Abstract
Clinical nonrandomized trials demonstrate some efficacy for ribavirin in the treatment of patients with severe Nipah virus-induced encephalitis. We report here that EICAR, the 5-ethynyl analogue of ribavirin, and the OMP-decarboxylase inhibitors 6-aza-uridine and pyrazofurin have strong antiviral activity against Nipah virus replication in vitro. Ribavirin and 6-aza-uridine were tested further in hamsters infected with a lethal dose of Nipah virus. The activity of these small-molecule inhibitors was compared with that of the interferon inducer poly(I)-poly(C12U). Both ribavirin and 6-aza-uridine were able to delay but not prevent Nipah virus-induced mortality. Poly(I)-poly(C12U), at 3 mg/kg of body weight daily from the day of infection to 10 days postinfection, prevented mortality in 5 of 6 infected animals.
A new paramyxovirus infecting humans emerged in Malaysia in 1998, causing 265 cases of encephalitis, with a mortality rate of 40% (4). This virus was called Nipah virus (NiV), and it causes symptoms ranging from banal febrile illness with headache to very severe acute encephalitis. Several of the patients who recovered from the initial infection suffered relapses of encephalitis several months or years later (31). NiV is a negative-strand RNA virus, closely related to the Hendra virus isolated in Australia in 1994 from horses and humans (28). Both viruses belong to the new genus Henipavirus, in the family Paramyxoviridae (34, 35). The natural reservoir of both viruses appears to be Pteropus bats, which eliminate the virus in their urine (5, 11, 13). The range of these bats extends from the Western Indian Ocean to Southeast Asia, Australia, and the Southwest Pacific Islands. Since the initial outbreak (which spread to Singapore) (22), two outbreaks of infection with NiV or a closely related virus were reported in Bangladesh and northern India in 2001 and four such outbreaks were recorded in Bangladesh in 2004 to 2005. These more recent outbreaks have revealed new epidemiological patterns, with the infection of humans through direct contact with fruit contaminated by infected bat excreta. The presence of antibodies against Nipah virus has been demonstrated in bats in Cambodia, and the virus has been isolated from one animal (17, 24). During the 1998 outbreak in Malaysia, an open trial was run in which NiV-infected patients were treated with ribavirin, a nucleoside analogue active against both positive- and negative-strand RNA viruses (8, 15, 23): ribavirin treatment was reported to reduce the mortality rate by 36% (3).
We recently developed a hamster model of NiV infection that reproduces the clinical manifestations observed in humans (36). This animal model has been used to evaluate a candidate vaccine and for serotherapy (9). We report here the in vitro and in vivo anti-NiV efficacy of selected molecules.
MATERIALS AND METHODS
Virus and cells.
NiV was amplified and titrated, and the antiviral activity of the molecules was tested in the Vero E6 cell line (ATCC CRL-1586) in maintenance medium (Dulbecco's minimum essential medium containing 2% fetal calf serum and antibiotics). Vero E6 cells were cultured to confluence in the same medium, but with 5% fetal calf serum (growth medium). NiV was isolated in 1999 from the cerebrospinal fluid of a patient and was kindly provided by K. B. Chua and S. Kim Lam (Kuala Lumpur, Malaysia). NiV stocks were produced by three passages of the virus in Vero E6 cells. All manipulations were carried out in the Jean Merieux INSERM biosafety level 4 (BSL-4) containment laboratory (Lyon, France). The titer of the virus stock was 107.64 50% tissue culture infectious doses (TCID50)/ml, as determined by plaque titration in 96-well culture microplates. Briefly, we prepared serial 10-fold dilutions of the virus preparation, and 200 μl of each dilution was added to eight wells of a 96-well culture microplate. The plates were incubated for 3 days at 37°C under an atmosphere containing 5% CO2, and the cultures were fixed and stained with crystal violet.
The in vitro efficacy of poly(I)-poly(C12U) was evaluated in HeLa cells by using a virus stock amplified on Vero E6 cells. The titer of this stock virus was evaluated in HeLa cells by plaque titration and was 107.26 TCID50/ml.
Compounds.
We evaluated the in vitro antiviral activity of various compounds reported to be active against paramyxoviruses. The compounds assessed were the S-adenosylhomocysteine (SAH) hydrolase inhibitors carbocyclic 3-deaza-adenosine (C-c3 Ado), (−)-5′-noraristeromycin, and 9-[(1′R,2′S,3′R)-2′,3′-dihydroxycyclopentanyl] adenine (DHCaA), the IMP dehydrogenase inhibitors ribavirin [1-(β-d-ribofuranosyl)-1,2,4-triazole-3-carboxamide] and EICAR [5-ethynyl-(1-β-d-ribofuranosylimidazole)-4-carboxamide], the OMP decarboxylase inhibitors pyrazofurin and 6-aza-uridine, and the CTP synthetase inhibitor carbodine. Stock solutions were prepared in dimethyl sulfoxide. Poly(I)-poly(C12U) (Ampligen; Hemispherix Biopharma, Inc.) is an interferon inducer. This compound was heated for 30 min at 55°C and then slowly cooled before use.
In vitro cytotoxicity.
The minimal cytotoxic concentration was defined as the lowest concentration causing a microscopically detectable change in cell morphology 7 days after infection.
Animals.
In vivo studies were carried out in 2-month-old male golden hamsters (Janvier, Le Fenest St. Isle, France). Animal experiments were approved by the regional ethics committee for animal experimentation. Hamsters were housed in a BSL-4 containment facility in ventilated containers equipped with HEPA filters and were handled according to French regulations for animal maintenance. All animal manipulations were carried out under isoflurane anesthesia in an anesthesia chamber. Blood was collected by orbital or cardiac puncture.
In vitro drug screening.
Antiviral efficacy in vitro was evaluated by means of cytopathic effect (CPE) reduction assays with Nipah virus. Vero E6 cells were seeded in 96-well culture plates at a density of 3 × 104 cells/well. The cells were incubated with growth medium to a confluence density of about 5 × 104 cells/well, 24 h later. The medium was then removed, and the cells in each well were infected with 50 TCID50 of Nipah virus in 100 μl of maintenance medium. Serial twofold dilutions of each compound were prepared in maintenance medium, and 100 μl of each dilution was added to eight wells of the culture plate. Plates were then incubated for 3 days at 37°C under an atmosphere containing 5% CO2, and the cells were fixed and stained with crystal violet. The inhibitory concentration was defined as the minimum concentration at which no CPE was observed in any of the eight wells tested.
In vivo toxicity assay.
Selected compounds found to be effective in vitro were further evaluated in vivo in the Nipah virus hamster model. Toxicity data for ribavirin and poly(I)-poly(C12U) were obtained from previous studies (12, 30). Alzet osmotic pumps (model 2ML2) capable of delivering 120 μl/day were filled with 5 ml of the test compound and implanted subcutaneously in anesthetized (ketamine [40 mg/kg of body weight] and xylazine [16 mg/kg of body weight]) animals. Body weight and temperature were monitored for 14 days.
In vivo efficacy tests.
We carried out two experiments. In the first, compounds were administered via osmotic pumps, and animals were infected with 350 50% lethal doses (LD50) of NiV immediately after insertion of the pump. Six animals were used for each compound studied; six control animals received virus only.
In the second experiment, three groups of six animals were infected via the intraperitoneal (i.p.) route. One group of six animals received phosphate-buffered saline and served as controls, another group received ribavirin (25 mg/kg) twice daily by the i.p. route, and a third group received daily i.p. injections of poly(I)-poly(C12U). Treatment was initiated immediately after infection and continued for 10 consecutive days.
Virological and serological studies.
Viral load in the various organs of the animals was monitored by both real-time reverse transcription-PCR (RT-PCR) and titration of infectious viruses on Vero E6 cells, as previously described (10, 36).
Sera were tested by enzyme-linked immunosorbent assay for immunoglobulin G (IgG) antibodies, using crude virus-infected cell lysate as the antigen (36).
RESULTS
In vitro assay.
C-c3 Ado, (−)-5′-noraristeromycin, and DHCaA, all inhibitors of SAH reported to be active against other paramyxoviruses, were inactive against NiV replication. Carbodine, a CTP synthase inhibitor with antiparamyxovirus activity, was inactive against NiV. Two IMP-DH inhibitors, ribavirin and its 5-ethynyl analogue EICAR, and two OMP-decarboxylase inhibitors, 6-aza-uridine and pyrazofurin, completely inhibited the in vitro replication of NiV. The minimum effective concentrations were 100 μg/ml for ribavirin, 0.12 μg/ml for pyrazofurin, 0.25 μg/ml for 6-aza-uridine, and 1 μg/ml for EICAR. At a concentration of 6.25 μg/ml, poly(I)-poly(C12U) completely abolished the CPE of NiV in HeLa cells (Table 1). None of the compounds was toxic at the effective antiviral concentration.
TABLE 1.
In vitro efficacy of various compounds against Nipah virus replicationa
| Compound | Concn range (μg/ml) | Inhibitory concn (μg/ml)b |
|---|---|---|
| C-c3 Ado* | 0.250-8 | >8 |
| (−)-5′ Noraristeromycin* | 0.125-4 | >4 |
| DHCaA* | 0.125-4 | >4 |
| Ribavirin* | 1.56-100 | 100 |
| EICAR* | 0.250-8 | 1 |
| Pyrazofurin | 0.125-4 | 0.125 |
| Carbodine* | 0.250-8 | >8 |
| 6-Aza-uridine* | 0.125-4 | 0.25 |
| Poly(I)-poly(C12U)† | 6.25-200 | ≤6.25 |
The effect on Nipah virus replication was tested in Vero E6 cells (*) or HeLa (†) cells in 96-well microplates, and the effects on CPE were evaluated. Each concentration was tested in 8 wells.
The inhibitory concentration was defined as the lowest concentration completely abolishing CPE in the 8 wells tested. These results were obtained in two experiments.
In vivo toxicity assay.
The doses and treatment schedules used for ribavirin and poly(I)-poly(C12U) were deduced from previous studies (12, 16). We evaluated the toxicity of 6-aza-uridine and EICAR, using osmotic pumps delivering the drug continuously, 24 h per day, for 14 days. Two hamsters received either 100 mg or 200 mg/kg 6-aza-uridine daily for a period of 12 days. Twelve days after the beginning of treatment, all animals appeared healthy, and body weight gain was normal (data not shown). However, EICAR, delivered at a daily dose of 20 or 40 mg/kg, was not well tolerated; the animals lost weight and died within 6 days of treatment initiation. We were unable to continue these assays further in hamsters due to the low availability of this compound.
In vivo efficacy tests.
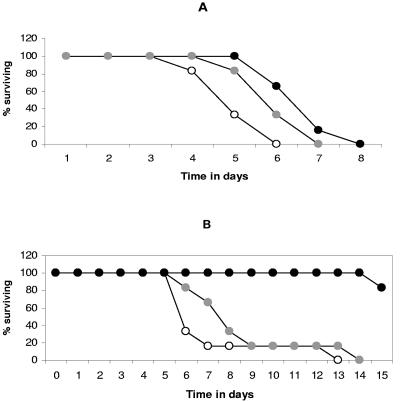
In the first experiment, animals were infected with 350 LD50 of NiV following the implantation of osmotic pumps containing the compound to be tested. Six animals received ribavirin at a dose of 50 mg/kg/day, six animals received 6-aza-uridine at a dose of 175 mg/kg/day, and six received phosphate-buffered saline only and served as controls. The mean day of death was 5.1 ± 0.7 days after infection for control animals, 6.1 ± 0.7 days (P < 0.05) for the 6-aza-uridine group, and 6.8 ± 0.7 days (P < 0.01) for the ribavirin group (Fig. 1A).
FIG. 1.
Effects of ribavirin, 6-aza-uridine, and the interferon inducer poly(I)-poly(C12U) on the survival of NiV-infected hamsters. Six animals were included in each group. (A) For experiment 1, animals were infected with 350 LD50 of Nipah virus i.p. They were then treated with placebo (white circles) or with a dose of 50 mg/kg/day ribavirin (black circles) or 175 mg/kg/day of 6-aza-uridine (gray circles). Drugs were administrated via osmotic pumps. (B) For experiment 2, animals received 35 LD50 of Nipah virus i.p. They were then treated i.p. with placebo (white circles), 25 mg/kg ribavirin twice daily (gray circles), or 3 mg/kg poly(I)-poly(C12U) once daily (black circles).
Due to the unexpected absence of a curative effect of ribavirin in the first experiment, we decided to use a lower infective dose in the second experiment and infected the hamsters with 35 LD50 of NiV. As poly(I)-poly(C12U) would not have been active if administered via an osmotic pump, treatment was instead given by i.p. injection. Six hamsters received 25 mg/kg ribavirin twice daily, six received 3 mg/kg poly(I)-poly(C12U) once daily, and six received placebo; 6-aza-uridine was not tested further. Treatment was initiated 2 h after infection and was continued for 10 consecutive days. The mean day of death was 7.3 ± 2.94 days after infection for placebo-treated animals infected with 35 LD50 and 8.6 ± 2.94 days after infection for animals treated with ribavirin (P > 0.05), with one animal in each group surviving until day 13 or 14. Poly(I)-poly(C12U) was highly protective: all of the poly(I)-poly(C12U)-treated animals were still alive on day 14, whereas all of the untreated animals were dead (Fig. 1B).
Virological data.
Unfortunately, due to the constraints of BSL-4 work, it was not possible to autopsy all infected animals after death. In experiment 1, samples were taken from two of the six control animals, two of the six animals of the ribavirin group, and three of the six animals of the 6-aza-uridine group at the time of death. Seven samples (two from the control group, three from the ribavirin group, and two from the 6-aza-uridine group) collected within the first 6 days of infection were tested for the presence of anti-NiV IgG. Only one sample, from the 6-aza-uridine group, scored positive.
The livers, lungs, kidneys, spleens, and brains of the dead animals were tested for the presence of NiV by real-time RT-PCR and titration of infectious virus. The results are presented in Table 2. The virus was detected by RT-PCR in all animal samples tested, independent of ribavirin or 6-aza-uridine treatment. Virus was undetectable by culture titration in only three samples.
TABLE 2.
Nipah virus detection by cell culture titration, RT-PCR, and serological testing in experiment 1c
| Hamster group | Animal no. | No. of days p.i.a | Liver
|
Lung
|
Kidney
|
Spleen
|
Brain
|
Serum IgG | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | ||||
| Control | M1 | 5 | 8.25 × 103 | + | 6.75 × 103 | + | 2.25 × 103 | + | 5.12 × 103 | + | <20 | + | NDb |
| M2 | 4 | 9.75 × 103 | + | 1.28 × 103 | + | 1.43 × 103 | + | 1.01 × 105 | + | 2.5 × 101 | + | − | |
| 6-Aza-uridine | A1 | 6 | 5 × 103 | + | 1.71 × 104 | + | 6.12 × 102 | + | 1.01 × 104 | + | 10 × 102 | + | − |
| A2 | 5 | 1.75 × 102 | + | ND | + | <20 | − | ND | + | ND | ND | ND | |
| A3 | 6 | 9 × 103 | + | 1.21 × 104 | + | 7.25 × 102 | + | 1.37 × 104 | + | 6.25 × 101 | + | − | |
| A5 | 5 | 4.25 × 103 | + | 1.4 × 104 | + | 4.25 × 102 | + | 4.37 × 103 | + | 3.75 × 101 | + | + | |
| Ribavirin | R1 | 5 | 1.75 × 103 | + | 8 × 103 | + | 1.10 × 103 | + | 1 × 104 | + | <20 | + | − |
| R2 | 7 | 1 × 102 | + | 1.45 × 104 | + | 8.62 × 103 | + | 1.91 × 103 | + | 3.13 × 102 | + | − | |
| R4 | 6 | 7.12 × 103 | + | 7.8 × 104 | + | 7 × 103 | + | 1.10 × 103 | + | 4.90 × 103 | + | − | |
Number of days postinfection at the time of the tests.
ND, test not done.
These tests were performed on the day of the animal's death.
In experiment 2, we were able to autopsy only one animal of the ribavirin group, which died on day 6, and the six animals of the poly(I)-poly(C12U) group. One of these animals was sick and was euthanatized for humane reasons on day 15; the other five were euthanatized on day 30, when they were still healthy. Two of the four animals that were sampled within the first week had no detectable anti-NiV IgG at the time of death, whereas a serological response was detected in all animals surviving on day 12. No infectious virus was detected in any of the five poly(I)-poly(C12U)-treated animals euthanatized on day 30 after infection, none of which had developed signs of disease. However, viral RNA was detected in the livers of three and in the kidney of one of these animals. The only poly(I)-poly(C12U)-treated animal to display symptoms died after 15 days of infection. For this animal, infectious virus and viral RNA were found in the brain, and viral RNA (but not infectious virus) was found in the liver, lungs, and spleen (Table 3).
TABLE 3.
Nipah virus detection by cell culture titration, RT-PCR, and serological testing in experiment 2c
| Hamster group | Animal no. | No. of days p.i.a | Liver
|
Lung
|
Kidney
|
Spleen
|
Brain
|
Serum IgG at day p.i.:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | Titer (PFU/ml) | RT-PCR | 6 | 12 | 30 | |||
| Control | H1 | 6 | NDb | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ||
| H6 | 12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | + | ||
| Ribavirin | H9 | 7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + (day 7) | ||
| H11 | 12 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | |||
| H12 | 6 | <20 | + | 2.3 × 102 | + | 3.4 × 102 | + | <20 | + | ND | ND | − | |||
| Poly(I)-poly(C12U) | H13 | 30 | <20 | + | <20 | − | <20 | − | <20 | − | <20 | ND | + | + | |
| H14 | 30 | <20 | − | <20 | − | <20 | − | <20 | − | <20 | − | + | + | ||
| H15 | 30 | <20 | − | <20 | − | <20 | − | <20 | − | <20 | − | + | + | ||
| H16 | 15 | <20 | + | <20 | + | <20 | + | <20 | + | 4.4 × 102 | + | + | |||
| H17 | 30 | <20 | + | <20 | − | <20 | − | <20 | − | <20 | − | − | + | ||
| H18 | 30 | <20 | + | <20 | − | <20 | − | <20 | − | <20 | − | + | + | ||
Number of days postinfection at the time of autopsy.
ND, test not done.
Autopsy was performed on none of the hamsters of the control group, one of the six hamsters in the ribavirin group, and all six hamsters in the poly(I)-poly(C12U) group. Blood samples were collected from two of the six hamsters of the control group and three of the six hamsters in the ribavirin group at the time of death and from all six hamsters in the poly(I)-poly(C12U) group 12 and 30 days after infection.
DISCUSSION
The primary aim of this study was to determine whether ribavirin could protect NiV-infected hamsters. We also tested the effect of EICAR—a closely related analogue of ribavirin reported to be 30 times more active than ribavirin against various paramyxoviruses—on NiV replication (6). In addition, we investigated whether various molecules with activity in vitro against various RNA viruses were active against NiV replication (1, 2, 6). Our secondary aim was to investigate whether the molecules that reduced Nipah virus replication in vitro could protect NiV-infected hamsters against fatal disease.
EICAR inhibited NiV replication in vitro about 100 times more efficiently than ribavirin. However, it was not well tolerated in vivo, and the efficacy of EICAR in infected hamsters was therefore not assessed. The OMP-decarboxylase inhibitors 6-aza-uridine and pyrazofurin were both highly active against NiV replication in cell culture. Hamsters tolerated 6-aza-uridine reasonably well, but treatment with this drug only slightly increased survival time after virus infection. SAH-hydrolase inhibitors, which are active against other paramyxoviruses, did not inhibit NiV replication in cell culture in vitro.
In our experimental conditions, ribavirin could not prevent the death of NiV-infected hamsters, and, at best, delayed death from viral disease by less than 2 days. An open label trial conducted in Malaysia with 140 ribavirin-treated patients and 54 untreated patients reported a 36% reduction in mortality from virus infection (P = 0.011) following ribavirin treatment (3). There are several possible reasons for the differences in protective activity of ribavirin in infected humans and infected hamsters. First, the infection may be more aggressive in experimentally infected hamsters than in humans, and the infective dose used in our two experiments may be much higher than that of natural infection in humans. Second, ribavirin metabolism may differ in humans and hamsters. Alternatively, the absence of randomization in the Malaysian study may have resulted in bias in the selection of patients for the treatment and nontreatment groups. Ribavirin has been reported to be active in hamster models for other viral infections, including a model of subacute sclerosis panencephalitis. Ribavirin was not active when administered via the intraperitoneal route but improved survival when administered intracranially (12). In a hamster model of yellow fever virus infections, ribavirin improved survival when administered within the first 5 days of infection (27).
Interferons (IFN) are major components of the host innate immune response to viral infections and are produced in response to such infections. Many viruses have strategies for counteracting this IFN response. One of these strategies involves inhibiting IFN signaling. Paramyxoviruses generally disrupt IFN signaling by directly targeting STAT proteins (7, 19-21, 33). The V proteins of the Nipah and Hendra viruses seem to inhibit IFN signaling in this way (25, 26). However, these results were obtained in vitro, using artificial constructs, rather than in a natural model of NiV-infected cells. In an in vitro model based on human HeLa cells, we found that poly(I)-poly(C12U), which induces IFN-α and -β production, completely blocked NiV replication. This suggests that type I IFNs are able to signal as part of the antiviral response in NiV-infected human cells. Thus, in the context of real NiV cell infection, the V protein of NiV probably cannot fully disrupt IFN signaling. We therefore investigated whether poly(I)-poly(C12U), which has a well-established safety profile in humans, displayed protective activity in the hamster model of lethal NiV infection (32). Daily injections of poly(I)-poly(C12U) for 10 consecutive days, starting at the time of i.p infection, gave substantial protection against death from NiV infection. These results, obtained in the hamster model, are consistent with those reported for HeLa cells. Indeed, the efficacy of poly(I)-poly(C12U) for controlling NiV infection in hamsters suggests that IFN signaling is efficient in this model. Poly(I)-poly(C12U), first administered as late as 48 h after infection, was recently demonstrated to have protective activity in a model of coxsackie B3 virus-induced myocarditis and in a model of lethal Punta Toro infection in mice (18, 29). Conversely, poly(I)-poly(C12U) was not protective in a hamster model of lethal Pichinde virus infection (30). Poly(I)-poly(C12U) has also been shown to be effective in animal models of flavivirus infections: mice infected with Modoc virus or mice or hamsters infected with the West Nile virus. However, in these flavivirus infection models, a prerequisite for potent activity was the initiation of treatment before infection (14, 16).
It will be of interest to explore various poly(I)-poly(C12U) treatment schedules in the hamster NiV model. These schedules may include delaying administration of the compound until the first symptoms appear. Relapses of NiV infection have been reported (31), so it would be of value to determine whether hamsters protected against the infection display subsequent relapse (31). Indeed, although we detected no infectious virus in the various organs of poly(I)-poly(C12U)-treated animals that survived the infection, a sensitive RT-PCR assay detected viral sequences in various organs. It would also be of interest to study the efficacy of combined poly(I)-poly(C12U) and ribavirin treatment.
Although many questions remain unanswered, a compound such as poly(I)-poly(C12U), which has been shown to be safe in humans (32), may be of value for the treatment of acute Nipah virus infection. Even if this compound decreases viral load by only a couple of log values in a clinical setting, this may be sufficient to allow the patient to mount a protective immune response, potentially resulting in a less severe infection and protection against death from viral infection.
Acknowledgments
We thank Kuo Bin Chua and Sait Kim Lam for providing Nipah virus.
REFERENCES
- 1.Andrei, G., and E. Declercq. 1990. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antivir. Res. 14:287-300. [DOI] [PubMed] [Google Scholar]
- 2.Canonico, P. G., P. B. Jahrling, and W. L. Pannier. 1982. Antiviral efficacy of pyrazofurin against selected RNA viruses. Antivir. Res. 2:331-337. [DOI] [PubMed] [Google Scholar]
- 3.Chong, H. T., A. Kamarulzaman, C. T. Tan, K. J. Goh, T. Thayaparan, R. Kunjapan, N. K. Chew, K. B. Chua, and S. K. Lam. 2001. Treatment of acute Nipah encephalitis with ribavirin. Ann. Neurol. 49:810-813. [DOI] [PubMed] [Google Scholar]
- 4.Chua, K. B., K. J. Goh, K. T. Wong, A. Kamarulzaman, P. S. K. Tan, T. G. Ksiazek, S. R. Zaki, G. Paul, S. K. Lam, and C. T. Tan. 1999. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 354:1257-1259. [DOI] [PubMed] [Google Scholar]
- 5.Chua, K. B., C. L. Koh, P. S. Hooi, K. F. Wee, J. H. Khong, B. H. Chua, Y. P. Chan, M. E. Lim, and S. K. Lam. 2002. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 4:145-151. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E., M. Cools, J. Balzarini, R. Snoeck, G. andrei, M. Hosoya, S. Shigeta, T. Ueda, N. Minakawa, and A. Matsuda 1991. Antiviral activities of 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob. Agents Chemother. 35:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisherhoch, S. P., J. A. Khan, S. Rehman, S. Mirza, M. Khurshid, and J. B. McCormick. 1995. Crimean-Congo hemorrhagic fever treated with oral ribavirin. Lancet 346:472-475. [DOI] [PubMed] [Google Scholar]
- 9.Guillaume, V., H. Contamin, P. Loth, M. C. Georges-Courbot, A. Lefeuvre, P. Marianneau, K. B. Chua, S. K. Lam, R. Buckland, V. Deubel, and T. F. Wild. 2004. Nipah virus: vaccination and passive protection studies in a hamster model. J. Virol. 78:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillaume, V., A. Lefeuvre, C. Faure, P. Marianneau, R. Buckland, S. K. Lam, T. F. Wild, and V. Deubel. 2004. Specific detection of Nipah virus using real-time RT-PCR (TaqMan). J. Virol. Methods 120:229-237. [DOI] [PubMed] [Google Scholar]
- 11.Halpin, K., P. L. Young, H. Field, and J. S. Mackenzie. 1999. Newly discovered viruses of flying foxes. Vet. Microbiol. 68:83-87. [DOI] [PubMed] [Google Scholar]
- 12.Honda, Y., M. Hosoya, T. Ishii, S. Shigeta, and H. Suzuki. 1994. Effect of ribavirin on subacute sclerosing panencephalitis virus infections in hamsters. Antimicrob. Agents Chemother. 38:653-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johara, M. Y., H. Field, A. M. Rashdi, C. Morrissy, B. van der Heide, P. Rota, A. bin Adzhar, J. White, P. Daniels, A. Jamaluddin, and T. Ksiazek. 2001. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 7:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leyssen, P., C. Drosten, M. Paning, N. Charlier, J. Paeshuyse, E. De Clercq, and J. Neyts. 2003. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob. Agents Chemother. 47:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Craven, K. M. Johnson, L. H. Elliott, and R. Belmontwilliams. 1986. Lassa fever-effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 16.Morrey, J. D., C. W. Day, J. G. Julander, L. M. Blatt, D. F. Smee, and R. W. Sidwell. 2004. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir. Chem. Chemother. 15:101-109. [DOI] [PubMed] [Google Scholar]
- 17.Olson, J. G., C. Rupprecht, P. E. Rollin, U. S. An, M. Niezgoda, T. Clemins, J. Walston, and T. G. Ksiazek. 2002. Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg. Infect. Dis. 8:987-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padalko, E., D. Nuyens, A. De Palma, E. Verbeken, J. L. Aerts, E. De Clercq, P. Carmeliet, and J. Neyts. 2004. The interferon inducer ampligen [poly(I)-poly(C12U)] markedly protects mice against coxsackie B3 virus-induced myocarditis. Antimicrob. Agents Chemother. 48:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palosaari, H., J. P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parisien, J. P., J. F. Lau, J. J. Rodriguez, B. M. Sullivan, A. Moscona, G. D. Parks, R. A. Lamb, and C. M. Horvath. 2001. The V protein of human parainfluenza virus 2 antagonizes type 1 interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283:230-239. [DOI] [PubMed] [Google Scholar]
- 21.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton, N. I., Y. S. Leo, S. R. Zaki, A. P. Auchus, K. E. Lee, A. E. Ling, S. K. Chew, B. Ang, P. E. Rollin, T. Umapathi, I. Sng, C. C. Lee, E. Lim, and T. G. Ksiazek. 1999. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354:1253-1256. [DOI] [PubMed] [Google Scholar]
- 23.Picardi, A., U. V. Gentilucci, E. M. Zardi, D. D'Avola, A. Amoroso, and A. Afeltra. 2004. The role of ribavirin in the combination therapy of hepatitis C virus infection. Curr. Pharm. Des. 10:2081-2092. [DOI] [PubMed] [Google Scholar]
- 24.Reynes, J. M., D. Counor, S. Ong, C. Faure, V. Seng, S. Molia, J. Walston, M. C. Georges-Courbot, V. Deubel, and J. L. Sarthou. 2005. Nipah virus in lyle's flying foxes, Cambodia. Emerg. Infect. Dis. 11:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez, J. J., J. P. Parisien, and C. M. Horvath. 2002. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 76:11476-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez, J. J., L. F. Wang, and C. A. Horvath. 2003. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 77:11842-11845. (Erratum, 77:13457.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sbrana, E., X. S., H. Guzman, M. Ye, A. P. A. Travassos Da Rosa, and R. B. Tesh. 2004. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am. J. Trop. Med. Hyg. 71:306-312. [PubMed] [Google Scholar]
- 28.Selvey, L. A., R. M. Wells, J. G. McCormack, A. J. Ansford, K. Murray, R. J. Rogers, P. S. Lavercombe, P. Selleck, and J. W. Sheridan. 1995. Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 162:642-645. [DOI] [PubMed] [Google Scholar]
- 29.Sidwell, R. W., J. H. Huffman, D. L. Barnard, D. F. Smee, R. P. Warren, M. A. Chirigos, M. Kende, and J. Huggins. 1994. Antiviral and immunomodulating inhibitors of experimentally induced Punta Toro virus infections. Antivir. Res. 25:105-122. [DOI] [PubMed] [Google Scholar]
- 30.Smee, D. F., J. Gilbert, J. A. Leonhardt, B. B. Barnett, J. H. Huggins, and R. W. Sidwell. 1993. Treatment of lethal Pichinde virus infections in weanling Lvg Lak hamsters with ribavirin, ribamidine, selenazofurin, and Ampligen. Antivir. Res. 20:57-70. [DOI] [PubMed] [Google Scholar]
- 31.Tan, C. T., K. J. Goh, K. T. Wong, S. A. Sarji, K. B. Chua, N. K. Chew, P. Murugasu, Y. L. Loh, H. T. Chong, K. S. Tan, T. Thayaparan, S. Kumar, and M. R. Jusoh. 2002. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 51:703-708. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, K. A., D. R. Strayer, P. D. Salvato, C. E. Thompson, N. Klimas, A. Molavi, A. K. Hamill, Z. Zheng, D. Ventura, and W. A. Carter. 1996. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:polyC(12U) in the treatment of HIV infection. Eur. J. Clin. Microbiol. Infect. Dis. 15:580-587. [DOI] [PubMed] [Google Scholar]
- 33.Ulane, C. M., J. J. Rodriguez, J. P. Parisien, and C. M. Horvath. 2003. STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling. J. Virol. 77:6385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L. F., B. H. Harcourt, M. Yu, A. Tamin, P. A. Rota, W. J. Bellini, and B. T. Eaton. 2001. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 3:279-287. [DOI] [PubMed] [Google Scholar]
- 35.Wang, L. F., M. Yu, E. Hansson, L. I. Pritchard, B. Shiell, W. P. Michalski, and B. T. Eaton. 2000. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J. Virol. 74:9972-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong, K. T., I. Grosjean, C. Brisson, B. Blanquier, M. Fevre-Montange, A. Bernard, P. Loth, M. C. Georges-Courbot, M. Chevallier, H. Akaoka, P. Marianneau, S. K. Lam, T. F. Wild, and V. Deubel. 2003. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol. 163:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]