Abstract
The NS3-4A serine protease of hepatitis C virus (HCV) is essential for viral replication and therefore has been one of the most attractive targets for developing specific antiviral agents against HCV. VX-950, a highly selective, reversible, and potent peptidomimetic inhibitor of the HCV NS3-4A protease, is currently in clinical development for the treatment of hepatitis C. In this report, we describe the in vitro characterization of anti-HCV activities of VX-950 in subgenomic HCV replicon cells. Incubation with VX-950 resulted in a time- and dose-dependent reduction of HCV RNA and proteins in replicon cells. Moreover, following a 2-week incubation with VX-950, a reduction in HCV RNA levels of 4.7 log10 was observed, and this reduction resulted in elimination of HCV RNA from replicon cells, since there was no rebound in replicon RNA after withdrawal of the inhibitor. The combination of VX-950 and alpha interferon was additive to moderately synergistic in reducing HCV RNA in replicon cells with no significant increase in cytotoxicity. The benefit of the combination was sustained over time: a 4-log10 reduction in HCV RNA level was achieved following a 9-day incubation with VX-950 and alpha interferon at lower concentrations than when either VX-950 or alpha interferon was used alone. The combination of VX-950 and alpha interferon also suppressed the emergence of in vitro resistance mutations against VX-950 in replicon cells.
The hepatitis C virus (HCV) epidemic continues to present a serious health challenge affecting 170 million people worldwide (28, 37). The current standard of care (SOC) for chronic hepatitis C is a combination of pegylated alpha interferon (IFN-α) and ribavirin (for a review, see references 30 and 38 and references therein), which can induce sustained viral response in less than half of genotype 1 patients, who account for the majority of the HCV-infected population in developed countries. Moreover, IFN-α is associated with serious adverse effects including leucopenia, thrombocytopenia, neutropenia, depression, fatigue, and “flu-like” symptoms. The addition of ribavirin, while enhancing the sustained viral response, is also associated with a serious side effect, hemolytic anemia. These side effects are sometimes dose limiting and may lead to discontinuation of treatment. Novel, safer and more effective drugs are urgently needed for the treatment of hepatitis C.
HCV, a member of the Flaviviridae family of viruses, has a 9.6-kb plus-strand RNA genome that encodes a polyprotein precursor of about 3,000 amino acids. This polyprotein precursor is processed proteolytically upon translation by both cellular and viral proteases to 10 individual proteins, including four structural proteins (C, E1, E2, and p7) and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) [for a review, see reference 25). Among the nonstructural proteins, NS5B RNA-dependent RNA polymerase and NS3, which consists of an N-terminal serine protease domain and a C-terminal helicase domain, are essential for viral replication (16) and thus are considered attractive targets for antiviral drugs (for a review, see reference 5). The serine protease activity of NS3, in a noncovalent complex with the NS4A cofactor, is responsible for the proteolytic cleavage at four junctions of the HCV polyprotein precursor: NS3/4A (self cleavage), NS4A/4B, NS4B/5A, and NS5A/5B (1, 2, 6, 11-13, 15, 21, 22, 39, 40).
The success of human immunodeficiency virus (HIV) protease inhibitors in treating HIV-infected patients has raised the hope that inhibitors of HCV NS3-4A serine protease could also become effective therapy options for hepatitis C patients. Indeed, significant progress has been made in recent years (for a review, see reference 5), and clinical proof of concept for this class of inhibitors has been demonstrated with three HCV NS3-4A protease inhibitors, BILN 2061 (14, 17), VX-950 (35, 36), and SCH 503034 (47). The average reduction in plasma viral load after a 2-day dosing for genotype 1 HCV-infected patients was ∼2.5 to 3.0 log10 for 500 mg twice daily (1,000 mg/day) BILN 2061 (14, 17), or ∼3.0 log10 for 750 mg every 8 h (2,250 mg/day) VX-950 (35, 36). The average maximal reduction in plasma viral load during a 14-day dosing was 4.65 log10 for 750 mg every 8 h VX-950 (35, 36), or 2.06 log10 for 400 mg thrice daily (1,200 mg/day) SCH 503034 (47). For some patients dosed with VX-950, the HCV plasma viral load dropped by more than 4 log10 to below the limit of detection (<10 IU/ml) during the 14 days of dosing (35, 36).
VX-950, a highly selective, potent inhibitor of HCV NS3-4A protease, was derived from the viral NS5A/5B substrate of the protease using structure-based drug design techniques. Unlike BILN 2061 (a noncovalent inhibitor), VX-950 is a covalent, reversible inhibitor of the NS3-4A protease with a slow-binding and slow-dissociation mechanism. As such, VX-950 exhibits significantly different kinetics in enzyme inhibition, which is most clearly exemplified by a very long half-life (58 min) of the bound enzyme-inhibitor complex (32, 33). The steady-state inhibitory constant (Ki*) of VX-950 was 7 nM against a genotype 1 (H strain) NS3 protease domain plus a NS4A cofactor peptide (32, 33). Here we demonstrate that VX-950 has excellent anti-HCV activities in vitro in HCV replicon cells. In addition, VX-950 is additive to moderately synergistic with IFN-α in inhibiting HCV replication and in suppressing the emergence of resistance in replicon cells.
MATERIALS AND METHODS
Materials.
VX-950 was prepared at Vertex Pharmaceuticals, Incorporated using a method described previously (46). BILN 2061 was discovered at Boehringer Ingelheim, and its synthesis has been described (7). Human recombinant IFN-α was purchased from Calbiochem (La Jolla, CA). Human serum was prepared from whole-blood donations and had tested negative for HCV (Bioreclamation, Hicksville, NY).
Cells.
Parental Huh-7 and HepG2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and nonessential amino acids. Stable Huh-7 cells containing the self-replicating, subgenomic HCV replicon, which was identical in sequence to the I377neo/NS3-3′/wt replicon described by Lohmann et al. (26), were selected and maintained in the presence of 0.25 mg/ml G418 (Invitrogen, Carlsbad, CA) and were used for anti-HCV assays. Peripheral blood mononuclear cells (PBMC) were isolated from fresh donor blood and cultured in RPMI-1640 medium (JRH Biosciences).
Determination of anti-HCV activity and cytotoxicity.
Determination of 50% inhibitory concentration (IC50), 90% inhibitory concentration (IC90), and 50% cytotoxic concentration (CC50) of VX-950 or IFN-α in HCV replicon cells was performed as described previously (23, 32). Briefly, 1 × 104 replicon cells per well were plated in 96-well plates. On the following day, replicon cells was incubated at 37°C for the indicated period of time with antiviral agents serially diluted in DMEM plus 2% FBS and 0.5% dimethyl sulfoxide (DMSO). Total cellular RNA was extracted using an RNeasy-96 kit (QIAGEN, Valencia, CA), and the copy number of HCV RNA was determined using a quantitative RT-PCR (QRT-PCR) assay (23). Each datum point represents the average of five replicates in cell culture. The cytotoxicity of VX-950 was measured under the same experimental settings using a tetrazolium (MTS)-based cell viability assay (Promega, Madison, WI). For the cytotoxicity assay with human hepatocyte cell lines, 1 × 104 parental Huh-7 cells per well or 4 × 104 HepG2 cells per well were used. To determine cytotoxicity of VX-950 against resting PBMC, 1 × 105 cells per well were incubated with VX-950 in RPMI-1640 medium (no serum) for 48 h, and the cell viability was determined by the MTS-based assay. To determine cytotoxicity of VX-950 against proliferating PBMC, 1× 105 cells per well in RPMI-1640 medium were added to a 96-well plate, which was precoated with anti-human CD3 antibody (Accurate Chemical & Scientific Corporation, Westbury, NY). The cells were incubated with VX-950 and anti-human CD28 antibody (Pharmingen/BD Biosciences, San Jose, CA) for 72 h at 37°C, and the cell growth was determined by [3H]thymidine update between the 48th and 72nd h.
Western blot analysis and immunofluorescence staining.
For Western blot analysis, cells were lysed after incubation with anti-HCV compounds. The concentration of total protein of cell lysates was determined using a BCA protein assay (Pierce, Rockford, IL). Equal amounts of protein were loaded on gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The presence of HCV NS3 and NS5A proteins in the replicon cells were detected by monoclonal antibodies specifically directed against NS3 (ViroStat, Portland, ME) or NS5A (BioDesign, Saco, ME). Purified NS3 antigen and NS5A antigen (ViroStat) were used as positive controls. An antibody directed against β-actin (Sigma, St. Louis, MO) was used to confirm equal loading of protein. For immunofluorescence staining, cells were fixed with methanol and an HCV NS3-specific monoclonal antibody (ViroStat) was used for detection of NS3 protein in the replicon cells.
Protein binding of VX-950 to human, dog, or rat plasma.
VX-950 was added to rat, dog, or human plasma to a final concentration of 5 or 50 μM in the presence of 0.2% DMSO and incubated for 15 min at 37°C. Atropine and propranolol were used as controls. The plasma protein-bound and protein-free fractions of VX-950 were separated by ultrafiltration. One quarter of the incubation was added to Amicon Centrifree Micropartition devices (n = 3) (Millipore, Billerica, MA). The devices were centrifuged, and the protein-bound and protein-free fractions of VX-950 were analyzed by high-pressure liquid chromatography.
Nine-day HCV replicon clearance assay.
The 9-day HCV replicon clearance assay was carried out in the absence of selection pressure from G418 as described previously (23). HCV replicon cells were plated at a density of 500 cells per well in a 96-well plate and incubated with antiviral agents (serially diluted in DMEM containing 10% FBS and 0.2% DMSO). The media and antiviral agents were replenished every 3 days. The number of cells in each well was determined by the tetrazolium (MTS)-based cell viability assay with an established standard curve. The level of HCV RNA remaining in the cells was determined by the QRT-PCR assay. The copy number of HCV replicon RNA per cell was calculated and normalized to that of no-compound control cells incubated with 0.2% DMSO for the same duration. Each datum point represents the average of five replicates in cell culture.
HCV replicon rebound study.
The HCV replicon rebound experiment was carried out as described previously (23). HCV replicon cells were plated in a six-well plate at a density of 2 × 105 cells per well. The cells were incubated for 13 days with no-compound control (0.2% DMSO) or antiviral agents (serially diluted in DMEM containing 10% FBS and 0.2% DMSO). The replicon cells were split every 3 to 4 days, the media and inhibitors were replenished, and a sample of cells was harvested. After 13 days of incubation, the cells were split and plated into fresh DMEM containing 10% FBS and 0.25 mg/ml G418 in the absence of antiviral agents. The culture was followed for three more weeks in the presence of G418, and cells were split or fresh medium was added every 3 to 4 days. During the 3 weeks, a cell sample was taken whenever the cells reached about 80% confluence and were split. For all the samples taken, the number of viable cells in each sample was determined using a ViaCount assay (Guava Technologies, Hayward, CA) according to the manufacturer's instruction. The level of HCV RNA in the cells was determined by the QRT-PCR assay, and then the copy number of HCV replicon RNA per viable cell in each sample was calculated.
Synergy and antagonism analysis of combination.
To quantitatively evaluate whether the effect of drug-drug combination was synergistic, additive, or antagonistic, HCV replicon cells were incubated with VX-950 or IFN-α at various concentrations either alone or in combination for 48 h. The experimental data were analyzed using CalcuSyn (Biosoft, Ferguson, MO), a computer program based on the method of Chou and Talalay (4). In this model, the dose-effect curves for each drug or drug combination were converted to median-effect plots with the program. Then the effect of combination was compared to that of a single agent. A combination index (CI) value for each experimental combination was calculated on the basis of the following equation: [(D)1/(Dx)1] + [(D)2/(Dx)2] + [(D)1(D)2/(Dx)1(Dx)2], where (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have x effect when each drug is used alone, respectively, and (D)1 and (D)2 are the doses of drug 1 and drug 2 that have the same x effect when used in combination. CI values of <1 indicate synergy, values of 1 indicate an additive effect, and values of >1 indicate antagonism.
Generation of resistance in replicon cells.
To evaluate generation of viral resistance against specific inhibitors, wild-type HCV replicon cells were plated in six-well plates at a density of 2 × 105 cells per well and serially passed in the presence of 0.25 mg/ml G418 and various concentrations of anti-HCV compounds. Fresh media and compounds were added to the monolayer every 3 to 4 days. Cells were split whenever they reached 70 to 80% confluence. For no-compound control cells or cells incubated with relatively low concentrations of the inhibitors, little cell death was observed, and a monolayer of the cells was maintained throughout the culture. For cells incubated with relatively high concentrations of inhibitors, most cells died after 2 to 3 weeks, while small colonies (in the order of 10 to 20 cells) started to appear, which were expanded for another 2 weeks and were analyzed for the presence of specific resistant mutations in the HCV NS3 protease domain.
RESULTS
VX-950 reduced HCV RNA levels in replicon cells in a time- and dose-dependent manner.
The anti-HCV activity of VX-950 (Fig. 1) was examined in Con1 (genotype 1b) subgenomic HCV replicon cells. In this system, inhibition of HCV NS3-4A protease by VX-950 was expected to block viral polyprotein processing and subsequently decrease viral RNA replication and total HCV RNA levels in the replicon cells. As shown in Fig. 2, VX-950 reduced HCV RNA levels in a time- and dose-dependent manner. The IC50s following a 24-, 48-, 72-, and 120-h incubation with VX-950 were determined to be 0.574, 0.488, 0.210, and 0.139 μM, respectively, indicating an increase in inhibitory effects with time. These results show that the IC50 declined as the culture time increased. Following three independent experiments using the 48-h incubation in the presence of 2% FBS, the average (±standard deviation [SD]) IC50 of VX-950 was determined to be 0.354 ± 0.035 μM, and the average (±SD) IC90 was 0.830 ± 0.190 μM. After a 48-h incubation with VX-950, no significant cytotoxicity, as evaluated in an MTS-based cell viability assay, was observed in the replicon cells. The average CC50 (±SD) of VX-950 in HCV replicon cells was 83 ± 27 μM, which resulted in a selective index, i.e., the n-fold value of CC50 over IC50, of 230 ± 59. In both parental Huh-7 and HepG2 cell lines, no significant cytotoxicity was observed after a 48-h incubation with up to 30 μM VX-950 in the presence of DMEM plus 2% FBS.
FIG. 1.
Chemical structure of VX-950.
FIG. 2.
Time- and dose-dependent reduction of HCV RNA in the replicon cells by VX-950. The replicon cells were incubated with various concentrations of VX-950 for 24, 48, 72, or 120 h. At the end of each incubation period, total RNA was extracted by RNeasy-96, and the levels of HCV RNA remaining were determined by the QRT-PCR assay and are shown as a percentage relative to the levels of HCV RNA in cells incubated with 0.5% DMSO (no-compound control). Each data point represents the average and SD for five cell culture replicates.
VX-950 reduced HCV protein levels in replicon cells.
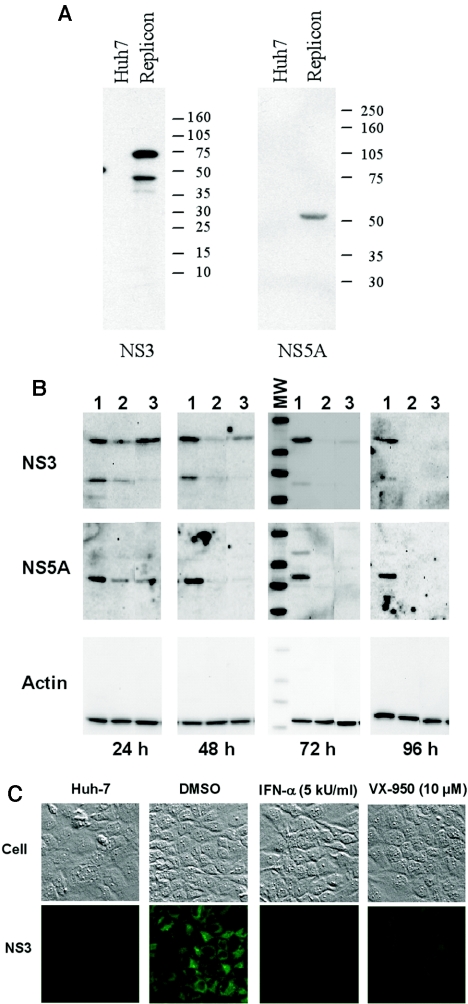
The anti-HCV activity of VX-950 in HCV replicon cells was also examined at the protein level using both Western blot analysis and immunofluorescence staining. The specificity of the antibodies against the HCV NS3 or NS5A protein was first confirmed using Western blot analysis, which showed that the HCV proteins were present in the replicon cells but not in the parental Huh-7 cells (Fig. 3A). To determine the effect of inhibitors on HCV proteins, replicon cells were incubated with 0.5% DMSO, 5 kU/ml IFN-α, or 10 μM VX-950 for 24, 48, 72, or 96 h. Equal amounts of proteins from the cell lysates were subjected to SDS-PAGE and Western blot analysis using specific antibodies against NS3, NS5A, or β-actin. As shown in Fig. 3B, the level of HCV proteins was relatively stable over 96 h in DMSO mock-incubated cells. In contrast, in cells incubated with VX-950 or IFN-α, there was a time-dependent decrease of HCV NS3 and NS5A proteins, while the level of β-actin remained constant. The HCV proteins became undetectable after a 96-h incubation of the replicon cells with the inhibitors. The reduction of HCV proteins was also demonstrated by immunofluorescence staining of the replicon cells using an NS3-specific antibody (Fig. 3C). The HCV NS3 protein was not present in the parental Huh-7 cells and was localized mostly on the endoplasmic reticulum membrane in the cytoplasm of replicon cells, which is consistent with what has been previously reported (10). After a 72-h incubation with 5 kU/ml IFN-α or 10 μM VX-950, there was little, if any, staining for the HCV NS3 protein.
FIG. 3.
Reduction of HCV proteins in the replicon cells by VX-950. (A) Specificity of the antibodies against HCV NS3 and NS5A proteins in Western blot analysis. Cell lysates from the parental Huh-7 (Huh7) or HCV replicon (Replicon) cells were analyzed by SDS-PAGE, transferred, and probed with specific antibody against NS3 (left) or NS5A (right) antigens. The molecular weight markers are indicated at the right. (B) Reduction of HCV proteins in a Western blot. Replicon cells were incubated with 0.2% DMSO (no-compound control) (lane 1), 5 kU/ml IFN-α (lane 2), or 10 μM VX-950 (lane 3) for 24, 48, 72, or 96 h. Equal amounts of protein from each cell extract were subjected to SDS-PAGE and subsequent Western blot analysis with specific antibodies against NS3 (top), NS5A (middle), or β-actin (bottom). The molecular weight markers (MW), shown in the left lane of the 72 h subpanel, are the same as those used in panel A. (C) Reduction of HCV proteins in immunofluorescence staining. The parental Huh-7 cells (left) or HCV replicon cells were either incubated with 0.2% DMSO (no-compound control), 5 kU/ml IFN-α, or 10 μM VX-950 for 72 h and then subjected to regular microscopy (top) or to immunofluorescence staining using an anti-NS3 antibody (bottom).
Effect of human serum on anti-HCV activity of VX-950.
Human serum contains relatively high amounts of protein, which could reduce the in vivo activity of drugs with high protein-binding properties by lowering the concentration of the free drug. To determine the degrees of binding to serum protein, VX-950 was incubated with rat, dog, or human plasma for 15 min at 37°C. The plasma protein-bound and protein-free fractions of VX-950 were separated by ultrafiltration using Amicon Centrifree Micropartition devices. At 5 μM, the fraction of plasma protein-bound VX-950 was 87%, 95%, or >99% for human, dog, or rat plasma, respectively. At 50 μM, the fraction of VX-950 bound to plasma protein was lower, with 71%, 66%, or 99% bound to human, dog, or rat plasma, respectively. Because the 48-h HCV replicon assay is performed in the presence of 2% FBS, we used higher concentrations of human serum to evaluate the effect of serum protein binding on antiviral activity. For these experiments, human serum was prepared from freshly donated blood that was HCV negative. HCV replicon cells were incubated for 48 h with 0.5% DMSO control or with 0.03, 0.1, 0.3, 1, 3, or 10 μM of VX-950 in the presence of 0%, 10%, 20%, or 40% (vol/vol) human serum. Anti-HCV activity was evaluated by determining reductions in HCV RNA levels, and cytotoxicity was evaluated in the MTS-based cell viability assay. The cytotoxicity of VX-950 was not significantly affected by the addition of human serum (Table 1), and the incubation of HCV replicon cells with up to 40% human serum had little, if any, effect on HCV RNA levels (data not shown). As the percentage of human serum increased, the 48-h IC50s and IC90s of VX-950 gradually increased (Table 1). At the highest percentage of human serum tested (40%), the IC50 of VX-950 was increased by 10.4-fold and the IC90 of VX-950 increased by 6.0-fold, compared with data obtained in the absence of human serum.
TABLE 1.
Effect of human serum on the 48-h IC50s and IC90s of VX-950a
| Concn of human serum (%) | IC50 (μM) | IC90 (μM) | CC50 (μM) |
|---|---|---|---|
| 0 | 0.33 | 0.79 | >30 |
| 10 | 0.88 | 1.37 | >30 |
| 20 | 1.40 | 2.52 | >30 |
| 40 | 3.45 | 4.76 | >100 |
Human serum was added to cell culture medium for a final concentration of 0%, 10%, 20%, or 40% (vol/vol), along with 0.03, 0.1, 0.3, 1, 3, or 10 μM VX-950 or 0.5% DMSO (no-compound control). The IC50s, IC90s, and CC50s were determined after a 48-h incubation.
The lack of cytotoxicity of VX-950 in PBMC.
The effect of VX-950 on nonproliferating cells was also examined in a standard in vitro cytotoxicity assay using resting PBMC. The cells were isolated from fresh blood donated by healthy volunteers and were incubated with various concentrations of VX-950 in the absence of serum so that the cells were not proliferating. The effect of VX-950 on cell viability rather than cell proliferation was determined after 48 h of incubation in an MTS-based assay. There was no reduction in cell viability in the presence of 3, 10, or 30 μM VX-950 in two independent experiments. In addition, VX-950 was incubated with proliferating PBMC for 72 h, which were preincubated and stimulated with anti-CD3 and anti-CD28 antibodies. Again, there was no reduction in cell viability in the presence of up to 30 μM VX-950 in two independent experiments.
VX-950 induced a multilog reduction of HCV RNA levels in replicon cells.
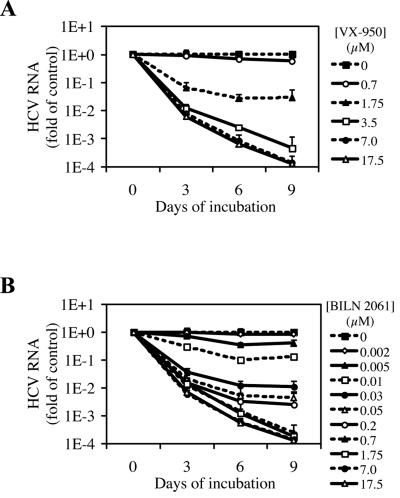
To investigate whether VX-950 or BILN 2061 is able to completely block HCV RNA replication and result in a multilog reduction in HCV replicon RNA levels, we tested these two protease inhibitors in a 9-day assay that we developed and described previously (23). In this case, replicon cells were incubated with various concentrations of VX-950 or BILN 2061 for three, six, or nine consecutive days. To preclude potential cytotoxic or cytostatic effects of these inhibitors, the HCV RNA levels in the cells were normalized by the number of viable cells. As shown in Fig. 4A, VX-950 reduced HCV RNA copies per cell in a time- and concentration-dependent manner. For example, a 3.3-log10 drop in HCV RNA levels was achieved after a 9-day incubation with 3.5 μM VX-950, which is 10 times the VX-950 IC50 in our 48-h replicon assay. With higher concentrations of VX-950, such as 7.0 or 17.5 μM, an approximately 4-log10 reduction in HCV RNA levels was observed after 9 days. BILN 2061 has an IC50 of 4 nM (20) in the 48-h HCV replicon assay. In our 9-day assay, BILN 2061 also reduced HCV RNA in a time- and concentration-dependent manner (Fig. 4B). However, a 3-log10 reduction in HCV RNA was not achieved after a 9-day incubation with concentrations of BILN 2061 up to 0.2 μM, which is 50 times its IC50 in the 48-h assay. A 3.6-log10 decrease in HCV RNA was achieved with 0.7 μM BILN 2061 after 9 days. These results suggest that although there is ∼90-fold difference in IC50s between VX-950 and BILN 2061 in the 48-h assay, there is only ∼5-fold or less difference in anti-HCV potency (multilog reduction of HCV RNA) in the 9-day replicon clearance assay.
FIG. 4.
Nine-day HCV replicon clearance assay. HCV replicon cells were incubated with various concentrations of VX-950 (A) or BILN 2061 (B) for 3, 6, or 9 days. Fresh protease inhibitors were added to the medium every 3 days. At the end of each incubation period, cell numbers were determined by the tetrazolium (MTS)-based cell viability assay with an established standard curve, and the level of HCV RNA in the cells was determined by the QRT-PCR assay. The HCV RNA copy number per cell in each sample is plotted as an n-fold value (on a log10 scale) of the copy number in replicon cells incubated with 0.2% DMSO (no-compound control) for the same period of time. Each datum point represents the average and SD for five cell culture replicates.
VX-950 prevents rebound of HCV replicon RNA after withdrawal of the inhibitor.
The goal of anti-HCV therapy is to completely eradicate the virus and to prevent a rebound after the end of drug treatment, also known as a sustained viral response. Previously, we developed a model system using replicon cells to determine the ability of antiviral compounds to completely eradicate HCV RNA from replicon cells and subsequently prevent rebound after the antiviral compounds were withdrawn (23). In this model system, HCV replicon cells were plated at a higher density in six-well culture plates than in the 9-day experiments described above, which used 96-well plates. Accordingly, in the rebound experiment, cells were split every 3 to 4 days as they grew to confluence. In the first part of the rebound experiment, HCV replicon cells were incubated with various concentrations of VX-950 for 13 days in the absence of G418, during which VX-950 was replenished whenever the cells were split. Again, to preclude potential cytotoxic or cytostatic effects, the HCV RNA levels in the cells were normalized by the number of viable cells. As shown in Fig. 5, the level of HCV RNA copies per cell remained stable in 0.2% DMSO mock-incubated cells. In contrast, there was a concentration- and time-dependent reduction of HCV RNA in VX-950-incubated cells. Incubation of replicon cells for 9 days with 3.5 or 17.5 μM VX-950 resulted in reduction in HCV RNA of 3.4- or 4.7-log10, respectively. After a 13-day incubation with 17.5 μM VX-950, the HCV RNA was not detectable by the QRT-PCR assay (Fig. 5). Since a 4.7-log10 reduction was at the detection limit of our QRT-PCR assay, the HCV replicon RNA may have been cleared from these cells.
FIG. 5.
VX-950 prevents rebound of HCV replicon upon withdrawal of inhibitor. HCV replicon cells were incubated with various concentrations of VX-950 in the absence of G418. Cells were split every 3 to 4 days, and fresh medium containing VX-950 was added at every split. After 13 days of incubation, VX-950 was withdrawn and 0.25 mg/ml G418 was added to enrich the remaining HCV replicon-positive cells that are capable of growing in the presence of G418 (rebound). The cultures were monitored for another 21 days in the presence of 0.25 mg/ml G418, and cell samples were collected whenever the cell monolayer reached confluence. The number of viable cells was determined by the ViaCount assay, and the level of HCV RNA in the cells was determined by the QRT-PCR assay. The absolute numbers of HCV replicon RNA copies per viable cell are shown. The dashed line indicates the detection limit of our QRT-PCR assay.
To confirm this finding, VX-950 was withdrawn after the 13-day incubation to allow rebound of any remaining HCV replicon RNA. The HCV replicon is a stable cell line maintained under G418 selection, which enriches the population of replicon-positive cells over that of replicon-negative cells. Therefore, to selectively expand any cell from which viable HCV replicon RNA had not been completely cleared, 0.25 mg/ml G418 was added to the medium upon withdrawal of VX-950. The cells were cultured for three additional weeks in the presence of G418, during which time the cells were split whenever they reached 80% confluence, and a cell sample was taken at the same time. Cells that had completely lost the HCV replicon died between 10 and 14 days in the presence of 0.25 mg/ml G418, just like their parental Huh-7 cells. Within 2 weeks after the withdrawal of VX-950, the level of HCV replicon RNA in cells that had been incubated with 1.0 or 3.5 μM VX-950 rebounded to the same level as that in no-compound control cells. In contrast, in cells incubated with 17.5 μM VX-950, no HCV replicon-positive cells were recovered, indicating that the HCV replicon RNA may have been effectively eliminated from these cells.
Combination of VX-950 with IFN-α is additive to moderately synergistic in replicon cells.
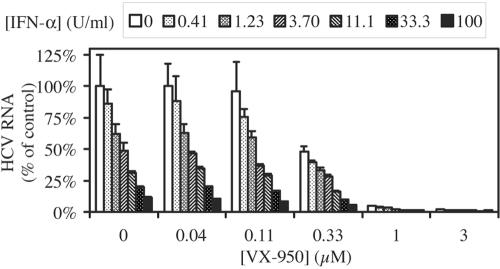
The current standard of care for the treatment of chronic hepatitis C is the combination of pegylated IFN-α and ribavirin. Since IFN-α, but not ribavirin, exhibits strong antiviral activities in replicon cells and in patients, we evaluated the effect of the combination of VX-950 and IFN-α in vitro using the replicon system. Replicon cells were incubated with various concentrations of VX-950, IFN-α, or combinations of both for 48 h, and the anti-HCV activity and cytotoxicity were examined. As shown in Fig. 6, the combination of VX-950 with IFN-α always resulted in a reduction in the level of HCV RNA greater than that achieved by each agent alone. There was no significant increase in cytotoxicity when VX-950 and IFN-α were used together. In fact, none of the incubations reduced cell viability by more than 5% (data not shown).
FIG. 6.
Combination of VX-950 with IFN-α in the 48-h HCV replicon cell assay. HCV replicon cells were incubated with various concentrations of VX-950 (indicated on the x axis) in combination with various concentration of IFN-α (indicated at the top) for 48 h. The level of HCV RNA remaining in replicon cells incubated with VX-950 and IFN-α in combination was determined by the QRT-PCR assay and is shown as a percentage of the levels in cells incubated with 0.5% DMSO (no-compound control). Each bar represents the average and SD for six cell culture replicates.
Next, we examined whether the effect of the combination was additive or synergistic. The data shown in Fig. 6 were analyzed using CalcuSyn, a computer program based on the mathematic model of Chou and Talalay (4). In this analysis, the anti-HCV effects produced by the combination of VX-950 and IFN-α at various ratios of concentrations were compared to those produced by either agent alone. A CI was calculated for each experimental combination using the equation described in Materials and Methods. A CI value of 1 indicates additive effect; a CI value of less than 1 indicates synergy; and a CI value of greater than 1 indicates antagonism. As shown in Table 2, most CI values were significantly less than 1 when the combinations of VX-950 and IFN-α at four different ratios were analyzed using this method. These results indicate that the combination of VX-950 and IFN-α has an additive to moderate synergistic effect on inhibition of HCV RNA replication in the replicon cells. The experimental data were also analyzed using a second mathematical model, MacSynergy (34), which also indicated an additive to moderate synergistic effect (data not shown).
TABLE 2.
CalcuSyn analysis of VX-950 and IFN-α combination
| Ratio (VX-950:IFN-α)a | Combination indexb:
|
||
|---|---|---|---|
| At IC50 | At IC75 | At IC90 | |
| 1:100 | 1.24 | 0.73 | 0.56 |
| 3:100 | 0.74 | 0.53 | 0.53 |
| 9:100 | 0.77 | 0.58 | 0.61 |
| 27:100 | 0.67 | 0.61 | 0.68 |
In each ratio, three to four different combinations of VX-950 and IFN-α were included. For example, the 1:100 ratio includes VX-950 plus IFN-α at 0.037 μM plus 3.7 U/ml, 0.11 μM plus 11.1 U/ml, 0.33 μM plus 33.3 U/ml, and 1.0 μM plus 100 U/ml.
IC50s, IC75s, or IC90s are concentrations of the compounds that achieve 50%, 75%, or 90% inhibition, respectively. The values of the combination index at various percentages of inhibition were calculated using the CalcuSyn program as described in Materials and Methods.
Enhancement of anti-HCV activity of VX-950 by the addition of IFN-α was sustained over time.
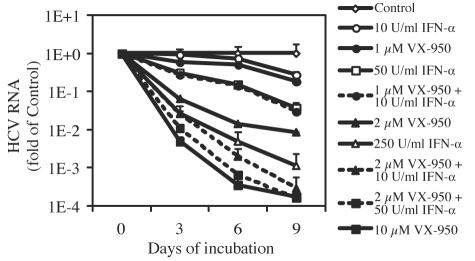
To investigate whether the enhancement of anti-HCV effect of VX-950 by IFN-α or vice versa can be sustained over time, we incubated the cells with VX-950, IFN-α, or combinations of both agents for three, six, or nine consecutive days and measured the amount of HCV replicon RNA remaining in the cells. Again, to preclude potential cytotoxic or cytostatic effects, HCV RNA levels in the cells were normalized by the number of viable cells. As shown in Fig. 7, there was a time-dependent decrease in the level of HCV RNA in replicon cells incubated with the anti-HCV compound regimens. After 9 days of incubation, 1.0 μM VX-950 reduced the level of HCV RNA by 0.7 log10, and 10 U/ml IFN-α reduced HCV RNA levels by 0.6-log10, whereas the combination of the two agents resulted in a slightly greater reduction (1.5 log10). Indeed, a combination of 1.0 μM VX-950 with 10 U/ml IFN-α achieved a degree of HCV RNA reduction that was comparable to that observed with a higher concentration of IFN-α (50 U/ml) at day 9 (1.4 log10). The combination of a slightly higher concentration of VX-950 (2.0 μM) with 10 U/ml IFN-α resulted in a 3.5-log10 reduction in HCV RNA at day 9, which is much more than the sum of either agent alone (2.1 log10 for 2.0 μM VX-950 alone and 0.6 log10 for 10 U/ml IFN-α alone). In fact, the HCV RNA reduction observed in the latter combination was comparable to that achieved at a much higher concentration of either agent alone (3.8 log10 with 10 μM VX-950 or 3.0 log10 with 250 U/ml IFN-α).
FIG. 7.
Combination of VX-950 and IFN-α in the 9-day HCV replicon clearance assay. The cells were incubated with various concentrations of VX-950 alone, various concentrations of IFN-α alone, or the two in combination for 3, 6, or 9 days. At the end of each incubation period, cell numbers were determined by the tetrazolium (MTS)-based cell viability assay with an established standard curve, and the level of HCV RNA in the cells was determined by the QRT-PCR assay. The HCV RNA copy number per cell in each sample is plotted as an n-fold value (on a log10 scale) of the copy number per cell in replicon cells incubated with 0.2% DMSO (no-compound control) for the same period of time. Each datum point represents the average and SD for five cell culture replicates.
Combination with IFN-α suppressed the emergence of VX-950-resistant variants in replicon cells.
In previous studies, we selected VX-950-resistant replicon cells using a stepwise, dose-escalating method (19, 20). The wild-type HCV replicon cells were serially passed in the presence of 0.25 mg/ml G418 and slowly increasing concentrations of VX-950. The dominant in vitro resistance variation against VX-950 was identified to be a substitution of Ala156 with Ser (A156S) in the protease domain (20). Two other variations at residue 156, A156T and A156V, were found to be cross-resistant to both VX-950 and BILN 2061 in replicon cells (19). Here, we investigated whether incubation of the wild-type HCV replicon with a combination of VX-950 and IFN-α would help suppress the emergence of these in vitro resistance variations against VX-950. Wild-type HCV replicon cells were plated in six-well plates and serially passed in the presence of 0.25 mg/ml G418 and various concentrations of VX-950, ranging from 0.35 μM to 7 μM. For each concentration, a duplicate set of cell culture in six-well plates was passed in parallel. Cells were split every 3 to 4 days upon reaching 70 to 80% confluence, and fresh compounds were added. After three passages, there was a dose-dependent decline in cell growth in the presence of VX-950. The higher the VX-950 concentration, the slower the cell growth was, presumably due to the inhibition of HCV RNA replication by VX-950 and the subsequent decrease in the expression of neomycin phosphotransferase protein. If cells did not reach 70 to 80% confluence, fresh media and compounds were added to the cell monolayer. After 18 days, small colonies (10 to 20 cells in size) were observed in the presence of 3.5 or 7.0 μM VX-950, whereas a monolayer of cells was recovered from incubation with 0, 0.35, 0.7, or 1.4 μM VX-950. In the latter case, the number of cells recovered inversely correlated with the concentrations of VX-950. In the presence of 3.5 μM VX-950, a total of 59 or 60 large colonies (with 50 to 100 cells per colony) were recovered in two separate wells at day 25. The few small colonies that were initially observed in the presence of 7.0 μM VX-950 at day 18 did not grow further and died by day 31. For cells recovered in the presence of antiviral agents, the nucleotide sequences of the HCV NS3 serine protease domain cDNA were determined after RNA extraction and RT-PCR, as described in Materials and Methods. VX-950 resistance mutations at Ala156 were observed in the large colonies recovered after incubation with 3.5 μM VX-950. At lower concentrations of VX-950, either 0.7 or 1.4 μM, a mixture of wild-type Ala and resistance mutations was seen at residue 156. No resistance mutations were found at residue 156 in the presence of 0.35 μM VX-950.
A parallel experiment was carried out as described above with the addition of 50 U/ml IFN-α to all the culture wells. IFN-α is expected to have a dual role in suppression of VX-950-resistant HCV replicons. First, IFN-α provides additional inhibitory pressure on HCV RNA replication and therefore decreases the probability of generation of resistance mutations. Second, the resistance mutants remain as sensitive to IFN-α as the wild-type replicon. A monolayer of cells was recovered in the presence of 50 U/ml IFN-α with or without 0.35 μM VX-950, and at residue 156, the wild-type Ala but not the resistance mutation was found. However, no HCV replicon cells were recovered by day 21 for 3.5 or 7.0 μM VX-950 plus 50 U/ml IFN-α, by day 27 for 1.4 μM VX-950 plus 50 U/ml IFN-α, or by day 31 for 0.7 μM VX-950 plus 50 U/ml IFN-α. These results demonstrated that the addition of IFN-α suppressed the emergence of in vitro resistance against VX-950 in cell culture.
DISCUSSION
In this report, we describe the in vitro anti-HCV activity of VX-950, a potent and specific inhibitor of the HCV NS3-4A serine protease, in subgenomic, genotype 1b HCV (Con1) replicon cells. VX-950 had an average IC50 of 0.354 μM in reducing HCV RNA in the genotype 1b (Con1) replicon cells following a 48-h incubation. No significant cytotoxicity was observed at a concentration up to 30 μM VX-950. Similarly, no cytotoxicity was observed against two human hepatocyte cell lines (Huh-7 and HepG2) and proliferating or resting PBMC at 30 μM VX-950. These data suggest that there could be an excellent therapeutic window for VX-950.
VX-950 is also an excellent inhibitor of genotype 2a HCV NS3 protease in the presence of a corresponding genotype 2a KK4A peptide (32). In another study using the NS4A cofactor peptide fused to the N terminus of the NS3 protease domain, VX-950 was shown to have equal potency against genotype 2 or 3 HCV protease as it does against genotype 1 enzyme (42). Although the potency of VX-950 in the recently developed HCV infectious cell culture using genotype 2 infectious cDNA clones (24, 44, 49) remains to be determined, PI-1, an α-ketoamide inhibitor that is related to VX-950, displayed potent antiviral activity in one of these infectious cell culture systems (24).
Most if not all drugs bind to human plasma proteins to a certain extent. It has been suggested that high protein binding could potentially limit the availability of free drug in blood and therefore its efficacy in vivo where high concentration of serum proteins are present in the circulation. Here we show that VX-950 had a 10-fold increase in the 48-h IC50 and 6-fold increase in the 48-h IC90 in the presence of 40% human serum, which is consistent with the fact that VX-950 was largely serum protein bound (87% bound to human serum at 5 μM). This serum effect was similar to that of another HCV protease inhibitor, BILN 2061, which also had a 10-fold increase in the 48-h IC50 in the presence of 40% human serum (data not shown). By analogy, HIV protease inhibitors are lipophilic organic bases and thus more than 90% protein bound, predominantly to α1-acid glycoprotein, except for indinavir (for a review, see reference 3). The IC50s of four other HIV protease inhibitors were reduced by 7- to 37-fold in the presence of 50% human serum compared to that in the absence of human serum. But the impact of protein binding on in vivo exposure of HIV protease inhibitors is complicated by many other factors, such as volume of distribution and metabolism. Since HCV replication occurs predominantly in infected liver and there is limited evidence of extrahepatic manifestation, the liver exposure of direct anti-HCV agents is likely much more important than that in blood. Therefore, binding to plasma proteins may have much less impact on HCV protease inhibitors than on HIV protease inhibitors.
One interesting observation is discrepancy in the comparison of potencies of VX-950 and BILN 2061 in the 48-h replicon cell assay versus the 9-day replicon cell assay. In the 48-h assay, VX-950 has an average IC50 of 0.354 μM, which is ∼90 times higher than that of BILN 2061 (IC50 = 4 nM). However, in the 9-day assay, a 3-log10 or more reduction in HCV RNA level was achieved with 3.5 μM VX-950 or 0.7 μM BILN 2061, only a fivefold difference. It is possible that the discrepancy of anti-HCV activities of these two inhibitors in the 48-h IC50 and 9-day clearance assays could be due, at least in part, to the difference in the mechanism of binding of the inhibitors to the HCV NS3-4A protease (32, 33). In our enzymatic assays, VX-950 inhibits HCV NS3-4A protease with a potency that is similar to that of BILN 2061, as evidenced by the similar steady-state constant for VX-950 (Ki* = 7 nM) (32) and BILN 2061 (Ki = 10 nM). BILN 2061 is a noncovalent and reversible inhibitor, for which the enzyme-inhibitor complex forms and dissociates quickly, presumably on the order of seconds. In contrast, formation and dissociation of the covalent complex between VX-950 and the HCV NS3-4A serine protease is a slow process (slow binding and slow dissociation). It has been determined that the half-life of the covalent complex is about 1 h (32, 33).
In addition, we demonstrated that incubation of HCV replicon cells with VX-950 for 2 weeks resulted in elimination of HCV RNA from the cells, as evidenced by the lack of rebound in HCV replicon RNA after the protease inhibitor was withdrawn. It is possible that cells containing a very small amount of HCV replicon RNA may not survive the selection pressure of G418 (although low at 0.25 mg/ml) and die during the 3-week selection period (in the absence of VX-950). Subculture during the 2-week VX-950 incubation period may also play a minor role in decline of number of cells containing HCV replicon RNA.
HCV has a very high replication rate in vivo, with an estimated daily production of 1012 virions in HCV-infected patients (31). Given the error-prone replication by the viral RNA-dependent RNA polymerase, preexisting variants that are resistant to specific anti-HCV drugs, such as HCV protease inhibitors, could get selected over wild-type HCV during therapy. In fact, such resistant variations have been selected in vitro and confirmed against several HCV NS3-4A protease inhibitors, including VX-950 (19, 20), BILN 2061 (19, 20, 27), SCH 503034 (41), SCH6 (45), and another inhibitor (43). Therefore, despite the potent anti-HCV activity of VX-950 demonstrated in the replicon cells, the optimal therapy could still involve the combination of VX-950 with other antiviral agents. This has been best illustrated with the treatment for HIV, where the combination of three or more drugs of various mechanisms is absolutely necessary to continuously suppress viral replication and the emergence of resistance. In this study, we demonstrated that the in vitro combination of IFN-α with VX-950 appeared to suppress the emergence of resistance variants against VX-950 in cell culture.
IFN-α is the main antiviral agent of current SOC for hepatitis C. The other component of the SOC therapy, ribavirin, increases the sustained antiviral response to IFN-α but does not exhibit a strong a direct antiviral activity by itself. In this study, we evaluated whether a combination with IFN-α will enhance the anti-HCV activity of VX-950 in replicon cells. First, the effect of the combination was quantitatively analyzed in a 48-h assay using mathematical analysis, which indicated an additive to moderate synergistic effect between the two antiviral agents. Importantly, there was no significant increase of cytotoxicity associated with the combination. Second, the enhanced antiviral activity was further demonstrated in the 9-day clearance assay, in which multilog reduction of viral RNA was achieved with the combination of the two antiviral agents at lower concentrations than those required for either single agent. These results for the combination of VX-950 and IFN-α in replicon cells suggest that the combination therapy should be an important strategy to explore in the clinical development of HCV NS3-4A protease inhibitors. It was recently reported that, in 14-day dosing trials, combination of SCH 503034 (48) or VX-950 with pegylated IFN-α resulted in a greater reduction of the plasma viral load for genotype 1 HCV-infected patients than that for patients dosed with either the pegylated IFN alone or the HCV protease inhibitor (SCH 503034 or VX-950) alone.
The additivity to moderate synergy we observed between HCV protease inhibitors and IFN-α (this report and reference 23) is consistent with several recent studies suggesting that the HCV NS3-4A protease could be one of the mechanisms that HCV uses to break down the host antiviral response. It was reported that HCV NS3-4A protease decreases phosphorylation of IFN regulatory factor 3 (IRF-3), thereby blocking translocation of IRF-3 into the nucleus, where it functions as a transcription activator (9). One of the HCV NS3-4A protease substrates in the IRF-3 signaling pathway was found to be Toll-IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF or TICAM-1). TRIF, an adaptor protein, is a critical component in the signal transduction cascade from toll-like receptor 3 (TLR-3) to the kinases responsible for activation of IRF-3 and NF-κB, two transcription activators that are essential for antiviral immune responses (18). TLR-3 is located on the cell surface and recognizes one of the pathogen-associated molecular patterns, double-stranded RNA, a replication intermediate of many RNA viruses. Double-stranded RNA is also recognized by intracellular DExD/H box RNA helicases, retinoic acid-inducible gene 1 (RIG-I) or Helicard (Mda5). The RIG-I signaling pathway, a TLR-3-independent pathway, is also disrupted by HCV NS3-4A protease (8). Recently it was shown that HCV NS3-4A protease cleaves Cardif, a new CARD domain-containing adaptor protein that interacts with RIG-I and recruits kinases to activate IRF-3 and NF-κB (29). These studies suggest that HCV NS3-4A protease inhibitors could have a dual anti-HCV function, blocking the HCV RNA replication and restoring the host antiviral response.
In conclusion, the data presented in this study demonstrate the excellent anti-HCV activities of VX-950. In the replicon cells, VX-950 is able to clear HCV RNA and prevent the rebound of viral RNA. In addition, our results indicate that the in vitro combination of VX-950 and IFN-α leads to a much stronger antiviral response than that seen with either agent alone. Finally, the in vitro combination of VX-950 and IFN-α suppresses the emergence of resistance variants against VX-950 in cell culture. Excellent antiviral activity observed in the recently completed phase 1b trial of VX-950 with chronic genotype 1 HCV-infected patients (35) suggests that VX-950 has the potential to become a novel therapeutic option for hepatitis C, and further evaluation as either a monotherapy or in combination is warranted.
Acknowledgments
We thank John Alford, Kevin Cottrell, Cynthia Gates, Yu-Ping Luong, Sue Ma, and John Maxwell for their contributions to the HCV protease drug discovery team, which enabled the study presented herein. We also thank Susan Almquist, Tara Kieffer, Sudipta Mahajan, Graeme Smith, Yee-Lan Wang, and Yi Zhou for their technical assistance. Finally, we thank Michael Briggs, Karen Eisenhauer, John Maxwell, Lindsay McNair, Mark Namchuk, John Randle, Scott Raybuck, and John Thomson for critical reading and editorial comments on the manuscript.
REFERENCES
- 1.Bartenschlager, R., L. Ahlborn-Laake, J. Mous, and H. Jacobsen. 1993. Nonstructural protein 3 of the hepatitis C virus encodes a serine-type proteinase required for cleavage at the NS3/4 and NS4/5 junctions. J. Virol. 67:3835-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager, R., V. Lohmann, T. Wilkinson, and J. O. Koch. 1995. Complex formation between the NS3 serine-type proteinase of the hepatitis C virus and NS4A and its importance for polyprotein maturation. J. Virol. 69:7519-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffito, M., D. J. Back, T. F. Blaschke, M. Rowland, R. J. Bertz, J. G. Gerber, and V. Miller. 2003. Protein binding in antiretroviral therapies. AIDS Res. Hum. Retrovir. 19:825-835. [DOI] [PubMed] [Google Scholar]
- 4.Chou, T. C., and P. Talalay. 1977. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J. Biol. Chem. 252:6438-6442. [PubMed] [Google Scholar]
- 5.De Francesco, R., L. Tomei, S. Altamura, V. Summa, and G. Migliaccio. 2003. Approaching a new era for hepatitis C virus therapy: inhibitors of the NS3-4A serine protease and the NS5B RNA-dependent RNA polymerase. Antivir. Res. 58:1-16. [DOI] [PubMed] [Google Scholar]
- 6.Failla, C., L. Tomei, and R. De Francesco. 1995. An amino-terminal domain of the hepatitis C virus NS3 protease is essential for interaction with NS4A. J. Virol. 69:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faucher, A. M., M. D. Bailey, P. L. Beaulieu, C. Brochu, J. S. Duceppe, J. M. Ferland, E. Ghiro, V. Gorys, T. Halmos, S. H. Kawai, M. Poirier, B. Simoneau, Y. S. Tsantrizos, and M. Llinas-Brunet. 2004. Synthesis of BILN 2061, an HCV NS3 protease inhibitor with proven antiviral effect in humans. Org. Lett. 6:2901-2904. [DOI] [PubMed] [Google Scholar]
- 8.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 10.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 77:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grakoui, A., D. W. McCourt, C. Wychowski, S. M. Feinstone, and C. M. Rice. 1993. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J. Virol. 67:2832-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijikata, M., H. Mizushima, Y. Tanji, Y. Komoda, Y. Hirowatari, T. Akagi, N. Kato, K. Kimura, and K. Shimotohno. 1993. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc. Natl. Acad. Sci. USA 90:10773-10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichsen, H., Y. Benhamou, H. Wedemeyer, M. Reiser, R. E. Sentjens, J. L. Calleja, X. Forns, A. Erhardt, J. Cronlein, R. L. Chaves, C. L. Yong, G. Nehmiz, and G. G. Steinmann. 2004. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology 127:1347-1355. [DOI] [PubMed] [Google Scholar]
- 15.Kim, J. L., K. A. Morgenstern, C. Lin, T. Fox, M. D. Dwyer, J. A. Landro, S. P. Chambers, W. Markland, C. A. Lepre, E. T. O'Malley, S. L. Harbeson, C. M. Rice, M. A. Murcko, P. R. Caron, and J. A. Thomson. 1996. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell 87:343-355. [DOI] [PubMed] [Google Scholar]
- 16.Kolykhalov, A. A., K. Mihalik, S. M. Feinstone, and C. M. Rice. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarre, D., P. C. Anderson, M. Bailey, P. Beaulieu, G. Bolger, P. Bonneau, M. Bos, D. R. Cameron, M. Cartier, M. G. Cordingley, A. M. Faucher, N. Goudreau, S. H. Kawai, G. Kukolj, L. Lagace, S. R. LaPlante, H. Narjes, M. A. Poupart, J. Rancourt, R. E. Sentjens, R. St George, B. Simoneau, G. Steinmann, D. Thibeault, Y. S. Tsantrizos, S. M. Weldon, C. L. Yong, and M. Llinas-Brunet. 2003. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426:186-189. [DOI] [PubMed] [Google Scholar]
- 18.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102:2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, C., C. A. Gates, B. G. Rao, D. L. Brennan, J. R. Fulghum, Y.-P. Luong, J. D. Frantz, K. Lin, S. Ma, Y.-Y. Wei, R. B. Perni, and A. D. Kwong. 2005. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J. Biol. Chem. 280:36784-36791. [DOI] [PubMed] [Google Scholar]
- 20.Lin, C., K. Lin, Y. P. Luong, B. G. Rao, Y. Y. Wei, D. L. Brennan, J. R. Fulghum, H. M. Hsiao, S. Ma, J. P. Maxwell, K. M. Cottrell, R. B. Perni, C. A. Gates, and A. D. Kwong. 2004. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 279:17508-17514. [DOI] [PubMed] [Google Scholar]
- 21.Lin, C., and C. M. Rice. 1995. The hepatitis C virus NS3 serine proteinase and NS4A cofactor: establishment of a cell-free trans-processing assay. Proc. Natl. Acad. Sci. USA 92:7622-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, C., J. A. Thomson, and C. M. Rice. 1995. A central region in the hepatitis C virus NS4A protein allows formation of an active NS3-NS4A serine proteinase complex in vivo and in vitro. J. Virol. 69:4373-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, K., A. D. Kwong, and C. Lin. 2004. Combination of a hepatitis C virus NS3-NS4A protease inhibitor and alpha interferon synergistically inhibits viral RNA replication and facilitates viral RNA clearance in replicon cells. Antimicrob. Agents Chemother. 48:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 25.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe, P. M. Howley, and D. E. Griffin (ed.), Fields virology, fourth ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 26.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 27.Lu, L., T. J. Pilot-Matias, K. D. Stewart, J. T. Randolph, R. Pithawalla, W. He, P. P. Huang, L. L. Klein, H. Mo, and A. Molla. 2004. Mutations conferring resistance to a potent hepatitis C virus serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 48:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHutchison, J. G., and K. Patel. 2002. Future therapy of hepatitis C. Hepatology 36:S245-S252. [DOI] [PubMed] [Google Scholar]
- 29.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health. 2002. Management of hepatitis C. Consensus Development Conference statement, June 10-12, 2002. Hepatology 36:S3-S20. [DOI] [PubMed] [Google Scholar]
- 31.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 32.Perni, R. B., S. J. Almquist, R. A. Byrn, G. Chandorkar, P. R. Chaturvedi, L. F. Courtney, C. J. Decker, K. Dinehart, C. A. Gates, S. L. Harbeson, A. Heiser, G. Kalkeri, E. Kolaczkowski, K. Lin, Y.-P. Luong, B. G. Rao, W. P. Taylor, J. A. Thomson, R. D. Tung, Y. Wei, A. D. Kwong, and C. Lin. 2006. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob. Agents Chemother. 50:899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perni, R. B., G. Chandorkar, P. R. Chaturvedi, L. F. Courtney, C. J. Decker, C. A. Gates, S. L. Harbeson, A. D. Kwong, C. Lin, Y.-P. Luong, W. Markland, B. G. Rao, R. D. Tung, and J. A. Thomson. 2003. VX-950: the discovery of an inhibitor of the hepatitis C virus NS3-4A protease and a potential hepatitis C virus therapeutic, abstr. 972. Hepatology 38:624A. [Google Scholar]
- 34.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 35.Reesink, H. W., S. Zeuzem, C. J. Weegink, N. Forestier, A. van Vliet, J. van de Wetering de Rooij, L. McNair, S. Purdy, H.-M. Chu, and P. L. M. Jansen. 2005. Initial results of a phase 1b, multiple dose study of VX-950, a hepatitis C virus protease inhibitor, abstr. 527. Presented at 36th Digestive Disease Week, Chicago, Ill.
- 36.Reesink, H. W., S. Zeuzem, C. J. Weegink, N. Forestier, A. van Vliet, J. van de Wetering de Rooij, L. A. McNair, S. Purdy, H.-M. Chu, and P. L. M. Jansen. 2005. Final results of a phase 1B, multiple-dose study of VX-950, a hepatitis C virus protease inhibitor, abstr. 96. Hepatology 42:234A. [Google Scholar]
- 37.Seeff, L. B., and J. H. Hoofnagle. 2003. Appendix: the National Institutes of Health Consensus Development Conference management of hepatitis C 2002. Clin. Liver Dis. 7:261-287. [DOI] [PubMed] [Google Scholar]
- 38.Strader, D. B., T. Wright, D. L. Thomas, and L. B. Seeff. 2004. Diagnosis, management, and treatment of hepatitis C. Hepatology 39:1147-1171. [DOI] [PubMed] [Google Scholar]
- 39.Tanji, Y., M. Hijikata, S. Satoh, T. Kaneko, and K. Shimotohno. 1995. Hepatitis C virus-encoded nonstructural protein NS4A has versatile functions in viral protein processing. J. Virol. 69:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomei, L., C. Failla, E. Santolini, R. De Francesco, and N. La Monica. 1993. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J. Virol. 67:4017-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong, X., R. Chase, A. Skelton, T. Chen, J. Wright-Minogue, and B. A. Malcolm. 2006. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antiviral. Res. (Epub ahead of print; doi: 10.1016/j.antiviral.2005.12.003.). [DOI] [PubMed]
- 42.Tong, X., Z. Guo, J. Wright-Minogue, E. Xia, A. Prongay, V. Madison, P. Qiu, S. Venkatraman, F. Velazquez, F. G. Njoroge, and B. A. Malcolm. 2006. Impact of naturally occurring variants of HCV protease on the binding of different classes of protease inhibitors. Biochemistry 45:1353-1361. [DOI] [PubMed] [Google Scholar]
- 43.Trozzi, C., L. Bartholomew, A. Ceccacci, G. Biasiol, L. Pacini, S. Altamura, F. Narjes, E. Muraglia, G. Paonessa, U. Koch, R. De Francesco, C. Steinkuhler, and G. Migliaccio. 2003. In vitro selection and characterization of hepatitis C virus serine protease variants resistant to an active-site peptide inhibitor. J. Virol. 77:3669-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi, M., X. Tong, A. Skelton, R. Chase, T. Chen, A. Prongay, S. L. Bogen, A. K. Saksena, F. G. Njoroge, R. L. Veselenak, R. B. Pyles, N. Bourne, B. A. Malcolm, and S. M. Lemon. 2005. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor: reduced RNA replication fitness and partial rescue by second-site mutations. J. Biol. Chem. (Epub ahead of print; doi: 10.1074/jbc.M510246200.). [DOI] [PubMed]
- 46.Yip, Y., F. Victor, J. Lamar, R. Johnson, Q. M. Wang, J. I. Glass, N. Yumibe, M. Wakulchik, J. Munroe, and S. H. Chen. 2004. P4 and P1′ optimization of bicycloproline P2 bearing tetrapeptidyl alpha-ketoamides as HCV protease inhibitors. Bioorg. Med. Chem. Lett. 14:5007-5011. [DOI] [PubMed] [Google Scholar]
- 47.Zeuzem, S., C. Sarrazin, R. Rouzier, A. Tarral, N. Brion, N. Forestier, S. Gupta, D. Deckman, K. Fellows, M. Hussain, D. L. Cutler, and J. Zhang. 2005. Anti-viral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype-1 (HCV-1) patients refractory to pegylated interferon (Peg-IFN-alpha), abstr. 94. Hepatology 42:233A. [Google Scholar]
- 48.Zeuzem, S., C. Sarrazin, F. Wagner, R. Rouzier, N. Forestier, S. Gupta, M. Hussain, A. Shah, D. L. Cutler, and J. Zhang. 2005. Combination therapy with the HCV protease inhibitor, SCH 503034, plus PEG-intron in hepatitis C genotype-1 PEG-intron non-responders: phase Ib results, abstr. 201. Hepatology 42:276A. [Google Scholar]
- 49.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]