.1 × 105d | 7.4 × 106 ± 2.3 × 106d |
| IMP (20) | 33.3 | 2.6 × 102 ± 0.5 × 102d,e | 4.0 × 104 ± 1.2 × 104d,e | 3.6 × 103 ± 0.7 × 103d,e | 3.8 × 103 ± 0.7 × 103d,e | 3.3 × 104 ± 0.5 × 104d,e |
| PIP (120) | 40.0 | 3.4 × 104 ± 0.4 × 104d | 3.1 × 106 ± 0.3 × 106d | 2.0 × 105 ± 9.7 × 104d | 3.5 × 105 ± 0.7 × 105d | 1.6 × 106 ± 8.3 × 105d |
POL-B, polymyxin B; IMP, imipenem; PIP, piperacillin.
Lethality was monitored for 72 h following the challenge.
Mean ± SD.
P < 0.05 (familywise significance level) versus the control group C1.
P < 0.05 (familywise significance level) versus LL-37-, PIP-, and POL-B-treated groups.
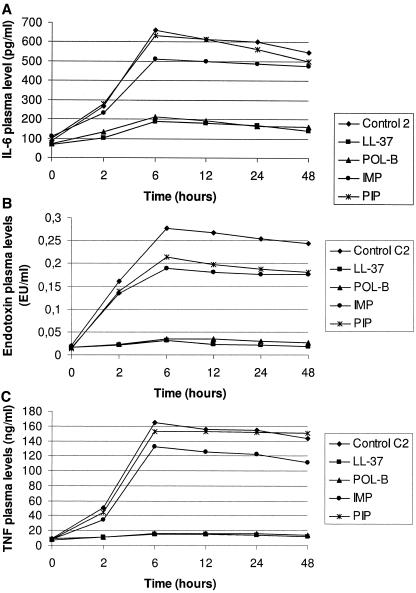
Endotoxin, TNF-α, and IL-6 kinetics were similar in model 3 to those described for the other models (Fig. 3). Similar effects on lethality rates (33.3% for LL-37, polymyxin B, and imipenem and 40% for piperacillin) and bacterial counts were observed for all compounds when the drugs were administered 360 min after intervention. In contrast, the administration of drugs at different times after surgical procedure had different impacts on plasma endotoxin, IL-6, and TNF-α levels. In fact, a constant decrease in these three parameters was produced only by the administration of LL-37 and polymyxin B 360 min after the surgical procedure, with maximal levels at the starting point (6 h). LL-37 and polymyxin B produced significant reductions in plasma endotoxin and cytokines levels compared to control and beta-lactam-treated groups, while no significant differences were observed between LL-37 and polymyxin B (not shown).
FIG. 3.
Plasma levels of IL-6 (top), endotoxin (middle), and TNF-α (bottom) after a surgical procedure. For all three parameters, LL-37 and polymyxin B levels were significantly different (P < 0.05) compared to imipenem and piperacillin levels after 2 h.
Finally, none of the LL-37-, polymyxin B-, piperacillin-, and imipenem-treated animals had clinical evidence of drug-related adverse effects; no changes in physiological parameters were observed in the supplementary 1-mg/kg LL-37-treated group without infection.
DISCUSSION
The role of antimicrobial peptides as effector molecules of innate immunity is widening from solely endogenous antibiotics to multifunctional mediators that provide a first line of host defense, modify the local inflammatory response, and activate adaptive immunity (4, 14, 15, 20, 38). Previous research has shown that LL-37 is a potent immunomodulator and that it stimulates the expression of a wide variety of genes involved in the innate immune response. It has been demonstrated to be a chemoattractant for human monocytes, T cells, and mast cells. Furthermore, LL-37 is a potent antiendotoxic agent and induces chemokine production. It also has a variety of other functions, including promotion of histamine release from mast cells, inhibition of tissue proteases, stimulation of wound healing, and angiogenesis (7, 28).
In light of these observations, we investigated its therapeutic potential in two rat models of E. coli infection and in a CLP model. Septic shock was induced by intraperitoneal administration of LPS and by bacterial challenge with E. coli. In addition, we performed a model of intra-abdominal sepsis induced via cecal ligation and puncture, since the presence of a bowel perforation and mixed bacterial infection of intestinal origin is a more realistic sepsis model and has clear parallels to clinical situations with human patients (29, 30). The antimicrobial and endotoxin-neutralizing effects of LL-37 were compared to those of beta-lactams such as imipenem and piperacillin and to that of polymyxin B. Beta-lactams are the antimicrobial agents most frequently used in empirical antibiotic therapy of septicemia and shock (21). Polymyxins, a group of cyclic cationic polypeptides originally derived from Bacillus polymyxa, share remarkable structural similarity with cationic peptides. Polymyxin B is considered the most potent antiendotoxin agent identified to date (11).
Our findings clearly show that LL-37 neutralizes the LPS-induced proinflammatory response. Compared to polymyxin B and the beta-lactams, LL-37 caused the highest inhibition of LPS-induced TNF-α and NO production in RAW 264.7 cells, and it was effective in vivo versus all parameters considered, regardless of the animal model utilized. Importantly, intravenous LL-37 produced a significant reduction in TNF-α plasma levels, compared to both control and beta-lactam-treated groups. In contrast, compared with polymyxin B, it produced a similar decrease in the concentration of plasma endotoxin. Since polymyxin B is frequently used as the standard against which novel antiendotoxin compounds are measured, it is significant that polymyxin B and LL-37 reduced the plasma levels of LPS to a similar extent and showed similar abilities in blocking induction of TNF in animal models. Models 2 and 3, which also evaluated the in vivo antimicrobial effects of the four compounds, showed that although LL-37 exhibited a somewhat lower antibacterial activity than other drugs, it decreased the rate of lethality at a level similar to those of imipenem and polymyxin B and better than that of piperacillin. It is important to observe in this regard that two key determinants of sepsis survival are bacterial clearance and the inflammatory response to the infection. Finally, comparable data were observed when the drugs were administered at 360 min (model 3).
The protective effect of LL-37 on animal survival is likely due to both the ability of this peptide to decrease the levels of endotoxin and cytokines in the plasma of septic animals and its bactericidal activity. Additional mechanisms by which LL-37 contributes to host defenses against microbial invasion have been previously described (7, 27, 28).
For instance, it has been shown that human blood-derived monocytes and T cells are chemotactic through formyl peptide receptor-like 1 at a concentration of approximately 50 μg/ml and toward mast cells through two different receptors at a concentration of between 5 and 10 μg/ml. Since leukocytes participate in both innate and adaptive immunity, the fact that LL-37 can chemoattract human leukocytes may provide one additional mechanism by which LL-37 contributes to host defense against microbial invasion by participating in the recruitment of leukocytes to sites of infection (9, 28).
Further studies are needed to investigate the feasibility of parenteral administration of LL-37 in animals and humans and better characterize its toxicity and potential therapeutic usefulness compared to similar compounds such as polymyxins.
Acknowledgments
This work was supported by grants from the Italian Ministry of Education, University and Research (PRIN 2003) and the Interuniversity Consortium for Biotechnology (CIB).
REFERENCES
- 1.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 3.Bochud, P. Y., and T. Calandra. 2003. Pathogenesis of sepsis: new concepts and implications for future treatment. Br. Med. J. 326:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boman, H. G. 2003. Antibacterial peptides: basic facts and emerging concepts. J. Int. Med. 254:197-215. [DOI] [PubMed] [Google Scholar]
- 5.Bone, R. C. 1991. Pathophysiology of sepsis. Ann. Intern. Med. 115:457-469. [DOI] [PubMed] [Google Scholar]
- 6.Bowdish, D. M. E., D. J. Davidson, D. P. Speert, and R. E. Hancock. 2004. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 172:3758-3767. [DOI] [PubMed] [Google Scholar]
- 7.Bowdish, D. M. E., D. J. Davidson, M. G. Scott, and R. E. Hancock. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 49:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon, M. 1987. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 9.De Yang, L., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemo attract human peripheral blood neutrophils, monocytes, and cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diekema, D. J., M. A. Pfaller, R. N. Jones, G. V. Doern, P. L. Winokur, A. C. Gales, H. S. Sader, K. Kugler, and M. Beach. 1999. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 29:595-607. [DOI] [PubMed] [Google Scholar]
- 11.Drabick, J. J., A. K. Bhattacharjee, D. L. Hoover, G. E Siber, V. E. Morales, L. D. Young, S. L. Brown, and A. S. Cross. 1998. Covalent polymyxin B conjugate with human immunoglobulin G as an antiendotoxin reagent. Antimicrob. Agents Chemother. 42:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgeworth, J. D., D. F. Treacher, and S. J. Eykyn. 1999. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit. Care Med. 27:1421-1428. [DOI] [PubMed] [Google Scholar]
- 13.Giacometti, A., O. Cirioni, R. Ghiselli, C. Bergnach, F. Orlando, G. D'Amato, F. Mocchegiani, C. Silvestri, M. S. Del Prete, B. Skerlavaj, M. Zanetti, V. Saba, and G. Scalise. 2004. The antimicrobial peptide BMAP-28 reduces lethality in mouse models of staphylococcal sepsis. Crit. Care Med. 32:2485-2490. [DOI] [PubMed] [Google Scholar]
- 14.Giacometti, A., O. Cirioni, R. Ghiselli, F. Mocchegiani, G. D'Amato, R. Circo, F. Orlando, B. Skerlavaj, C. Silvestri, V. Saba, M. Zanetti, and G. Scalise. 2004. Cathelicidin peptide sheep myeloid antimicrobial peptide-29 prevents endotoxin-induced mortality in rat models of septic shock. Am. J. Respir. Crit. Care Med. 169:187-194. [DOI] [PubMed] [Google Scholar]
- 15.Giacometti, A., O. Cirioni, R. Ghiselli, F. Mocchegiani, G. D'Amato, M. S. Del Prete, F. Orlando, W. Kamysz, J. Łukasiak, V. Saba, and G. Scalise. 2003. Administration of protegrin peptide IB-367 to prevent endotoxin-induced mortality in bile duct-ligated rats. Gut 52:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glauser, M. P., G. Zanetti, J. D. Baumgartner, and J. Cohen. 1991. Septic shock: pathogenesis. Lancet 338:732-736. [DOI] [PubMed] [Google Scholar]
- 17.Gough, M., R. E. W. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hack, C. E., L. A. Aarden, and L. G. Thijs. 1997. Role of cytokines in sepsis. Adv. Immunol. 66:101-195. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 20.Hancock, R. E. W., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaccard, C., N. Troillet, S. Harbarth, G. Zanetti, D. Aymon, R. Schneider, R. Chiolero, B. Ricou, J. Romand, O. Huber, P. Ambrosetti, G. Praz, D. Lew, J. Bille, M. P. Glauser, and A. Cometta. 1998. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob. Agents Chemother. 42:2966-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, A., and R. L. Anel. 2001. Experimental and emerging therapies for sepsis and septic shock. Expert Opin. Investig. Drugs 10:1471-1485. [DOI] [PubMed] [Google Scholar]
- 23.Mallen-St. Clair, J., C. T. Pham, S. A. Villalta, G. H. Caughey, and P. J. Wolters. 2004. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J. Clin. Investig. 113:628-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manocha, S., D. Feinstein, A. Kumar, and A. Kumar. 2002. Novel therapies for sepsis: antiendotoxin therapies. Expert Opin. Investig. Drugs 11:1795-1812. [DOI] [PubMed] [Google Scholar]
- 25.Meng, G., M. Rutz, M. Schiemann, J. Metzger, A. Gabriec, R. Shawndner, P. B. Luppa, F. Ebel, D. H. Busch, S. Bauer, H. Wagner, and C. J. Kirschning. 2004. Antagonist antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Investig. 113:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. National Committee for Clinical Laboratory Standards , Wayne, PA.
- 27.Oren, Z., J. C. Lerman, H. Gudmundsson, B. Agerberth, and Y. Shai. 1999. Structure and organization of the human peptide LL-37 in phospholipids membranes: relevance to the molecular basis for its non-cell selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 28.Oren, Z., and Y. Shai. 1998. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 47:451-463. [DOI] [PubMed] [Google Scholar]
- 29.Parker, S. J., and P. E. Watkins. 2000. Experimental models of gram-negative sepsis. Br. J. Surg. 88:22-30. [DOI] [PubMed] [Google Scholar]
- 30.Remick, D. G., D. E. Newcomb, G. L. Bolgos, and D. R. Call. 2000. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13:110-116. [DOI] [PubMed] [Google Scholar]
- 31.Sharma, V. K., and R. P. Dellinger. 2003. Recent developments in the treatment of sepsis. Expert Opin. Investig. Drugs 12:139-152. [DOI] [PubMed] [Google Scholar]
- 32.Supajatura, V., H. Ushio, A. Nakao, K. Okumura, C. Ra, and H. Ogawa. 2001. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 167:2250-2256. [DOI] [PubMed] [Google Scholar]
- 33.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel, R. P., M. R. Pinsky, R. J. Ulevitch, and L. Young. 1996. Current understanding of sepsis. Clin. Infect. Dis. 22:407-413. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler, A. P., and G. R. Bernard. 1999. Treating patients with severe sepsis. N. Engl. J. Med. 340:207-214. [DOI] [PubMed] [Google Scholar]
- 36.Yethon, J. A., and C. Whitfield. 2001. Lipopolysaccharide as a target for the development of novel therapeutics in gram-negative bacteria. Curr. Drug Targets Infect. Disord. 1:91-106. [DOI] [PubMed] [Google Scholar]
- 37.Yibin, G., Z. Jiang, Z. Hong, L. Gengfa, W. Liangxi, W. Guo, and L. Yongling. 2005. A synthetized cationic tetradecapeptide from hornet venom kills bacteria and neutralizes lipopolysaccharide in vivo and in vitro. Biochem. Pharmacol. 70:209-219. [DOI] [PubMed] [Google Scholar]
- 38.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 39.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]