Abstract
An influenza B virus from an infant with no history of treatment or contact with neuraminidase inhibitors demonstrated a significant reduction in sensitivity to these drugs. Here, we describe the analysis of a mixed viral population that contained a novel D197E amino acid substitution that was responsible for this reduction.
The neuraminidase (NA) inhibitor drugs zanamivir (Relenza) and oseltamivir (Tamiflu) are effective inhibitors of replication of both influenza A and B viruses (13). While reports of in vivo resistance following treatment with the NA inhibitors are significantly lower than with the adamantane antiviral drugs (8, 14), resistance has been observed in around 4% of children treated with oseltamivir (11, 16). Kiso et al. (12), however, using a molecular cloning approach to detect resistance, found that resistant viruses were present in 18% of children undergoing short-term treatment with oseltamivir.
Previously, we reported detecting resistance to NA inhibitors in an influenza B isolate (B/Perth/211/2001) from an 8-month-old infant girl with an acute respiratory illness with no history of treatment or contact with either zanamivir or oseltamivir (9). Although in an NA enzyme inhibition assay the B/Perth/211/2001 isolate demonstrated significantly higher 50% inhibitory concentrations (IC50s) (mean IC50 ± 1 standard deviation [SD] for zanamivir, 13.8 ± 1.7 nM; oseltamivir carboxylate, 233.9 ± 31.8 nM) than 128 other circulating influenza B viruses isolated between 1998 and 2002 (mean IC50 ± 1 SD for zanamivir, 1.4 ± 0.6 nM; oseltamivir carboxylate, 14.8 ± 9.6 nM) (8), sequence analysis of the NA gene did not reveal any amino acid changes in sites that had been reported to confer resistance. Here, we describe further analysis of the virus by baculovirus cloning and plaque purification that identified a mixed viral population in the specimen, with two species having an amino acid difference at position 197 (influenza B numbering).
To determine the drug sensitivity of the B/Perth/211/2001 NA in isolation from the hemagglutinin and other influenza virus components, the Bac-to-Bac Baculovirus Expression System (Invitrogen, Australia) was used to generate the full-length membrane-anchored recombinant B/Perth/211/2001 NA protein according to the manufacturer's instructions. During the cloning process, sequence analysis revealed glutamic acid (E) at position 197 rather than the aspartic acid (D) that was obtained in the sequence of the original isolate, strongly suggesting that the B/Perth/211/2001 isolate was composed of a mixed population of different viral species.
Because initial plaque purification had failed to identify a mixed population in the B/Perth/211/2001 isolate in our earlier study (9), a more rigorous protocol was implemented, involving the random selection of larger numbers of plaques with each plaque “plaque-to-plaque” passaged prior to further analysis. In a fluorescence-based NA enzyme inhibition assay (8), 14 out of 16 plaques selected from the MDCK1 passage, and 7 out 7 plaques from the MDCK3 passage, displayed IC50s (mean IC50 ± 1 SD [n = 21]: zanamivir, 19.2 ± 5.8 nM; oseltamivir carboxylate, 217.5 ± 35.3 nM) similar to those of the unpurified B/Perth/211/2001 isolate. However, two plaques from the MDCK1 passage had IC50s that were significantly lower than those of the other plaques (mean IC50 ± 1 SD [n = 2]: zanamivir, 2.3 ± 0.2 nM; oseltamivir carboxylate, 14.9 ± 0.6 nM) and more similar to the IC50s of normal circulating influenza B strains (values described earlier) (8). Sequence analysis, following methods described previously (9), demonstrated a D residue at position 197 for the two plaques with low IC50s, while the 21 other plaques had E at position 197.
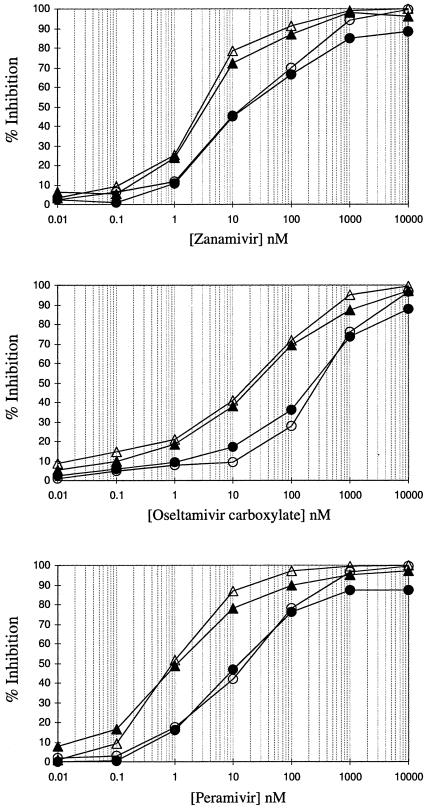
To confirm the role of amino acid 197 in NA inhibitor drug sensitivity, site-directed mutagenesis of this amino acid was performed in the baculovirus system using the QuikChange Site-Directed Mutagenesis method (Stratagene). The rBaculo E197 and rBaculo D197 recombinant NAs were then compared in the NA inhibition assay with the plaque-purified E197 and D197 influenza viruses (Fig. 1). NA inhibition graphs and IC50s (Table 1) demonstrated that the recombinant NA (baculovirus-infected cell lysates treated with TX100) and the plaque-purified influenza virus with the same mutation (either D197 or E197) had very similar IC50s for zanamivir, oseltamivir carboxylate, and peramivir (a currently unlicensed NA inhibitor) (1). The baculovirus system demonstrated unequivocally that the 197 residue in B/Perth/211/2001 affected the sensitivity of the influenza virus to the NA inhibitors (Table 1).
FIG. 1.
Fluorescence-based NA inhibition curves of plaque-purified B/Perth/211/2001 clones and recombinant baculoviruses expressing B/Perth/211/2001 neuraminidase for zanamivir, oseltamivir carboxylate, and peramivir. The line markers are as follows: plaque-purified B/Perth/211/2001 clones D197 (▵) and E197 (○), and recombinant baculoviruses expressing B/Perth/211/2001 neuraminidase with either D197 (▴) or E197 (•).
TABLE 1.
IC50s and increases between D197 and E197 viruses for plaque-purified B/Perth/211/2001 clones and recombinant baculoviruses expressing B/Perth/211/2001 neuraminidase for zanamivir, oseltamivir carboxylate, and peramivir
| NA inhibitor | Plaque-purified influenza viruses
|
Recombinant baculoviruses
|
||||
|---|---|---|---|---|---|---|
| IC50 (nM)
|
D:E increase (n-fold) | IC50 (nM)
|
D:E increase (n-fold) | |||
| D197 | E197 | D197 | E197 | |||
| Zanamivir | 2.8 | 18.0 | 6.4 | 3.2 | 15.7 | 4.9 |
| Oseltamivir carboxylate | 17.8 | 209.3 | 11.8 | 23.7 | 221.1 | 9.3 |
| Peramivir | 0.9 | 16.3 | 18.1 | 1.0 | 10.8 | 10.8 |
The aspartic acid residue (D) at position 197 of influenza B viruses is equivalent to the D198 for A(N2) strains, is located in the “second shell” of the enzyme active site (2, 15), and is believed to provide a structural framework for functional residues in the enzyme active site, rather than directly interacting with the substrate (4). While this residue is conserved among type B viruses, the equivalent D198 is not strictly conserved among influenza A subtypes, with N6, N7, and N9 having an N at this position. An NA-resistant virus with a D-to-N mutation has been reported (5) from a virus isolated from an immunocompromised patient undergoing treatment with oseltamivir (5). However, unlike the virus from that study, and other previously reported resistant viruses isolated from humans (3, 5-7, 10), it appears that B/Perth/211/2001 represents the first influenza virus reported to have acquired a mutation resulting in reduced NA inhibitor sensitivity in the absence of drug treatment. Given the very low usage of NA inhibitors in Australia, the likelihood that a virus from a drug-treated individual infected the patient is extremely low.
A significant finding of this study and previous studies (7, 12) is the presence of a mixture of NA inhibitor-sensitive and -resistant viral species within an infected individual that might not be detected using standard virus isolation and sequencing procedures.
Molecular cloning of the NA and hemagglutinin genes of viruses from Japanese children undergoing treatment with oseltamivir (12) revealed a significantly higher proportion of individuals with resistant viruses than had been identified in previous studies. Findings from this study and others suggest that new approaches to the study of in vivo resistance to the neuraminidase inhibitor drugs may need to be employed to provide a clearer picture of the occurrence and transmission of resistant strains.
While the clinical significance of the B/Perth/211/2001 strain, and those detected by Kiso et al. (12), is unknown, it is particularly noteworthy that these resistant variants upon in vitro passage either outgrew the wild type or maintained the major proportion of viruses within the mixture. This was observed for B/Perth/211/2001, for which no sensitive plaques were detected in the MDCK3 passage. Further studies to test the transmissibility of these variants remain important to gain a better understanding of the clinical significance of the viruses. Given that the B/Perth/211/2001 strain was isolated from an untreated patient, it remains imperative to investigate both untreated and treated populations, using these more sensitive techniques to improve our knowledge about potential resistance generation, particularly given that the NA inhibitor drugs are likely to be important as a first-line response in a pandemic situation.
Nucleotide sequence accession numbers.
Full-length NA sequences of the plaque-purified D197 and E197 viruses obtained from B/Perth/211/2001 are available from GenBank (accession numbers DQ174088 for clone D197 and DQ174087 for clone E197).
Acknowledgments
We thank Peter McMinn from the Princess Margaret Hospital for Children, Western Australia, for the submission of the B/Perth/211/2001 influenza virus specimen used in this study. Oseltamivir carboxylate (GS 4071), the active form of the ethyl ester prodrug oseltamivir phosphate, was kindly provided by Noel Roberts, Roche Products, Welwyn Garden City, United Kingdom.
The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Commonwealth Department of Health and Ageing.
REFERENCES
- 1.Anonymous. 2002. Biocryst influenza drug fails in phase III trial; development halted. Antiviral Agents Bull. 15:162-163. [Google Scholar]
- 2.Burmeister, W. P., R. W. Ruigrok, and S. Cusack. 1992. The 2.2 Å resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 11:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr, J., J. Ives, L. Kelly, R. Lambkin, J. Oxford, D. Mendel, L. Tai, and N. Roberts. 2002. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 54:79-88. [DOI] [PubMed] [Google Scholar]
- 4.Colman, P. M. 1994. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 3:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubareva, L. V. 2004. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 103:199-203. [DOI] [PubMed] [Google Scholar]
- 6.Gubareva, L. V., L. Kaiser, M. N. Matrosovich, Y. Soo-Hoo, and F. G. Hayden. 2001. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J. Infect. Dis. 183:523-531. [DOI] [PubMed] [Google Scholar]
- 7.Gubareva, L. V., M. N. Matrosovich, M. K. Brenner, R. C. Bethell, and R. G. Webster. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257-1262. [DOI] [PubMed] [Google Scholar]
- 8.Hurt, A. C., I. G. Barr, G. Hartel, and A. W. Hampson. 2004. Susceptibility of human influenza viruses from Australasia and South East Asia to the neuraminidase inhibitors zanamivir and oseltamivir. Antiviral Res. 62:37-45. [DOI] [PubMed] [Google Scholar]
- 9.Hurt, A. C., J. L. McKimm-Breschkin, M. McDonald, I. G. Barr, N. Komadina, and A. W. Hampson. 2004. Identification of a human influenza type B strain with reduced sensitivity to neuraminidase inhibitor drugs. Virus Res. 103:205-211. [DOI] [PubMed] [Google Scholar]
- 10.Ives, J. A. L., J. A. Carr, D. B. Mendel, C. Y. Tai, R. Lambkin, L. Kelly, J. S. Oxford, F. G. Hayden, and N. A. Roberts. 2002. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 55:307-317. [DOI] [PubMed] [Google Scholar]
- 11.Jackson, H. C., N. Roberts, Z. M. Wang, and R. Belshe. 2000. Management of influenza: use of new antivirals and resistance in perspective. Clin. Drug Investig. 20:447-454. [Google Scholar]
- 12.Kiso, M., K. Mitamura, Y. Sakai-Tagawa, K. Shiraishi, C. Kawakami, K. Kimura, F. G. Hayden, N. Sugaya, and Y. Kawaoka. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759-765. [DOI] [PubMed] [Google Scholar]
- 13.McKimm-Breschkin, J. L. 2000. Resistance of influenza viruses to neuraminidase inhibitors—a review. Antiviral Res. 47:1-17. [DOI] [PubMed] [Google Scholar]
- 14.Roberts, N. A. 2001. Treatment of influenza with neuraminidase inhibitors: virological implications. Phil. Trans. R. Soc. Lond. B 356:1895-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varghese, J. N., J. L. McKimm-Breschkin, J. B. Caldwell, A. A. Kortt, and P. M. Colman. 1992. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins 14:327-332. [DOI] [PubMed] [Google Scholar]
- 16.Whitley, R. J., F. G. Hayden, K. S. Reisinger, N. Young, R. Dutkowski, D. Ipe, R. G. Mills, and P. Ward. 2001. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 20:127-133. [DOI] [PubMed] [Google Scholar]