Abstract
Ciprofloxacin is the substrate for a multidrug resistance-related protein (MRP)-like multidrug transporter in J774 mouse macrophages, which also modestly affects levofloxacin but only marginally affects garenoxacin and moxifloxacin (J.-M. Michot et al., Antimicrob. Agents Chemother. 49:2429-2437, 2005). Two clones of ciprofloxacin-resistant cells were obtained by a stepwise increase in drug concentration (from 34 to 51 to 68 mg/liter) in the culture fluid. Compared to wild-type cells, ciprofloxacin-resistant cells showed (i) a markedly reduced ciprofloxacin accumulation (12% of control) and (ii) a two- to threefold lower sensitivity to the enhancing effect exerted by MRP-inhibitors (probenecid and MK571) on ciprofloxacin accumulation or by ciprofloxacin itself. ATP-depletion brought ciprofloxacin accumulation to similarly high levels in both wild-type and ciprofloxacin-resistant cells. Garenoxacin and moxifloxacin accumulation remained unaffected, and levofloxacin showed an intermediate behavior. DNA and protein synthesis were not impaired in ciprofloxacin-resistant cells for ciprofloxacin concentrations up to 100 mg/liter (∼85 and 55% inhibition, respectively, in wild-type cells). In Listeria monocytogenes-infected ciprofloxacin-resistant cells, 12-fold higher extracellular concentrations of ciprofloxacin were needed to show a bacteriostatic effect in comparison with wild-type cells. The data suggest that the resistance mechanism is mediated by an overexpression and/or increased activity of the MRP-like ciprofloxacin transporter expressed at a basal level in wild-type J774 macrophages, which modulates both the intracellular pharmacokinetics and activity of ciprofloxacin.
Active efflux is a general means developed by cells for protection against invasion by amphiphilic, potentially harmful molecules (18). In this context, overexpression of multidrug efflux pumps is now recognized as a common and widespread mechanism of resistance to anticancer agents in eukaryotic cells (for a review, see reference 3). These pumps often display broad substrate specificities (7, 15). It is therefore not surprising that they also transport other amphipathic drugs like antibiotics (for a review, see reference 19). In this context, we showed that the fluoroquinolone ciprofloxacin is subject to active efflux by a multidrug resistance-related protein (MRP)-like transporter in J774 mouse macrophages (11). The activity of this transporter significantly reduces the accumulation of ciprofloxacin in comparison with other drugs of the same class (10) and, as a consequence, impairs its activity against intracellular bacteria such as Listeria monocytogenes (13).
In an attempt to better characterize the transporter of ciprofloxacin, we have generated J774 macrophages with increased efflux capabilities toward this drug. We applied a procedure commonly used to select resistance to anticancer drugs (6), namely, the continuous exposure to progressively increasing concentrations of the drug under study. This methodology, which can select multifactorial resistance, is intended to mimic to some extent what may develop in vivo upon chronic exposure to the corresponding drug (6). We obtained stable cell lines resistant to 68 mg/liter (0.2 mM) ciprofloxacin. The present paper deals with a description of the quinolone pharmacokinetics and pharmacodynamics in these cells in comparison with the wild-type, parent cell line.
MATERIALS AND METHODS
Cell culture and selection of ciprofloxacin-resistant J774 macrophages.
All experiments were performed with J774 mouse macrophages. Wild-type cells were maintained exactly as reported previously (11). To select ciprofloxacin-resistant cells, we used a stepwise approach similar to that described previously for obtaining probenecid-resistant J774 macrophages (1, 11). Based on preliminary experiments evaluating ciprofloxacin cytotoxicity in wild-type cells, a concentration of 34 mg/liter (0.1 mM) was used as a first selection step. After 4 weeks (i.e., up to passage 6), the ciprofloxacin concentration was increased to 51 mg/liter (0.15 mM) for 3 weeks (from passage 7 to 9) and then further increased to 68 mg/liter (0.2 mM) for 6 months (up to passage 30). At each increase in ciprofloxacin concentration, cells showed a marked but transient (1 to 2 passages) decrease in their multiplication rates, after which, however, they resumed at almost normal growth. Cells were then used for experiments up to the 120th passage, while being maintained in the continuous presence of 68 mg/liter ciprofloxacin. During this period, cells were regularly checked for ciprofloxacin accumulation in the absence and in the presence of probenecid (with no differences in the corresponding accumulation levels). Revertant cells were obtained by transferring ciprofloxacin-resistant cells to drug-free medium and cultivating them in the absence of ciprofloxacin for up to 90 passages.
Measurement of antibiotic accumulation and modulation by transporter inhibitors and ATP depletion.
The incubation of cells with antibiotics was performed as described elsewhere (11). Ciprofloxacin-resistant cells, routinely cultivated in the presence of 68 mg/liter ciprofloxacin, were rinsed twice in phosphate-buffered saline prior to the start of the experiments. The addition of inhibitors of efflux transporters and ATP-depletion (by addition of 60 mM deoxyglucose and 5 mM NaN3) were performed as described previously (11), except that the preincubation in ATP-depleting conditions was set at 45 min after preliminary experiments had disclosed that the residual ATP level remained similar for preincubation times between 15 and 60 min.
Assay of cell-associated quinolones.
Quinolones were assayed by fluorimetry, using procedures previously described in detail (10, 11). We checked previous studies to ensure that J774 cells do not metabolize quinolones to a significant extent and that the amount of quinolones detected by fluorimetry matches that detected by bioassay (2). The cell antibiotic content was expressed by reference to the cell total protein concentration of each sample.
Protein and DNA synthesis.
Incorporation of [3H]thymidine or [3H]leucine was determined by measuring the amount of trichloroacetic acid-precipitable radioactivity after incubation with the radiolabeled tracers (200 nM [3H]thymidine; 80 nM [3H]leucine) as previously described (17). The time of incubation of the cells with the radioactive tracers was set to 3 h, which yielded a similar incorporation in wild-type and ciprofloxacin-resistant cells (387.1 ± 63.4 versus 424.4 ± 13.3 pmol/mg of protein for [3H]thymidine and 18.2 ± 0.6 versus 18.8 ± 1.2 pmol/mg of protein for [3H]leucine).
Assay of total cell ATP content.
Total ATP was measured by luciferase assay (Boehringer Manheim ATP-bioluminescence assay kit CLS II; Roche Diagnostics, F. Hoffman-la Roche Ltd., Basel, Switzerland) as described previously (11). The lowest value measured in ATP-depleted cells was still 2.99-fold (95% confidence interval, 2.57- to 3.31-fold) higher than the background.
Assessment of cellular viability.
Cell viability was assessed by measuring the release of lactate dehydrogenase in the culture medium, assayed by the method of Vassault (20). This release was 3.7% ± 0.3% for wild-type cells and 2.7% ± 1.3% for resistant cells in the absence of other treatment and remained lower than 10% in all experimental conditions.
Cell infection and measurement of intracellular activity.
Intracellular activity of ciprofloxacin was determined toward a hemolysin-producing strain (EGD serotype 1/2a) of L. monocytogenes (obtained from P. Berche, Laboratoire de Microbiologie, Faculté de Médecine, Necker, Paris, France). Experiments were conducted as described earlier (13), except that we used an initial inoculum of 7 bacteria per macrophage.
Materials.
Antibiotics were obtained as microbiological standards from their corresponding manufacturers as follows: ciprofloxacin (purity, 98.0%) and moxifloxacin (purity, 99.8%) from Bayer A.G., Leverkusen, Germany; levofloxacin (purity, 99.7%) from Aventis Pharma, Antony, France; and garenoxacin (purity, 99.8%) from Bristol Myers Squibb, New Brunswick, Conn. Verapamil and 2-d-deoxyglucose were supplied from Fluka Chemie, Buchs, Switzerland; probenecid was from Sigma-Aldrich Chemie, Steinheim, Germany; and MK571 (3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid) was from Alexis Corporation, San Diego, Calif. Cell culture medium and serum were from Gibco Invitrogen Corporation (Paisley, Scotland). [3H]thymidine (41 Ci/mmol) and [3H]leucine (63 Ci/mmol) were obtained from Amersham PLC, Little Chalfont, Buckingamshire, United Kingdom. All other reagents were from E. Merck AG (Darmstadt, Germany) or Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Curve-fitting analyses (including calculations of regression parameters and 95% confidence intervals) were made with GraphPad Prism, version 4.00, for Windows (San Diego, CA). Statistical analyses were performed using Graphpad Instat and XLSTAT Pro (version 7.5.2; Addinsoft SARL, Paris, France).
RESULTS
Two clones of ciprofloxacin-resistant cells (CR1 and CR2) with similarly reduced capacity to accumulate ciprofloxacin were obtained at two 2-year intervals (in 2001 and 2003). Compared to wild-type cells, these cells did not reveal a constant difference in morphology based on examination in the optic microscope, and growth rates were essentially similar. Electron microscopy of CR2 cells showed a well-dilated Golgi apparatus and an abundance of mitochondria, denoting the maintenance of active metabolic activity. The specific change in phenotype with respect to tolerance to ciprofloxacin was assessed by examining the microscopic appearance and the protein content of wild-type cultures exposed abruptly to 68 mg/liter ciprofloxacin. This resulted in a large loss of the cells from the culture (with a protein content decreasing to ∼50% and ∼25% of the control after 24 and 48 h, respectively). Most critical pharmacokinetic experiments were performed with both clones, yielding similar results. A brief description of CR1 cells and of their main properties was reported earlier (J.-M. Michot et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A486, 2002). We therefore present here only the phenotypic characterization of CR2 cells, which were studied in more detail.
Accumulation of quinolones in wild-type and resistant cells and effect of ATP depletion.
In a first series of experiments, we compared the accumulation of the quinolones in wild-type versus ciprofloxacin-resistant cells. Pilot experiments showed that an incubation time of 2 h allowed cultures to reach a stable cellular concentration for all four of the quinolones studied, and this exposure time was therefore systematically used thereafter. Figure 1 (left graph) shows the following ranking for quinolone accumulation by wild-type cells: ciprofloxacin < levofloxacin < garenoxacin < moxifloxacin, as previously reported (10) with P < 0.05 for all drugs (by analysis of variance [ANOVA]). In resistant cells, the cellular concentrations of ciprofloxacin and levofloxacin were markedly reduced (12 and 18% of the values measured in wild-type cells, respectively; P < 0.001 for wild-type versus ciprofloxacin-resistant cells by ANOVA), and differences between drugs remained significant (P < 0.05 by ANOVA).
FIG. 1.
Cellular concentration of quinolones each at an extracellular concentration of 17 mg/liter in wild-type (WT) and in ciprofloxacin-resistant (RS) macrophages after 2 h of incubation. All values are the means of three independent determinations ± standard deviations. CIP, ciprofloxacin; LVX, levofloxacin; GRN, garenoxacin; MXF, moxifloxacin.
We previously reported that ciprofloxacin accumulation is markedly increased in J774 macrophages by ATP depletion (11). We therefore examined the influence of ATP depletion in ciprofloxacin-resistant cells in comparison with wild-type cells. Basal ATP levels were 25 ± 3 and 32 ± 2 nmol/mg of protein in wild-type and ciprofloxacin-resistant cells, respectively, and were reduced to 3.5 ± 1.0 and 7.9 ± 1.0 nmol/mg of protein, respectively, after a 45-min incubation with 60 mM deoxyglucose and 5 mM NaN3. Figure 1 (right graph) shows that ATP depletion indeed increased ciprofloxacin accumulation in wild-type cells (P < 0.001), as anticipated, but had no significant effect on the accumulation of the three other quinolones. It also increased the accumulation of ciprofloxacin and levofloxacin in ciprofloxacin-resistant cells (P < 0.001). Most interestingly, ATP depletion caused each quinolone to actually accumulate to a similar level in wild-type and ciprofloxacin-resistant cells.
Influence of efflux pump inhibitors on quinolone accumulation.
The cellular accumulation of ciprofloxacin and levofloxacin is increased in the presence of probenecid and MK571 (10). The influence of these inhibitors on the accumulation of quinolones in wild-type and ciprofloxacin-resistant cells was therefore examined here. Results are shown in Fig. 2. Looking at ciprofloxacin first, we see that probenecid and MK571 caused an increase in cell accumulation of this quinolone in both wild-type and ciprofloxacin-resistant cells. Ciprofloxacin-resistant cells, however, were two- to threefold less sensitive to either inhibitor when these were used at concentrations lower than the maximal value tested (differences between wild-type and ciprofloxacin-resistant cells became nonsignificant by ANOVA at concentrations of 15 mM probenecid and 200 μM MK571 or higher). Similar observations were made for levofloxacin but with less difference between wild-type and ciprofloxacin-resistant cells, especially for MK571 (differences between the two cell types became nonsignificant at concentrations of 15 mM probenecid and 100 μM MK571 or higher). Garenoxacin accumulation in wild-type and ciprofloxacin-resistant cells was only weakly affected by probenecid and MK571 (and was not different from that measured in wild-type cells with 15 mM probenecid and 100 μM MK571 or higher), and that of moxifloxacin was not significantly modified. The influence of verapamil (50 and 100 μM) on quinolone accumulation in wild-type and ciprofloxacin-resistant cells was tested in parallel. No significant effect was observed (data not shown).
FIG. 2.
Influence of efflux pump inhibitors on the cellular concentration of quinolones in wild-type and in ciprofloxacin-resistant macrophages. Cells were incubated for 2 h in the simultaneous presence of 17 mg/liter of each quinolone and increasing concentrations of each inhibitor. Results are expressed as percentages of the maximal values measured in each individual experiment. All values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol). CIP, ciprofloxacin; LVX, levofloxacin; GRN, garenoxacin; MXF, moxifloxacin.
Influence of the extracellular concentration of quinolones on their accumulation.
Ciprofloxacin exerts a facilitating effect on its own accumulation, which has been interpreted as denoting a concentration-dependent inhibition of its efflux (11) (other hypotheses like unbalanced influx at high extracellular concentrations were excluded based on the observation that high extracellular concentrations in moxifloxacin were also able to increase ciprofloxacin accumulation [10]). As shown in Fig. 3, this effect was reproduced with wild-type cells here, since the cellular concentration of ciprofloxacin increased in an exponential fashion with respect to its extracellular concentration, yielding cellular-to-extracellular concentration ratios about 10-fold higher for extracellular ciprofloxacin concentrations of 200 mg/liter versus 5 mg/liter (200 mg/liter was the limit of solubility of ciprofloxacin under our conditions). A similar phenomenon was seen in ciprofloxacin-resistant cells, but its magnitude was considerably lower (threefold) in the range of concentrations investigated. Accordingly, a one-phase exponential association function was fitted to the data for ciprofloxacin (R2 of 0.987 and 1.000 for wild-type and ciprofloxacin-resistant cells, respectively). In sharp contrast, the cellular contents of garenoxacin and moxifloxacin were strictly proportional to their extracellular concentration up to 500 mg/liter, with no difference between wild-type and ciprofloxacin-resistant cells (R2 and slope of linear regressions, respectively: 0.981 and 30.4 ± 1.6 × 103 for wild-type cells and 0.997 and 26.1 ± 0.5 × 103 for ciprofloxacin-resistant cells for garenoxacin; 0.989 and 42.7 ± 1.6 × 103 for wild-type cells and 0.987 and 44.9 ± 1.9 × 103 ciprofloxacin-resistant cells for moxifloxacin). For levofloxacin, a minor effect of the drug concentration on its own uptake was seen in ciprofloxacin-resistant cells in the concentration range of 3 to 20 mg/liter (Fig. 3, LVX inset) (R2 of 0.999 for one-phase association), but this effect became insignificant at higher concentrations (R2 and slope of linear regressions, respectively: 1.000 and 29.7 ± 0.1 × 103 for wild-type cells and 0.998 and 28.7 ± 0.6 × 103 for ciprofloxacin-resistant cells).
FIG. 3.

Influence of the extracellular concentration of quinolones on their cellular concentrations in wild-type cells or ciprofloxacin-resistant cells measured after 2 h of incubation. All values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol). Details of the data for low extracellular concentrations of levofloxacin, garenoxacin, and moxifloxacin are shown in the insets. CIP, ciprofloxacin; LVX, levofloxacin; GRN, garenoxacin; MXF, moxifloxacin.
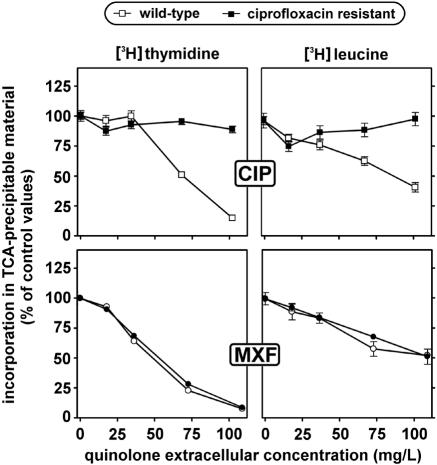
Influence of quinolones on protein and DNA synthesis in wild-type and resistant cells.
Quinolones impair DNA synthesis in eukaryotic cells when used at high concentrations such as those used here (12). We therefore compared wild-type and ciprofloxacin-resistant cells for this effect and also examined the influence exerted by ciprofloxacin on protein synthesis. Cells were incubated for 24 h with increasing concentrations of ciprofloxacin and then exposed to either [3H]thymidine or [3H]leucine for 3 h. Results are shown in Fig. 4 (top graphs, CIP). Ciprofloxacin caused a concentration-dependent decrease of both DNA (for ciprofloxacin concentrations of >34 mg/liter) and protein synthesis in wild-type cells. In contrast, no marked inhibition of either DNA or protein synthesis was seen in ciprofloxacin-resistant cells. Moxifloxacin was also studied in view of its contrasting behavior with ciprofloxacin for accumulation by wild-type versus ciprofloxacin-resistant cells. As shown in Fig. 4 (bottom graphs), moxifloxacin caused a concentration-dependent decrease in both DNA and protein synthesis that was essentially similar in both cell types.
FIG. 4.
Incorporation of [3H]thymidine and [3H]leucine in trichloroacetic acid (TCA)-precipitable material of wild-type and ciprofloxacin-resistant macrophages. Cells were incubated during 24 h with increasing concentrations of ciprofloxacin (CIP) or moxifloxacin (MXF) and then exposed for 3 h to an 80 nM concentration radiolabeled thymidine or a 200 nM concentration of radiolabeled leucine. All values are expressed as a percentage of the control and are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol).
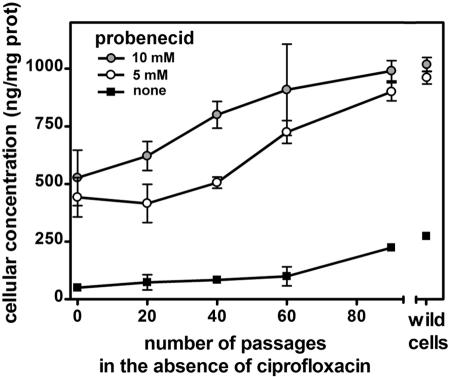
Reversibility of the resistance phenotype.
In the next series of experiments, we examined whether and at what rate cultivation of ciprofloxacin-resistant cells in a ciprofloxacin-free medium would allow cells to revert to a wild-type phenotype with respect to the accumulation of ciprofloxacin (Fig. 5). Statistical analysis (ANOVA) showed that cellular content in ciprofloxacin became significantly different (P < 0.01) from that measured in ciprofloxacin-resistant cells (passage 0; 51 ± 6 ng/mg of protein) after 90 passages for cells incubated with ciprofloxacin alone (224 ± 4 ng/mg of protein) and after 60 passages for cells incubated with ciprofloxacin and probenecid (5 or 10 mM). Complete reversion (no significant difference versus wild-type cells; 274 ± 1 ng/mg of protein) was obtained at passage 90 for cells challenged with ciprofloxacin in the absence of probenecid or in the presence of 5 mM probenecid and at passage 40 for cells challenged with ciprofloxacin and 10 mM probenecid.
FIG. 5.
Cellular concentration of ciprofloxacin in ciprofloxacin-resistant macrophages cultivated for an increasing number of passages in the absence of ciprofloxacin (each passage was made at a 3-day interval). Cells were incubated for 2 h with 17 mg/liter ciprofloxacin alone (filled squares) or with ciprofloxacin plus either 5 mM probenecid (open circles) or 10 mM probenecid (gray circles). Reference values for wild-type cells are shown on the right of the graph. All values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol).
Activity of ciprofloxacin against intracellular L. monocytogenes in wild-type and resistant cells.
Ciprofloxacin has only limited activity against intracellular L. monocytogenes, which, however, can be improved in the presence of efflux pump inhibitors, like gemfibrozil (13). Here, we examined the influence of probenecid on this activity in wild-type and ciprofloxacin-resistant cells. For this purpose, macrophages were infected with L. monocytogenes and incubated in the presence of increasing concentrations of ciprofloxacin for 5 h after phagocytosis. Results are shown in Fig. 6 (left graph). In wild-type cells, the activity of ciprofloxacin followed a typical sigmoidal concentration-response curve (R2 = 0.845 for the curve fitting), with a static effect observed at an extracellular concentration corresponding to 2.5× MIC. In resistant cells, the concentration-response curve (R2 = 0.979 for the curve fitting) was markedly shifted toward higher extracellular concentrations, and a static effect was reached only at an extracellular concentration of 30× MIC (P < 0.05 between wild-type and resistant cells by analysis of covariance of the curves). In subsequent experiments, we observed that the addition of probenecid simultaneously with ciprofloxacin caused a shift of the curves toward lower extracellular concentrations for both wild-type and ciprofloxacin-resistant cells (data not shown), suggesting that activity was directly correlated to the intracellular drug content. We tested this hypothesis by running an experiment in which the dose response of the ciprofloxacin antibacterial effect toward L. monocytogenes was examined in wild-type cells exposed to 5 mM probenecid versus ciprofloxacin-resistant cells exposed to 10 mM probenecid, which should create roughly similar ciprofloxacin cell contents in both cell types (Fig. 2). As shown in Fig. 6 (right graph), the responses seen in wild-type and ciprofloxacin-resistant cells again followed sigmoidal dose-response curves (with R2 of 0.945 in wild-type cells plus 5 mM probenecid and 0.964 in ciprofloxacin-resistant cells plus 10 mM probenecid) and were quite similar, with static effects observed for ciprofloxacin extracellular concentrations corresponding to 0.8× and 1.1× MIC and with maximal effects of about 0.5 log under the value of the original inoculum in both cases (no significant difference by analysis of covariance between these two curves or between curves obtained in the absence or in the presence of probenecid for wild-type cells; P < 0.05 for curves obtained with ciprofloxacin-resistant cells in the absence and in the presence of probenecid).
FIG. 6.
Concentration killing curves of ciprofloxacin toward L. monocytogenes in wild-type (WT) cells or ciprofloxacin-resistant (RS) cells. In the right panel, cells were incubated with probenecid as indicated (the probenecid concentrations were selected to obtain a grossly similar content in ciprofloxacin based on data from Fig. 2). The abscissas show the extracellular concentrations of ciprofloxacin in multiples of its MIC in broth (2 mg/liter). The arrow points to the concentration corresponding to the maximum concentration (Cmax; peak concentration in serum, 4.3 mg/liter [9]) commonly observed in the serum of patients treated with ciprofloxacin. The ordinates show the changes in CFU (log10) per mg of cell protein as observed after 5 h of incubation in comparison with the original inocula (horizontal dotted lines). All values are the means of three independent determinations ± standard deviations (when not visible, error bars are smaller than the size of the symbol).
DISCUSSION
This study shows that stepwise increases in ciprofloxacin concentration allow J774 macrophages to survive in the presence of drug concentrations that would cause almost complete cell death in wild-type cells. This property can reasonably be ascribed to an increased expression and/or activity of the ciprofloxacin efflux transporter previously described in J774 macrophages (11) for the following reasons. First, the cellular concentration of ciprofloxacin is markedly reduced in ciprofloxacin-resistant cells, but this can be reversed by (i) ATP depletion, (ii) high concentrations of efflux pump inhibitors (probenecid or MK571), or (iii) an increase in ciprofloxacin concentration. Of note is the fact that the efflux pump inhibitors or ciprofloxacin were considerably less effective in ciprofloxacin-resistant cells than in wild-type cells. Second, no effect was seen on the pattern of accumulation of garenoxacin or moxifloxacin, two quinolones that are not affected by the ciprofloxacin transporter in J774 macrophages (10). Levofloxacin, which is partially affected by this transporter (10), actually showed intermediate behavior in most of the experiments reported here. All of these elements are consistent with increased expression or activity of a ciprofloxacin efflux transporter present in J774 macrophages and tentatively identified as a member of the MRP family of ATP-energized multidrug transporters (11). Even though increased expression of the transporter is the most probable reason for these changes, increased activity or modified substrate specificity cannot be excluded, based on the observation that levofloxacin accumulation seems to be more affected by active efflux in resistant cells than in wild-type cells. A contrary, alternative hypothesis such as impairment of quinolone influx, reduction of sequestering capabilities, or cell survival through a protective mechanism at the level of DNA replication of function would not account for these observations. Conversely, it is intriguing in this context that ciprofloxacin-resistant cells showed a higher basal level of ATP and also retained a higher level after exposure to 2-deoxyglucose and NaN3, which could be interpreted as an adaptation of the metabolism to energize an ATP-dependent mechanism essential for cell survival.
The role of efflux in allowing ciprofloxacin-resistant cells to survive in the presence of high concentrations of ciprofloxacin is evident from the fact that ciprofloxacin markedly affects DNA and protein synthesis in wild-type cells but not in ciprofloxacin-resistant cells. We also see that moxifloxacin, which is not affected by the transporter, alters these two parameters similarly in both cell lines.
The present data do not provide indications as to the molecular mechanism by which ciprofloxacin could modify efflux pump expression. Regulation of the expression of MRP transporters is indeed complex, variable among different members of this family, and still poorly understood (7). Yet, it has been demonstrated that a 1- to 3-day exposure of lymphocytes to ciprofloxacin at concentrations ranging from 5 to 80 mg/liter ciprofloxacin interferes with several gene programs, which suggests a massive stress response (4). This can create a typical situation of multiple mutations or modulation of gene expression allowing for the successful selection of cells adapted to the new environment. The slow reversibility of the high-efflux phenotype is consistent with a high level of overexpression, since the stability of a multifactorial resistance phenotype is related to the level of gene expression it requires (6). Yet it may also simply indicate that the metabolic cost of the resistance has remained low, yielding little advantage to revertant cells upon suppression of the inducer. We must also stress that other changes than those affecting active efflux transport may have been selected in our ciprofloxacin-resistant cells, as is often observed with the mode of selection applied here (see reference 6 for general principles and references 8 and 14 for typical examples).
The observation that exposure of cells to ciprofloxacin may lead to an overexpression of its own efflux may be of importance in chemotherapy. First, the reduction in its cellular concentration may significantly reduce the level of activity of the drug against intracellular bacteria, as demonstrated here for cells infected with L. monocytogenes. Future studies will need to examine to what extent our observations apply to other cells than the murine macrophages studied here and whether the conclusions made from our data with L. monocytogenes are also valid for other intracellular pathogens. Second, the overexpression of the ciprofloxacin transporter may confer resistance to other drugs, since the transporter probably belongs to a family characterized by a broad substrate specificity, transporting drugs of variable chemical structure and belonging to unrelated pharmacological classes (for a recent review, see reference 7). In this context, quinolones have been shown to reverse resistance to anticancer agents by competing for their transport in malignant cell lines (5, 16). Supratherapeutic concentrations of ciprofloxacin were needed to select for resistance in the present study. However, other cell types may be more susceptible, especially if they are coexposed to drugs interacting with the same transporter. The present data may, therefore, raise interesting questions about potential, expected consequences of a prolonged use of quinolones for therapeutic or prophylactic purposes.
Acknowledgments
We are particularly grateful to M. C. Cambier for dedicated help in obtaining and maintaining the resistant and revertant cell lines. We also thank M. Vergauwen for skillful technical assistance.
J.-M.M. was, successively, recipient of a fellowship of the Belgian Bourse Belge de la Vocation/Belgische Stichting Roeping and Aspirant of the Fonds Spécial de Recherches of the Université catholique de Louvain. N.E.C. is a postdoctoral fellow, and F.V.B. is a Chercheur Qualifié of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grants 3.4549.00 and 3.4542.02) and by a grant-in-aid from Bayer AG, Leverkusen, Germany.
We thank the manufacturers for the kind gifts of their respective antibiotics.
REFERENCES
- 1.Cao, C., T. H. Steinberg, H. C. Neu, D. Cohen, S. B. Horwitz, S. Hickman, and S. C. Silverstein. 1993. Probenecid-resistant J774 cell expression of enhanced organic anion transport by a mechanism distinct from multidrug resistance. Infect. Agents Dis. 2:193-200. [PubMed] [Google Scholar]
- 2.Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. [DOI] [PubMed] [Google Scholar]
- 3.Deng, L., S. Tatebe, Y. C. Lin-Lee, T. Ishikawa, and M. T. Kuo. 2002. MDR and MRP gene families as cellular determinant factors for resistance to clinical anticancer agents. Cancer Treat. Res. 112:49-66. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson, E., A. Forsgren, and K. Riesbeck. 2003. Several gene programs are induced in ciprofloxacin-treated human lymphocytes as revealed by microarray analysis. J. Leukoc. Biol. 74:456-463. [DOI] [PubMed] [Google Scholar]
- 5.Gollapudi, S., F. Thadepalli, C. H. Kim, and S. Gupta. 1995. Difloxacin reverses multidrug resistance in HL-60/AR cells that overexpress the multidrug resistance-related protein (MRP) gene. Oncol. Res. 7:213-225. [PubMed] [Google Scholar]
- 6.Gottesman, M. M., C. Cardarelli, S. Goldenberg, T. Licht, and I. Pastan. 1998. Selection and maintenance of multidrug-resistant cells. Methods Enzymol. 292:248-258. [DOI] [PubMed] [Google Scholar]
- 7.Haimeur, A., G. Conseil, R. G. Deeley, and S. P. Cole. 2004. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr. Drug Metab. 5:21-53. [DOI] [PubMed] [Google Scholar]
- 8.Huang, Y., A. M. Ibrado, J. C. Reed, G. Bullock, S. Ray, C. Tang, and K. Bhalla. 1997. Co-expression of several molecular mechanisms of multidrug resistance and their significance for paclitaxel cytotoxicity in human AML HL-60 cells. Leukemia 11:253-257. [DOI] [PubMed] [Google Scholar]
- 9.Israel, D., J. G. Gillum, M. Turik, K. Harvey, J. Ford, H. Dalton, M. Towle, R. Echols, A. H. Heller, and R. Polk. 1993. Pharmacokinetics and serum bactericidal titers of ciprofloxacin and ofloxacin following multiple oral doses in healthy volunteers. Antimicrob. Agents Chemother. 37:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michot, J.-M., C. Seral, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Influence of efflux transporters on the accumulation and efflux of 4 quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 49:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michot, J.-M., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2004. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob. Agents Chemother. 48:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seay, T. M., S. J. Peretsman, and P. S. Dixon. 1996. Inhibition of human transitional cell carcinoma in vitro proliferation by fluoroquinolone antibiotics. J. Urol. 155:757-762. [PubMed] [Google Scholar]
- 13.Seral, C., S. Carryn, P. M. Tulkens, and F. Van Bambeke. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167-1173. [DOI] [PubMed] [Google Scholar]
- 14.Sinha, P., J. Poland, S. Kohl, M. Schnolzer, H. Helmbach, G. Hutter, H. Lage, and D. Schadendorf. 2003. Study of the development of chemoresistance in melanoma cell lines using proteome analysis. Electrophoresis 24:2386-2404. [DOI] [PubMed] [Google Scholar]
- 15.Sun, J., Z. G. He, G. Cheng, S. J. Wang, X. H. Hao, and M. J. Zou. 2004. Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med. Sci. Monit. 10:RA5-RA14. [PubMed] [Google Scholar]
- 16.Terashi, K., M. Oka, H. Soda, M. Fukuda, S. Kawabata, K. Nakatomi, K. Shiozawa, T. Nakamura, K. Tsukamoto, Y. Noguchi, M. Suenaga, C. Tei, and S. Kohno. 2000. Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells. Antimicrob. Agents Chemother. 44:1697-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyteca, D., S. P. Van Der, F. Van Bambeke, K. Leys, P. M. Tulkens, P. J. Courtoy, and M. P. Mingeot-Leclercq. 2001. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur. J. Cell Biol. 80:466-478. [DOI] [PubMed] [Google Scholar]
- 18.Van Bambeke, F., E. Balzi, and P. M. Tulkens. 2000. Antibiotic efflux pumps. Biochem. Pharmacol. 60:457-470. [DOI] [PubMed] [Google Scholar]
- 19.Van Bambeke, F., J. M. Michot, and P. M. Tulkens. 2003. Antibiotic efflux pumps in eukaryotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 51:1067-1077. [DOI] [PubMed] [Google Scholar]
- 20.Vassault, A. 1987. Lactate dehydrogenase, p. 118-126. In H. U. Bergemeyer (ed.), Methods in enzymatic analysis. VHC Publishers, Veinheim, Federal Republic of Germany.