Abstract
Artemisinin and curcumin show an additive interaction in killing Plasmodium falciparum in culture. In vivo, 3 oral doses of curcumin following a single injection of α,β-arteether to Plasmodium berghei-infected mice are able to prevent recrudescence due to α,β-arteether monotherapy and ensure almost 100% survival of the animals.
Artemisinin derivative-based combination therapy (ACT) has been advocated as the therapy of choice to handle widespread drug resistance in Plasmodium falciparum malaria, at the same time preventing recrudescence due to artemisinin monotherapy. However, most of the combinations are less than ideal because of side effects, pharmacokinetic mismatch, and cost (8, 15). Studies in this laboratory have shown that curcumin isolated from the roots of Curcuma longa (turmeric) has antimalarial activity in P. falciparum culture and in Plasmodium berghei-infected mice (10). In this study, we propose a novel artemisinin-curcumin therapy to treat malaria.
Artemisinin and curcumin (98% curcuminoid content) were purchased from Sigma Chemicals, Bangalore, India. α,β-Arteether (EMAL, a synthetic derivative of artemisinin) developed by Central Drug Research Institute, Lucknow, India, was a kind gift from IPCA Laboratories Ltd., Mumbai, India. [3H]hypoxanthine was purchased from Perkin-Elmer, Singapore.
The P. falciparum FCK strain, a local chloroquine-resistant isolate, was cultured in human O-positive washed erythrocytes using standard protocols (14). Parasites were synchronized using 5% (wt/vol) d-sorbitol, and the cultures were pooled and the parasites released from the erythrocyte with 0.15% (wt/vol) saponin (3) for further processing. The 50% inhibitory concentrations (IC50s) for artemisinin and curcumin were determined in P. falciparum cultures by measuring [3H]hypoxanthine uptake into the parasite as a measure of viability (2). Fractional inhibitory concentrations (FIC) were calculated using the formula
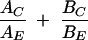 |
where AC and BC are the concentrations of A and B in the combination associated with a particular level of effect, e.g., IC60, and AE and BE are the concentrations of A and B when used singly to produce the same level of effect, on the basis that a simple additive interaction should lead to a value of 1 (7). FIC were derived from graphs drawn using GraphPad Prism 4. Means ± standard deviations and Student's t test were analyzed using Microsoft Excel.
For in vivo studies, Swiss mice (25 to 28 g) were injected intraperitoneally with P. berghei-infected mouse blood (60 to 70% parasitemia) on day 0, such that the animals developed high parasitemia and died in about 5 to 8 days. The artemisinin derivative α,β-arteether was injected intramuscularly at different doses on day 1. This was followed by oral feeding of curcumin in dimethyl sulfoxide on days 1, 2, and 3 (100 mg/kg of body weight). The mice were observed for external symptoms and mortality when the drugs were given alone or in combination. Blood from the tail vein was analyzed on different days for parasitemia using Giemsa to stain the slides.
Detailed studies indicate that the IC50s for artemisinin and curcumin are 45 to 50 nM and 15 to 18 μM, respectively. On this basis, several combinations of artemisinin and curcumin were used to generate dose-response curves, and the data were used to calculate the FIC. The results presented in Table 1 indicate that the FIC at all the ICs tested are <1, and the results are statistically significant. The values are >0.5, and the interaction between curcumin and artemisinin in P. falciparum culture would be termed nonsynergistic (7).
TABLE 1.
FIC for curcumin-artemisinin combination in P. falciparum culture
| Inhibition level | FIC (mean ± SD)a | P value |
|---|---|---|
| IC60 | 0.81 ± 0.06 | <0.05 |
| IC75 | 0.79 ± 0.05 | <0.05 |
| IC80 | 0.83 ± 0.06 | <0.05 |
| IC90 | 0.78 ± 0.07 | <0.01 |
FIC obtained are statistically lower than 1, the value indicative of additive interaction.
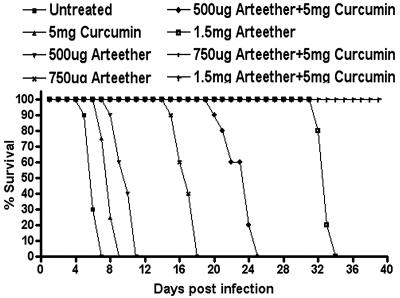
The efficacy of the curcumin-artemisinin combination in P. falciparum culture led us to evaluate its efficacy in vivo. The results presented in Fig. 1 indicate that α,β-arteether or curcumin monotherapy at the indicated doses prolongs the survival of P. berghei-infected mice (25 to 28 g) but does not confer complete protection. Thus, while the P. berghei-infected animals die between 5 to 7 days, a single injection of α,β-arteether at 500 μg, 750 μg, and 1.5 mg results in the death of animals between 9 to 11, 16 to 18, and 32 to 34 days, respectively. However, treatment of the infected mice with a combination of α,β-arteether and curcumin results in better survival rates, and a 3-day oral regimen of curcumin with a single injection of α,β-arteether at 750 μg or 1.5 mg per infected mouse led to complete protection of animals against recrudescence and 100% survival.
FIG. 1.
Effect of curcumin and arteether on mortality of P. berghei-infected mice.
The artemisinin derivative-based combinations under development are artesunate plus amodiaquine, artesunate plus sulfadoxine-pyrimethamine, artesunate plus mefloquine, dihydroartemisinin plus piperaquine, and artemether plus lumefantrine (8). Artesunate plus mefloquine is well tolerated and highly effective. It has been used in Thailand for almost a decade (9). Dihydroartemisinin plus piperaquine is under development and has given favorable results in Vietnamese patients (4). The artemether plus lumefantrine combination is the only formulation that has been registered for use in Africa, although a study has shown irreversible hearing impairment (13). The combination also needs fat for better absorption (15). A general issue with many of these combinations is side effects and pharmacokinetic mismatch between artemisinin and the other drugs in the combination. Another major concern of all these combinations is their high cost.
The curcumin-artemisinin combination may prove superior from several perspectives. Both are from natural sources of long-term use, and as such, no resistance is known to curcumin that is present in a dietary supplement. Artemisinin runs the risk of resistance development when used widely as monotherapy (5). Curcumin is tolerated at very high doses, and as much as 8 g/day has been given for 3 months to cancer patients on trial without toxic side effects (1). A single dose of 750 μg of arteether in the curcumin combination that gives complete protection in P. berghei-infected mice, when extrapolated to the human dose (8), would work out to nearly one-third of the dose recommended for the artemisinin-lumefantrine combination in the patients. Thus, there is potential to decrease the dose of artemisinin and lower the cost of therapy. Curcumin itself is a cheap compound. It is interesting to note the effectiveness of curcumin in combination with α,β-arteether, although curcumin is reported to manifest low bioavailability and rapid metabolism in rodents and humans (6). In a sense, the rapid clearance of artemisinin (12) and curcumin (6) overcomes the problem of pharmacokinetic mismatch. However, if necessary, strategies are available to increase the bioavailability of curcumin in humans. For example, a low dose of piperine from pepper can enhance curcumin uptake by 2,000% in humans, and the combination is well tolerated (11). In view of all these characteristics, the artemisinin derivative-curcumin combination is worth serious consideration for trial in humans.
Acknowledgments
This study was supported by grants from the Department of Biotechnology, New Delhi, India.
REFERENCES
- 1.Cheng, A. L., J. K. Hsu, M. Lin, Y. F. Hsu, T. S. Ho, J. Y. Shen, J. T. Ko, B. R. Lin, W. Lin, H. S. Ming-Shiang, et al. 2001. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high risk or premalignant lesion. Anticancer Res. 21:2895-2900. [PubMed] [Google Scholar]
- 2.Desjardins, R. E., R. E. Canefield, C. J. Hayness, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitch, C. D., R. Chevli, H. S. Banyal, G. Phillips, M. A. Pfaller, and D. J. Krogstad. 1982. Lysis of Plasmodium falciparum by ferriprotoporpyrin IX and chloroquine-ferriprotoporphyrin IX complex. Antimicrob. Agents Chemother. 21:819-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Artemisinin Study Group. 2004. Artesunate combination for treatment of malaria: meta-analysis. Lancet 363:9-17. [DOI] [PubMed] [Google Scholar]
- 5.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in vitro artemeether and point mutations of the SERCA-type PfATPase 6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 6.Joe, B., M. Vijayakumar, and B. R. Lokesh. 2004. Biological properties of curcumin—cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 44:97-111. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, M. D., C. Mac Dougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremsner, P. G., and S. Krishna. 2004. Antimalarial combinations. Lancet 364:285-294. [DOI] [PubMed] [Google Scholar]
- 9.Nosten, F. M., M. van Vugt, R. Price, C. Luxemberger, K. L. Thway, A. Brockman, R. McGready, F. ter kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 10.Reddy, R. C., P. G. Vathsala, V. G. Keshamouni, G. Padmanaban, and P. N. Rangarajan. 2005. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 326:472-474. [DOI] [PubMed] [Google Scholar]
- 11.Shoba, G., D. Joy, T. Joseph, M. Majeed, R. Rajendran, and P. S. Srinivas. 1998. Influence of Piperine on the pharmakinetics of curcumin in animals and human volunteers. Planta Med. 64:353-356. [DOI] [PubMed] [Google Scholar]
- 12.ter Kuile, F., N. J. White, P. H. Halloway, G. Pasvol, and S. Krishna. 1993. In vitro studies of the pharmacodynamic properties of drugs used for severe malaria. Exp. Parasitol. 76:85-95. [DOI] [PubMed] [Google Scholar]
- 13.Toovey, S., and A. Jamieson. 2004. Audiometric changes associated with the treatment of uncomplicated falciparum malaria with co-artemether. Trans. R. Soc. Trop. Med. Hyg. 98:261-267. [DOI] [PubMed] [Google Scholar]
- 14.Trager, W., and B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 15.Yeung, S., W. Pongtavornpinyo, I. M. Hastings, A. J. Mills, and N. J. White. 2004. Antimalarial drug resistance, artemisinin-based combination therapy and the contribution of modeling to elucidating policy choices. Am. J. Trop. Med. Hyg. 71:179-186. [PubMed] [Google Scholar]