Abstract
The administration of macrolides such as azithromycin for chronic pulmonary infection of cystic fibrosis patients has been reported to be of benefit. Although the mechanisms of action remain obscure, anti-inflammatory effects as well as interference of the macrolide with Pseudomonas aeruginosa virulence factor production have been suggested to contribute to an improved clinical outcome. In this study we used a systematic approach and analyzed the impact of azithromycin on the global transcriptional pattern and the protein expression profile of P. aeruginosa PAO1 cultures versus those in untreated controls. The most remarkable result of this study is the finding that azithromycin exhibited extensive quorum-sensing antagonistic activities. In accordance with the inhibition of the quorum-sensing systems, virulence factor production was diminished and the oxidative stress response was impaired, whereas the type III secretion system was strongly induced. Moreover, P. aeruginosa motility was reduced, which probably accounts for the previously observed impaired biofilm formation capabilities of azithromycin-treated cultures. The interference of azithromycin with quorum-sensing-dependent virulence factor production, biofilm formation, and oxidative stress resistance in P. aeruginosa holds great promise for macrolide therapy in cystic fibrosis. Clearly quorum-sensing antagonist macrolides should be paid more attention in the management of chronic P. aeruginosa infections, and as quorum-sensing antagonists, macrolides might gain vital importance for more general application against chronic infections.
The lung of cystic fibrosis (CF) patients has a unique susceptibility to chronic Pseudomonas aeruginosa infection, which is still the major cause of morbidity and mortality in these patients (4, 24, 36). Although in past decades antibiotic therapy has greatly increased life expectancy, only limited therapeutic options are available, and chronic P. aeruginosa infection is rarely eradicated (5, 20, 37). Hence, there is a is an urgent need to develop alternative treatment regimens to improve lung function and thus the prognosis of the disease. Although the principles concerning therapeutic strategies in the treatment of chronic lung infection have not changed significantly in the last 10 years, the use of azithromycin (AZM) for infection control and inflammation modulation is one new aspect (2, 11, 38, 44, 53).
By conventional standards P. aeruginosa is insensitive to therapeutic concentrations of macrolides; however, recently macrolides have been reported to positively influence the clinical outcome in patients suffering from chronic P. aeruginosa infection in diffuse panbronchiolitis (13, 15, 21, 40, 41). Diffuse panbronchiolitis was first reported in Japan and is characterized by an inflammatory cell infiltration in the respiratory bronchioles, leading to their obstruction and dilatation (35). As disease progresses, patients typically become colonized with mucoid strains of P. aeruginosa accompanied by cystic changes of the lung and by poor clinical prognosis due to progressive deterioration of respiratory function.
The remarkable parallels between diffuse panbronchiolitis and CF led to the question of whether macrolide antibiotics would also be of benefit in patients with CF and to large-scale randomized controlled trials to elucidate the properties of macrolides for chronic P. aeruginosa infection of the lung in CF patients (30, 34, 39, 52). The majority of clinical studies report positive trends concerning the therapeutic potential of macrolide therapy (44). However, the mechanisms of action in chronic P. aeruginosa infection remain obscure (54). Immunomodulatory effects are postulated to account for some of the beneficial effects (9, 16), in addition to altered airway epithelial chloride transport and inhibition of P. aeruginosa virulence factor production by interference with interbacterial communication (33, 45).
Interbacterial communication is also referred to as quorum sensing (QS) and is a very sophisticated mechanism by which signal molecules act as autoinducers and trigger a variety of biological functions when microbial populations attain certain cell densities. QS controls not only virulence factor production but also biofilm formation in P. aeruginosa (3) and thus contributes significantly to pathogenesis and persistence of infection. The QS system in P. aeruginosa comprises two hierarchically organized systems, each consisting of an autoinducer synthetase (LasI/RhlI) and a corresponding regulator protein (LasR/RhlR). Both the las and the rhl QS systems have been shown to be transcriptionally repressed by sublethal azithromycin concentrations (45). Moreover, a QS mutant was shown to be less responsive to AZM inhibition (48).
In this study we applied a systematic approach and analyzed the transcriptome and proteome profiles of P. aeruginosa in response to sublethal concentrations of AZM. Using this global approach, we aimed to identify the influence of AZM on QS-regulated genes and proteins and thus to gain background data on therapy with macrolides for purposes other than their bactericidal properties.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
For the isolation of RNA and for the preparation of cellular and extracellular protein extracts, P. aeruginosa PAO1 (DSM 1707) was grown in brain heart infusion (BHI) medium at 37°C with shaking with or without the addition of 2 μg/ml AZM (Pfizer, Germany) until early stationary phase.
RNA extraction and preparation of protein samples.
Total RNA was extracted from 10 ml of four AZM-treated and untreated PAO1 cultures each, cDNA was synthesized from the RNA pooled from two independent cultures, and subsequently two GeneChips were hybridized for each culture condition. RNA isolation, cDNA generation, fragmentation, biotinylation, and GeneChip hybridization and analysis were performed according to the Affymetrix guidelines and conform to the MIAME requirements (Minimum Information About a Microarray Experiment; experimental details are available at http://www.ncbi.nlm.nih.gov/projects/geo/submission/login/under accession number GSE2430). Three independent protein extracts from pooled supernatants and cell pellets of four 150-ml AZM-treated and untreated PAO1 cultures each were prepared and used immediately for two-dimensional gel electrophoresis. The preparation of protein extracts, two-dimensional gel electrophoresis, and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MALDI-TOF MS) analysis were performed as described previously (51). The gels were stained with ruthenium II Tris(bathophenanthroline disulfonate) (RuBPS) (22) and differentially expressed proteins as detected in duplicate gels were quantified by ProteomWeaver 2.1 (DEFINIENS). Proteins were considered to be significantly affected when their spot intensities changed at least twofold.
Lactate dehydrogenase release assay.
The bacteria were grown in BHI medium with and without the addition of 2 μg/ml AZM and harvested in log phase (optical density at 600 nm [OD600], ∼0.6) and stationary phase (OD600, ∼2.7), respectively. J774.A1 cells were grown to confluence in flat-bottom 96-well plates in Dulbecco's modified Eagle's medium with 10% fetal calf serum and infected with 20 μl of the bacterial suspension in 200 μl fresh Dulbecco's modified Eagle's medium with 10% fetal calf serum. J774.A1 cell viability was assessed by the determination of lactate dehydrogenase in the supernatant fraction of six parallel wells by using a lactate dehydrogenase cytotoxicity detection kit according to the manufacturer's instructions (Roche, Mannheim, Germany). All experiments were performed in triplicate.
H2O2 sensitivity assay.
The H2O2 sensitivity disk assay was adapted from that of Hassett et al. (7). Briefly, Pseudomonas PAO1 was grown at 37°C in BHI medium for various incubation periods with and without the addition of 2 μg/ml AZM; 100 μl of the bacterial culture was suspended in 3 ml of LB soft agar at 40°C in 0.6% (wt/vol) agar, mixed, and poured on LB agar plates with 1.5% (wt/vol) agar. Sterile filter paper disks were placed on the soft solid agar, and the disks were spotted with 8 μl of 30% H2O2. Plates were incubated at 37°C for 24 h, and the diameter of the zone of growth inhibition was measured. All experiments were performed in triplicate.
Motility assays.
Media for swimming and twitching assays were LB containing 0.3% (wt/vol) and 1.5% (wt/vol) Bacto Agar (Difco) with and without the addition of 2 μg/ml AZM. Plates were inoculated with bacteria from an overnight LB culture grown in the presence and absence of 2 μg/ml AZM. The swimming plates were inoculated with 15 μl of the bacterial suspension and incubated at 37°C for 24 h, whereas the twitching plates were inoculated with a sterile toothpick at the bottom of the petri dish and incubated at 37°C for at least 24 h.
RESULTS AND DISCUSSION
Influence of sublethal azithromycin concentrations on the PAO1 transcriptome and proteome.
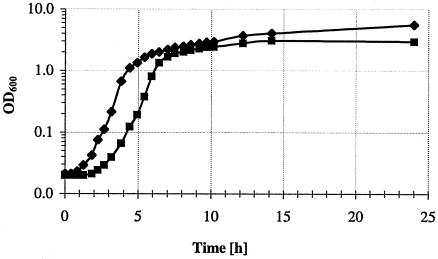
Since interference with virulence factor production in P. aeruginosa has been postulated to be responsible for the observed beneficial effect of macrolide therapy, we aimed to analyze the effect of AZM on bacteria at the early stationary growth phase, when virulence factor production is high. As observed previously (45), the addition of 2 μg/ml azithromycin (1/64th of the MIC) led to a prolonged lag phase and an only minimally affected exponential and stationary phase of growth compared to that with the PAO1 control culture (Fig. 1).
FIG. 1.
Growth of PAO1 in LB broth with (▪) and without (⧫) the addition of 2 μg/ml azithromycin.
A comparison of the gene expression profiles when the PAO1 strain cultured with and without exogenous AZM reached an OD600 of 2.8 revealed 107 genes that were differentially expressed (78 of those genes were shown to be up-regulated and 29 genes were repressed), representing 1.9% of the entire genome (Table 1). Many of the AZM-induced genes were genes encoding ribosomal subunits. In addition, an initiation factor (infA PA2619) and an elongation factor (efp PA2851) were overexpressed in AZM-treated cultures. Since the macrolides exhibit their antibacterial activity by binding to the 50S ribosomal subunit, resulting in the blockage of transpeptidation and/or translation, this implies these genes may be overexpressed to compensate for impaired translation due to sublethal AZM concentrations. Moreover, the ribosome modulation factor rmf was strongly repressed upon AZM addition. The PAO1 rmf gene exhibits high homology to the rmf gene in Escherichia coli, in which rmf has been shown to be important for survival under stationary-phase conditions (56).
TABLE 1.
Azithromycin-regulated genes in PAO1
| Genea | Gene name | Up-regulated (+) or down-regulated (−) in response to AZM (fold) | Protein |
|---|---|---|---|
| PA0044b | exoT | +13.54 | Exoenzyme T |
| PA0263c | hcpC | +3.83 | Secreted protein Hcp |
| PA0409 | pilH | +3.24 | Twitching motility protein PilH |
| PA0422 | +6.27 | Conserved hypothetical protein | |
| PA0456 | +3.38 | Probable cold shock protein | |
| PA0555 | fda | +5.16 | Fructose-1,6-bisphosphate aldolase |
| PA0649 | trpG | +12.08 | Anthranilate synthase component II |
| PA0650 | trpD | +7.38 | Anthranilate phosphoribosyltransferase |
| PA0651 | trpC | +5.90 | Indole-3-glycerolphosphate synthase |
| PA0805 | +8.74 | Hypothetical protein | |
| PA0943 | +3.51 | Hypothetical protein | |
| PA0996 | pqsA | +3.78 | Probable coenzyme A ligase |
| PA1440 | +5.38 | Hypothetical protein | |
| PA1493d | cysP | +3.50 | Sulfate-binding protein of ABC transporter |
| PA1494 | +2.61 | Conserved hypothetical protein | |
| PA1564 | +4.64 | Conserved hypothetical protein | |
| PA1696b | pscO | +8.38 | Translocation protein in type III secretion |
| PA1706b,d | pcrV | +91.88 | Type III secretion protein PcrV |
| PA1707b | pcrH | +14.16 | Regulatory protein PcrH |
| PA1708b | popB | +6.77 | Translocator protein PopB |
| PA1709b | popD | +6.25 | Translocator outer membrane protein PopD precursor |
| PA1710b | exsC | +8.99 | Exoenzyme S synthesis protein C precursor |
| PA1711b | +11.87 | Hypothetical protein | |
| PA1712b | exsB | +8.59 | Exoenzyme S synthesis protein B |
| PA1714b | +4.45 | Hypothetical protein | |
| PA1715b | pscB | +16.46 | Type III export apparatus protein |
| PA1754 | cysB | +3.24 | Transcriptional regulator CysB |
| PA2015 | +2.78 | Probable acyl-coenzyme A dehydrogenase | |
| PA2016 | +8.72 | Probable transcriptional regulator | |
| PA2023 | galU | +4.90 | UTP-glucose-l-phosphate uridylyltransferase |
| PA2191b | exoY | +20.12 | Adenylate cyclase ExoY |
| PA2193e | hcnA | +2.62 | Hydrogen cyanide synthase HcnA |
| PA2423e | +5.55 | Hypothetical protein | |
| PA2464 | +3.42 | Hypothetical protein | |
| PA2619 | infA | +18.96 | Initiation factor |
| PA2747 | +4.85 | Hypothetical protein | |
| PA2755 | eco | +4.69 | Ecotin precursor |
| PA2830 | htpX | +6.97 | Heat shock protein HtpX |
| PA2851 | efp | +4.60 | Translation elongation factor P |
| PA2895 | +2.70 | Hypothetical protein | |
| PA2900 | +5.85 | Probable outer membrane protein precursor | |
| PA2901 | +14.03 | Hypothetical protein | |
| PA3244d | minD | +5.71 | Cell division inhibitor MinD |
| PA3262 | +4.72 | Probable peptidyl-prolyl cis-trans isomerase, FkbP type | |
| PA3369 | +6.90 | Hypothetical protein | |
| PA3370 | +6.28 | Hypothetical protein | |
| PA3371f | +11.50 | Hypothetical protein | |
| PA3531 | bfrB | +4.88 | Bacterioferritin B |
| PA3656 | rpsB | +4.74 | 30S ribosomal protein S2 |
| PA3686 | adk | +5.59 | Adenylate kinase |
| PA3742 | rplS | +2.88 | 50S ribosomal protein L19 |
| PA3841b | exoS | +9.56 | Exoenzyme S |
| PA3842 | +14.72 | Probable chaperone | |
| PA3976 | thiE | +7.89 | Thiamine-phosphate pyrophosphorylase |
| PA3988 | +2.67 | Hypothetical protein | |
| PA4004 | +4.36 | Conserved hypothetical protein | |
| PA4006 | nadD | +4.19 | Nicotinic acid mononucleotide adenylyltransferase |
| PA4059 | +6.47 | Hypothetical protein | |
| PA4114 | +8.51 | Spermidine acetyltransferase | |
| PA4235 | bfrA | +4.35 | Bacterioferritin A |
| PA4263 | rplC | +3.56 | 50S ribosomal protein L3 |
| PA4441 | +3.18 | Hypothetical protein | |
| PA4460 | +3.56 | Conserved hypothetical protein | |
| PA4495 | +3.45 | Hypothetical protein | |
| PA4525 | pilA | +4.89 | Type 4 fimbrial precursor PilA |
| PA4567 | rpmA | +5.05 | 50S ribosomal protein L27 |
| PA4568 | rplU | +9.85 | 50S ribosomal protein L21 |
| PA4605 | +8.72 | Conserved hypothetical protein | |
| PA4670 | prs | +7.09 | Ribose-phosphate pyrophosphokinase |
| PA4671 | +3.70 | Probable ribosomal protein L25 | |
| PA4751 | ftsH | +3.21 | Cell division protein FtsH |
| PA4764 | fur | +3.06 | Ferric uptake regulation protein |
| PA4765 | omlA | +8.35 | Outer membrane lipoprotein OmlA precursor |
| PA5130 | +11.23 | Conserved hypothetical protein | |
| PA5191 | +2.79 | Hypothetical protein | |
| PA5316 | rpmB | +5.23 | 50S ribosomal protein L28 |
| PA5481c | +5.46 | Hypothetical protein | |
| PA5482 | +6.22 | Hypothetical protein | |
| PA0139d | ahpC | −2.97 | Alkyl hydroperoxide reductase subunit C |
| PA0586 | −4.61 | Conserved hypothetical protein | |
| PA0852d,e | cpbD | −3.31 | Chitin-binding protein CbpD precursor |
| PA1048 | −2.81 | Probable outer membrane protein precursor | |
| PA1244 | −6.62 | Hypothetical protein | |
| PA1871e | lasA | −8.33 | LasA protease precursor |
| PA2031 | −3.88 | Hypothetical protein | |
| PA2146c | −6.30 | Conserved hypothetical protein | |
| PA2171c | −7.61 | Hypothetical protein | |
| PA2190c | −3.13 | Conserved hypothetical protein | |
| PA2259 | ptxS | −2.93 | Transcriptional regulator PtxS |
| PA2274 | −2.73 | Hypothetical protein | |
| PA2300e | chiC | −4.38 | Chitinase |
| PA2564e | −3.77 | Hypothetical protein | |
| PA2565 | −3.23 | Hypothetical protein | |
| PA3049 | rmf | −12.49 | Ribosome modulation factor |
| PA3478e | rhlB | −4.33 | Rhamnosyltransferase chain B |
| PA3529d | −4.86 | Probable peroxidase | |
| PA3533 | −3.98 | Conserved hypothetical protein | |
| PA4078f | −18.35 | Probable nonribosomal peptide synthetase | |
| PA4205 | mexG | −4.78 | Hypothetical protein |
| PA4206 | mexH | −3.53 | Probable RND efflux membrane fusion protein precursor |
| PA4236g | katA | −10.78 | Catalase KatA |
| PA4306e | −3.63 | Hypothetical protein | |
| PA4366d,g | sodB | −4.42 | Superoxide dismutase |
| PA4377 | −6.80 | Hypothetical protein | |
| PA4385d | groEL | −4.07 | GroEL protein |
| PA4386 | groES | −4.09 | GroES protein |
| PA4611 | −4.46 | Hypothetical protein |
Only open reading frames which were found in all four GeneChip pairings defined by the Affymetrix microarray suite software as having significant changes in their signal intensities and were at least twofold up- or down-regulated in each of the four pairings (the arithmetic middle of all four pairings is given) are listed. Genes identified previously as being QS regulated are in boldface.
The TTSS was identified as QS repressed by Hogardt et al. (10).
Conditional QS-induced genes; identified as QS regulated by Schuster et al. (42).
Genes/proteins that were shown to be regulated by both proteomics and transcriptomics.
General QS regulon, based on the results of three microarray studies: Hentzer et al. (8), Schuster et al. (42), and Wagner et al. (49).
Conditional QS-induced genes; identified as QS regulated by Hentzer et al. (8).
Identified as QS induced by Hassett et al. (7).
An exposure-dependent bactericidal activity of macrolides has been described previously (46) and was observed in this study (after 24 h and 48 h of incubation, significantly fewer bacteria were isolated from the AZM-treated culture than from controls; paired t test, P < 0.001). In this context the AZM-dependent down-regulation of rmf might contribute to the observed impaired survival of AZM-treated PAO1 under stationary-phase conditions.
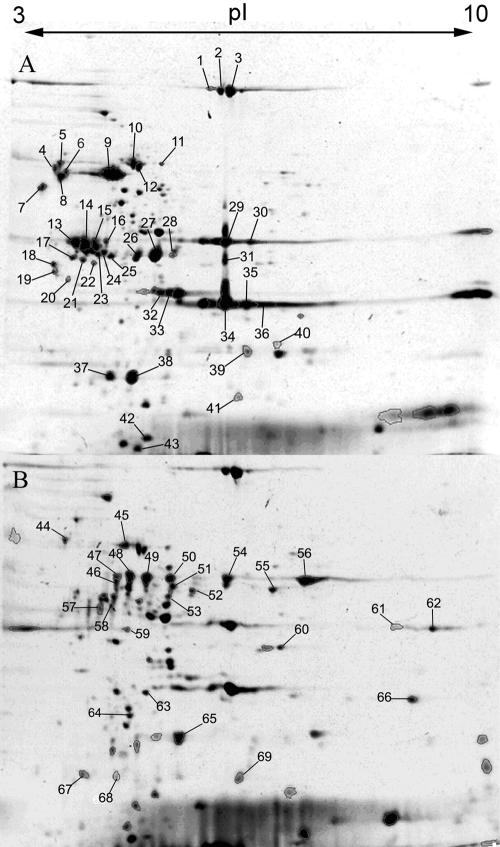
In a complementary approach to the identification of the global transcriptional pattern we analyzed the protein expression profile of AZM-treated in comparison to nontreated PAO1 cultures. Comparative analyses of the secretome and of cellular extracts of AZM-treated versus nontreated PAO1 cultures disclosed a total of 43 differentially expressed proteins (Table 2); 11 proteins were up-regulated and 15 proteins were down-regulated in the secretome of the PAO1 cultures after AZM addition (Fig. 2). Whereas the two-dimensional gels of the secretome comprised approximately 645 protein spots, 714 protein spots were detected within the cellular fraction. Six proteins were up-regulated and 11 proteins were down-regulated in the cellular fraction of the PAO1 cultures after AZM addition.
TABLE 2.
Azithromycin-regulated proteins in PAO1
| Group and spot no.a | Geneb | Gene name | Protein | Up- or down-regulationc
|
||
|---|---|---|---|---|---|---|
| This study | Nouwens et al. (29) | Arevalo-Ferro et al. (1) | ||||
| Secretome | ||||||
| 51, 63 | PA0026 | Hypothetical protein | ↑ | ↑ | ||
| 65 | PA0888 | aotJ | Arginine/ornithine binding protein AotJ | ↑ | ↑ | ↑ |
| 54 | PA1065 | Conserved hypothetical protein | ↑ | |||
| 66 | PA1342 | Probable binding protein component of ABC transporter | ↑ | ↑ | ||
| 60d | PA1493 | cysP | Sulfate-binding protein of ABC transporter | ↑ | ||
| 45 | PA1673 | Hypothetical protein | ↑ | |||
| 64d | PA1706 | pcrV | Type III secretion protein PcrV | ↑ | ||
| 55 | PA4175 | prpL | PvdS-regulated endoprotease, lysyl class | ↑ | ↓ | ↑ |
| 69 | PA5489 | dsbA | Thiol:disulfide interchange protein DsbA | ↑ | ↑ | |
| 59 | PA4265 | tufA | Elongation factor Tu, TufA | ↑ | ||
| PA4277 | tufB | Elongation factor Tu, TufB | ||||
| 41d | PA0139 | ahpC | Alkyl hydroperoxide reductase subunit C | ↓ | ||
| 1, 2, 3 | PA0572 | Hypothetical protein | ↓ | ↓ | ||
| 29, 30, 32d | PA0852 | cbpD | Chitin-binding protein CbpD precursor | ↓ | ↓ | ↑ |
| 4, 5, 6, 8, 19, 20, 44 | PA1086 | flgK | Flagellar hook-associated protein 1 FlgK | ↓ | ↑ | |
| 18 | PA1087 | flgL | Flagellar hook-associated protein type 3 FlgL | ↓ | ↑ | |
| 13, 14, 15, 16, 17, 21, 22, 23, 24, 25, 46, 47, 48, 49, 50, 57, 58, 67, 68 | PA1092 | fliC | Flagellin type B | ↓ | ↑ | ↑ |
| 26, 27, 28, 37, 38, 39, 52, 53, 56 | PA1094 | fliD | Flagellar capping protein FliD | ↓ | ↑ | |
| 33 | PA1158 | Probable two-component sensor | ↓ | |||
| 7 | PA1294 | aprA | Alkaline metalloproteinase precursor AprA | ↓ | ↓ | |
| 42 | PA1784 | Hypothetical protein | ↓ | |||
| 9 | PA2939 | pepB | Probable aminopeptidase | ↓ | ↓ | ↑ |
| 31, 34, 35, 36 | PA3724 | lasB | Elastase LasB | ↓ | ↓ | ↑ |
| 40 | PA3746 | ffh | Signal recognition particle protein Ffh | ↓ | ||
| 10, 12d | PA4385 | groEL | GroEL protein | ↓ | ||
| 11 | PA5192 | pckA | Phosphoenolpyruvate carboxykinase PckA | ↓ | ||
| Cytosolic proteins | ||||||
| 82 | PA0609 | trpE | Anthranilate synthase (EC 4.1.3.27) alpha chain | ↑ | ||
| 81 | PA1596 | htpG | Heat shock protein HtpG | ↑ | ||
| 86d | PA3244 | minD | Cell division inhibitor MinD | ↑ | ||
| 87 | PA3397 | fpr | Ferredoxin-NADP+ reductase | ↑ | ||
| 84 | PA3635 | eno | Enolase | ↑ | ||
| 85 | PA4602 | glyA3 | Serine hydroxymethyltransferase GlyA3 | ↑ | ||
| 83 | PA0139 | ahpC | Alkyl hydroperoxide reductase subunit C | ↓ | ||
| 73 | PA0230 | pcaB | 3-Carboxy-cis,cis-muconate cycloisomerase PcaB | ↓ | ||
| 71 | PA0766 | mucD | Serine protease MucD precursor | ↓ | ||
| 76 | PA0837 | slyD | Peptidyl-prolyl cis-trans isomerase SlyD | ↓ | ||
| 74 | PA1337 | ansB | Glutaminase asparaginase AnsB | ↓ | ||
| 75 | PA1344 | yvaG | Probable short-chain dehydrogenase | ↓ | ||
| 79 | PA1584 | sdhB | Succinate dehydrogenase (B subunit) SdhB | ↓ | ||
| 80 | PA1900 | phzB2 | Probable phenazine biosynthesis protein PhzB2 | ↓ | ↑ | |
| 77d | PA3529 | tsaA | Probable peroxidase | ↓ | ||
| 78d | PA4366 | sodB | Superoxide dismutase SodB | ↓ | ||
| 72 | PA5173 | arcC | Carbamate kinase ArcC | ↓ | ||
See Fig. 2.
Data generated from peptide mass maps were compared to the complete translated open reading frames of the PAO1 genome (www.pseudomonas.com).
In order to detect differential protein expression, the spot intensities of the entirety of the fragments of one protein were compared between AZM-treated and nontreated PAO1 cultures. Except for protein spots that were detected only in gels of cultures with or without AZM, the differences in protein spot intensity were between 2- and 10-fold.
Genes shown to be regulated by both proteomics and transcriptomics.
FIG. 2.
Secretome of PAO1 cultures without (A) and with (B) the addition of 2 μg/ml AZM. Forty-three differentially expressed protein spots (1 to 43) were identified by mass spectrometry from the gels of the secretome of the PAO1 control cultures (A) and 26 protein spots (44 to 69) were identified from the gels of the AZM-treated PAO1 secretome (B).
The finding that only eight genes and proteins (ahpC, cbpD, cysP, pcrV, minD, tsaA, sodB, and groEL) were shown to be affected at both the transcriptional and the protein levels emphasizes that transcriptomics and proteomics are complementary approaches, and combining their particular strengths should give maximal relevant results. Major differences between proteome and transcriptome data have been documented in previous studies to map the P. aeruginosa QS regulon: the two proteome studies of Arevalo-Ferro et al. (1) and Nouwens et al. (29) identified 47 and 27 QS-regulated proteins, respectively, and only 11 and 8, respectively, of the corresponding genes were found to be regulated at the transcriptional level in three independent transcriptome studies analyzing QS-dependent P. aeruginosa gene expression (8, 42, 49).
Effect of azithromycin on quorum sensing in PAO1.
Eight out of 77 genes (10.4%) of the general QS regulon (identified as QS-dependent genes in all three recent microarray studies (8, 42, 49) were identified as being influenced by AZM addition (Table 1). Overall, 15 AZM- and QS-dependent genes (identified as QS dependent in at least one of the three microarray studies) were found. Ten of the 15 QS- and AZM-dependent genes were shown to be down-regulated in this study in response to AZM, but five genes were up-regulated. An up-regulation is opposite to what is expected when assuming that AZM inhibits QS. However, QS-regulated proteins have previously been found to be oppositely regulated in a comparison between two previous proteomic studies (1, 29) and it was speculated that some of the observed discrepancies are caused by differences in experimental conditions. Further work will be required to address this issue. Moreover, we found genes of the QS-dependent type III secretion system (TTSS) (10) and QS-controlled katA and sodB (7) to be AZM dependent.
In addition to the identification of a common subset between QS- and AZM-regulated genes, we compared differential protein expression due to AZM treatment with QS-dependent protein expression in the two previous proteome studies. There was a large common subset of QS- and AZM-regulated proteins: 15 proteins that were shown to be affected by AZM addition were among the 47 proteins previously identified as being QS dependent (31.9%) (Table 2). The best congruence was found in the secretome, supporting the recent findings of Wagner et al. (50), who reported the constant expression of QS-regulated virulence factors under various culture conditions.
Since chronic P. aeruginosa infection in CF is rarely eradicated despite intensive antimicrobial therapy, interference with or blocking of QS systems has been considered an attractive alternative therapeutic strategy. Recently, halogenated furanones have been shown to control P. aeruginosa infections in animal models (8, 55). The finding that QS antagonists are effective is of considerable importance, since it demonstrates that QS is a useful and promising drug target in vivo. For treatment of humans, macrolides seem to be a promising alternative to the toxic halogenated furanones that might block the QS systems within therapeutic concentration ranges. How precisely the macrolides interfere with the transcription of QS-regulated genes remains poorly defined and will be an important question to be addressed in the future.
Azithromycin enhances the expression of the type III secretion system.
Several studies have demonstrated that macrolide antibiotics suppress the expression of substances that contribute to P. aeruginosa virulence, such as exoenzymes, exopolysaccharides, and pigments (17, 25, 26, 48), and it has been hypothesized that CF patients benefit from AZM treatment due to the negative effect on virulence factor production. However, one of the major findings of this study is that AZM treatment of PAO1 led to increased expression of the TTSS. We found increased expression of 10 of 36 genes of the TTSS gene cluster (PA1690 to PA1725) in the AZM-treated cultures. Moreover, the expression of genes encoding the secreted effector proteins ExoT, ExoY, and ExoS, which are located outside the TTSS gene cluster, was enhanced. The pcrV gene, encoding a TTSS-secreted protein, exhibited the highest differential gene expression (91.9-fold), and accordingly, PcrV was overproduced in the AZM-treated cultures.
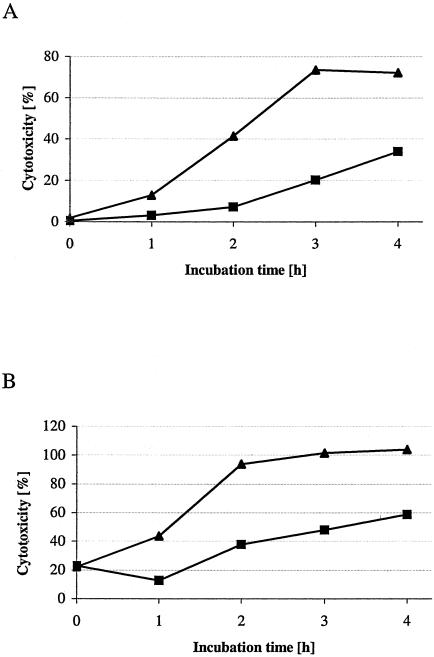
In order to test whether TTSS overproduction in P. aeruginosa has a biological effect, we determined the in vitro cytotoxicity of AZM-treated bacteria on the murine macrophage cell line J774.A1. As shown in Fig. 3 P. aeruginosa PAO1 that was cultured in medium containing 2 μg/ml AZM until the log and early stationary phases of growth exhibited increased cytotoxicity in comparison to bacteria cultured without AZM. These effects were not observed when PAO1 was grown with sublethal gentamicin or ceftazidime concentrations (data not shown). Remarkably, it has previously been demonstrated that treatment of P. aeruginosa with macrolides, including AZM, significantly enhances virulence in mice (19). An involvement of acute toxic effects rather than multiplication of the bacteria was suggested.
FIG. 3.
Cytotoxicity as determined by lactate dehydrogenase release of J774.A1 cells of AZM-treated bacteria (▴) versus the untreated PAO1 control (▪). The bacteria were harvested from the log phase of growth (A) and the stationary phase of growth (B). One representative experiment out of three is shown.
The results of our study imply that enhanced expression of the TTSS might account for these previously observed effects, as the TTSS has been shown to enhance P. aeruginosa virulence significantly (43). However, only inoculation of macrolide-treated bacteria had been shown to be associated with increased mortality in mice, whereas the administration of macrolides after inoculation of P. aeruginosa did not increase mortality (19, 28). The impact of the TTSS-induced cytotoxicity on therapeutic macrolide administration remains to be clarified in future investigations.
Azithromycin affects P. aeruginosa motility.
AZM-treated PAO1 cultures exhibited reduced expression of various proteins required for flagellum biosynthesis (Table 2) and demonstrated reduced flagellum-driven motility on swimming agar plates. The mean value of the radius of the untreated PAO1 and AZM-treated PAO1 cultures was 3.58 (±0.17) cm and 1.18 (±0.09) cm, respectively (P < 0.01). Although macrolides have been previously demonstrated to inhibit not only swimming motility (14) but also twitching motility (14, 54), we observed increased expression of pilA and pilH, involved in type IV pilus biogenesis (Table 1). No effects of sublethal AZM concentrations on type IV pili could be observed by proteomics.
Analysis of the twitching motility of AZM-treated PAO1 revealed that twitching motility was significantly reduced. The mean value of the radius of the untreated PAO1 and AZM-treated PAO1 cultures was 0.67 (±0.06) cm and 0.51 (±0.06) cm, respectively (P < 0.01). While flagellum-mediated motility has been implicated to be required to bring P. aeruginosa within proximity of a surface, type IV pili by virtue of twitching motility enable P. aeruginosa to migrate across a surface, recruit cells from adjacent monolayers, and form cell aggregates (31), thus contributing to biofilm formation. The observed impaired swimming and twitching motility of AZM-treated PAO1 could explain the previous observations that AZM delays biofilm formation, as evidenced by decreased biomass (6) and impaired alginate production (12, 27).
Azithromycin affects the oxidative stress response in PAO1.
Another major finding of this study is that AZM treatment obviously led to an impaired oxidative stress response in P. aeruginosa. The superoxide dismutase SodB, the catalase KatA, and the alkylhydroperoxide reductase AhpC, all of which contribute significantly to the stress response in P. aeruginosa (32), were shown to be repressed at the transcriptional level (sodB, ahpC, and katA) and the protein level (SodB and AhpC) upon AZM addition. We also observed an up-regulation of the fur gene in response to sublethal AZM concentrations. fur is involved in the regulation of iron uptake under iron-limiting conditions. However, fur has also been shown to be up-regulated under oxidative stress (32) and simultaneous overexpression of the bfrB gene indicates a sufficient intracellular iron storage (47).
Another important element affected by oxidative stress is sulfur, since iron-sulfur proteins have been shown to play a protective role against oxidative stress (18). The sulfate binding protein of an ABC transporter (CysP) was one of the eight proteins that were demonstrated to be up-regulated upon AZM addition both at the transcriptional level and at the protein level. PA3262, a putative peptidyl-prolyl isomerase that probably corrects misfolding caused by the damage of reactive oxygen intermediates, was up-regulated at the transcriptional level. Similar, the thiol-disulfide oxireductase (DsbA), which has been shown to be responsible for protein thiol modifications in Escherichia coli (23), was up-regulated in the secretome of AZM-treated PAO1. Thiol-disulfide interconversion plays a crucial role in the control of cellular redox potential and the prevention of oxidative damage.
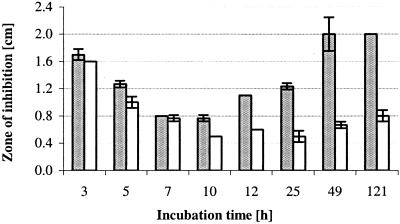
PA3529, encoding a probable peroxidase, as well as the genes encoding the heat shock proteins GroEL (also shown to be differentially expressed at the protein level) and GroES were down-regulated by AZM treatment. The observed effects of AZM on several genes and proteins that are involved in the oxidative stress response implied that AZM might affect long-term survival of P. aeruginosa during chronic infection. Thus, we tested whether AZM-exposed cultures were more sensitive to H2O2 treatment than the untreated controls. As shown in Fig. 4 bacteria that were treated with AZM were significantly more susceptible when exposed to H2O2 on solid agar. Prolonged growth of PAO1 in AZM-supplemented cultures increased bacterial susceptibility to H2O2, whereas gentamicin or ceftazidime pretreatment had no effect (data not shown).
FIG. 4.
Growth inhibition by H2O2 as determined by agar diffusion assays. Prolonged cultivation (≥10 h) of PAO1 in AZM-supplemented medium significantly increased sensitivity to H2O2 (t test; P < 0.0017). AZM-treated PAO1 (gray bars) was compared with untreated PAO1 (white bars). Results are given as the mean ± standard deviation of three determinations.
Concluding remarks.
Both the antimicrobial and anti-inflammatory effects of AZM have been implicated as being responsible for the improvement in CF patient outcome. The results of this study clearly indicate that there is an antipseudomonal effect of AZM that is linked to reduced virulence factor production, biofilm formation, and survival under stressful conditions due to interference with QS in P. aeruginosa. We identified a large common subset of QS- and AZM-regulated genes/proteins, in particular within the secretome, comprising many virulence factors. Moreover, the TTSS, which was previously shown to be negatively regulated by QS (10), was induced upon AZM addition, whereas in accordance with the results of a study reporting QS control of genes essential for relieving oxidative stress (7), we found markedly increased sensitivity of AZM-treated PAO1 cultures to H2O2. Moreover, our data on P. aeruginosa motility are in accordance with the observation that AZM retards biofilm formation (6), which has been reported to be dependent on the QS systems.
Our in vitro data imply that the QS-antagonistic activity of AZM contributes to the improvement of CF patient health. Apart from the reduced expression of virulence factors, interference with the bacterial oxidative stress response might be of major relevance. One vitally adaptive response of P. aeruginosa is the ability to resist the oxidative stress that is induced during phagocytosis, when the bacteria are confronted with reactive oxygen intermediates such as H2O2, O2−, and OH− from the respiratory burst of human phagocytes. Polymorphonuclear cells are the major effector cells responsible for the clearance of P. aeruginosa from the site of infection, and the inflammatory response in the chronically infected CF lung in particular is accompanied by very high levels of reactive oxygen intermediates that the bacteria must survive to be able to persist. Thus, the impaired oxidative stress response might account for the observed beneficial effects of AZM treatment and for the significant reduction of PAO1 viability after prolonged incubation with sub-MIC concentrations of AZM that has been reported previously (46).
Macrolides inhibit protein synthesis at the ribosomal level, and it is conceivable that unidentified stress responses, bacterial regulons, or signal transduction processes are responsible for the observed effects of sublethal concentrations on gene expression. Furthermore, future studies will have to elucidate whether the observed effects of sublethal AZM concentrations are also relevant in vivo. However, the results of this in vitro study and the fact that AZM exhibits beneficial effects in the treatment of CF patients give us reason to assume that the administration of AZM for CF will have a great impact on the management of chronic infection due to its interference with P. aeruginosa QS and thus with virulence factor production, biofilm formation, and persistence during chronic infection.
Acknowledgments
Financial support by the DFG-sponsored European Graduate School program “Pseudomonas: Pathogenicity and Biotechnology” is gratefully acknowledged.
We thank Tanja Töpfer, Jaqueline Majewski, and Reiner Munder for excellent technical assistance and Jürgen Wehland for continuous encouraging support.
REFERENCES
- 1.Arevalo-Ferro, C., M. Hentzer, G. Reil, A. Gorg, S. Kjelleberg, M. Givskov, K. Riedel, and L. Eberl. 2003. Identification of quorum-sensing regulated proteins in the opportunistic pathogen Pseudomonas aeruginosa by proteomics. Environ. Microbiol. 5:1350-1369. [DOI] [PubMed] [Google Scholar]
- 2.Carr, R. R., and M. C. Nahata. 2004. Azithromycin for improving pulmonary function in cystic fibrosis. Ann. Pharmacother. 38:1520-1524. [DOI] [PubMed] [Google Scholar]
- 3.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 4.Davies, J. C. 2002. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr. Respir. Rev. 3:128-134. [DOI] [PubMed] [Google Scholar]
- 5.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 6.Gillis, R. J., and B. H. Iglewski. 2004. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 42:5842-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassett, D. J., J. F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. West, C. T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 8.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirakata, Y., M. Kaku, R. Mizukane, K. Ishida, N. Furuya, T. Matsumoto, K. Tateda, and K. Yamaguchi. 1992. Potential effects of erythromycin on host defense systems and virulence of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogardt, M., M. Roeder, A. M. Schreff, L. Eberl, and J. Heesemann. 2004. Expression of Pseudomonas aeruginosa exoS is controlled by quorum sensing and RpoS. Microbiology 150:843-851. [DOI] [PubMed] [Google Scholar]
- 11.Hoiby, N. 2002. New antimicrobials in the management of cystic fibrosis. J. Antimicrob. Chemother. 49:235-238. [DOI] [PubMed] [Google Scholar]
- 12.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 13.Kadota, J., H. Mukae, H. Ishii, T. Nagata, H. Kaida, K. Tomono, and S. Kohno. 2003. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir. Med. 97:844-850. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura-Sato, K., Y. Iinuma, T. Hasegawa, T. Horii, T. Yamashino, and M. Ohta. 2000. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob. Agents Chemother. 44:2869-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keicho, N., and S. Kudoh. 2002. Diffuse panbronchiolitis: role of macrolides in therapy. Am. J. Respir. Med. 1:119-131. [DOI] [PubMed] [Google Scholar]
- 16.Kita, E., M. Sawaki, F. Nishikawa, K. Mikasa, Y. Yagyu, S. Takeuchi, K. Yasui, N. Narita, and S. Kashiba. 1990. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology 41:177-183. [DOI] [PubMed] [Google Scholar]
- 17.Kita, E., M. Sawaki, D. Oku, A. Hamuro, K. Mikasa, M. Konishi, M. Emoto, S. Takeuchi, N. Narita, and S. Kashiba. 1991. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J. Antimicrob. Chemother. 27:273-284. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, K., and S. Tagawa. 2004. Activation of SoxR-dependent transcription in Pseudomonas aeruginosa. J. Biochem. (Tokyo) 136:607-615. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, T., K. Tateda, T. Matsumoto, S. Miyazaki, A. Watanabe, T. Nukiwa, and K. Yamaguchi. 2002. Macrolide-treated Pseudomonas aeruginosa induces paradoxical host responses in the lungs of mice and a high mortality rate. J. Antimicrob. Chemother. 50:59-66. [DOI] [PubMed] [Google Scholar]
- 20.Koch, C., and N. Hoiby. 2000. Diagnosis and treatment of cystic fibrosis. Respiration 67:239-247. [DOI] [PubMed] [Google Scholar]
- 21.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 22.Lamanda, A., A. Zahn, D. Roder, and H. Langen. 2004. Improved Ruthenium II tris (bathophenantroline disulfonate) staining and destaining protocol for a better signal-to-background ratio and improved baseline resolution. Proteomics 4:599-608. [DOI] [PubMed] [Google Scholar]
- 23.Leichert, L. I., and U. Jakob. 2004. Protein thiol modifications visualized in vivo. PLoS Biol. 2:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinari, G., C. A. Guzman, A. Pesce, and G. C. Schito. 1993. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J. Antimicrob. Chemother. 31:681-688. [DOI] [PubMed] [Google Scholar]
- 26.Molinari, G., P. Paglia, and G. C. Schito. 1992. Inhibition of motility of Pseudomonas aeruginosa and Proteus mirabilis by subinhibitory concentrations of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 11:469-471. [DOI] [PubMed] [Google Scholar]
- 27.Nagino, K., and H. Kobayashi. 1997. Influence of macrolides on mucoid alginate biosynthetic enzyme from Pseudomonas aeruginosa. Clin. Microbiol. Infect. 3:432-439. [DOI] [PubMed] [Google Scholar]
- 28.Nicolau, D. P., M. A. Banevicius, C. H. Nightingale, and R. Quintiliani. 1999. Beneficial effect of adjunctive azithromycin in treatment of mucoid Pseudomonas aeruginosa pneumonia in the murine model. Antimicrob. Agents Chemother. 43:3033-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nouwens, A. S., S. A. Beatson, C. B. Whitchurch, B. J. Walsh, H. P. Schweizer, J. S. Mattick, and S. J. Cordwell. 2003. Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology 149:1311-1322. [DOI] [PubMed] [Google Scholar]
- 30.Ordonez, C. L., M. Stulbarg, H. Grundland, J. T. Liu, and H. A. Boushey. 2001. Effect of clarithromycin on airway obstruction and inflammatory markers in induced sputum in cystic fibrosis: a pilot study. Pediatr. Pulmonol. 32:29-37. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 32.Palma, M., D. DeLuca, S. Worgall, and L. E. Quadri. 2004. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 186:248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pechere, J. C. 2001. Azithromycin reduces the production of virulence factors in Pseudomonas aeruginosa by inhibiting quorum sensing. Jpn. J. Antibiot. 54(Suppl. C):87-89. [PubMed] [Google Scholar]
- 34.Pirzada, O. M., J. McGaw, C. J. Taylor, and M. L. Everard. 2003. Improved lung function and body mass index associated with long-term use of macrolide antibiotics. J. Cyst. Fibros. 2:69-71. [DOI] [PubMed] [Google Scholar]
- 35.Poletti, V., M. Chilosi, G. Casoni, and T. V. Colby. 2004. Diffuse panbronchiolitis. Sarcoidosis Vasc. Diffuse Lung Dis. 21:94-104. [PubMed] [Google Scholar]
- 36.Rajan, S., and L. Saiman. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47-56. [DOI] [PubMed] [Google Scholar]
- 37.Ratjen, F. 2001. Changes in strategies for optimal antibacterial therapy in cystic fibrosis. Int. J. Antimicrob. Agents 17:93-96. [DOI] [PubMed] [Google Scholar]
- 38.Saiman, L. 2004. The use of macrolide antibiotics in patients with cystic fibrosis. Curr. Opin. Pulmon. Med. 10:515-523. [DOI] [PubMed] [Google Scholar]
- 39.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 40.Sawaki, M., R. Mikami, K. Mikasa, M. Kunimatsu, S. Ito, and N. Narita. 1986. The long term chemotherapy with erythromycin in chronic lower respiratory tract infections—first report: comparison with amoxicillin. Kansenshogaku Zasshi 60:37-44. [DOI] [PubMed] [Google Scholar]
- 41.Sawaki, M., R. Mikami, K. Mikasa, M. Kunimatsu, S. Ito, and N. Narita. 1986. The long term chemotherapy with erythromycin in chronic lower respiratory tract infections—second report: including cases with Pseudomonas infections. Kansenshogaku Zasshi 60:45-50. [DOI] [PubMed] [Google Scholar]
- 42.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Southern, K. W., P. M. Barker, and A. Solis. 2004. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. CD002203. [Online.] [DOI] [PubMed]
- 45.Tateda, K., R. Comte, J. C. Pechere, T. Kohler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tateda, K., Y. Ishii, T. Matsumoto, N. Furuya, M. Nagashima, T. Matsunaga, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1996. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob. Agents Chemother. 40:2271-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasil, M. L., and U. A. Ochsner. 1999. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol. Microbiol. 34:399-413. [DOI] [PubMed] [Google Scholar]
- 48.Wagner, T., G. Soong, S. Sokol, L. Saiman, and A. Prince. 2005. Effects of azithromycin on clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patients. Chest 128:912-919. [DOI] [PubMed] [Google Scholar]
- 49.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner, V. E., R. J. Gillis, and B. H. Iglewski. 2004. Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 22(Suppl. 1):S15-S20. [DOI] [PubMed] [Google Scholar]
- 51.Wehmhoner, D., S. Haussler, B. Tummler, L. Jansch, F. Bredenbruch, J. Wehland, and I. Steinmetz. 2003. Inter- and intraclonal diversity of the Pseudomonas aeruginosa proteome manifests within the secretome. J. Bacteriol. 185:5807-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolter, J. M., S. L. Seeney, and J. G. McCormack. 2002. Macrolides in cystic fibrosis: is there a role? Am. J. Respir. Med. 1:235-241. [DOI] [PubMed] [Google Scholar]
- 54.Wozniak, D. J., and R. Keyser. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125:62S-69S. [DOI] [PubMed] [Google Scholar]
- 55.Wu, H., Z. Song, M. Hentzer, J. B. Andersen, S. Molin, M. Givskov, and N. Hoiby. 2004. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 53:1054-1061. [DOI] [PubMed] [Google Scholar]
- 56.Yamagishi, M., H. Matsushima, A. Wada, M. Sakagami, N. Fujita, and A. Ishihama. 1993. Regulation of the Escherichia coli rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J. 12:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]