Abstract
The σS subunit of Escherichia coli RNA polymerase regulates the expression of stationary phase and stress response genes. σS is highly unstable in exponentially growing cells, whereas its stability increases dramatically upon starvation or under certain stress conditions. The degradation of σS is controlled by the phosphorylatable adaptor protein RssB and the ClpXP protease. RssB specifically directs σS to ClpXP. An unanswered question is how RssB-mediated degradation of σS is blocked by conditions such as glucose or phosphate starvation. We report here the identification and characterization of a new regulator of σS stability, IraP (inhibitor of RssB activity during phosphate starvation), that stabilizes σS both in vivo and in vitro. Deletion of iraP interferes with σS stabilization during phosphate starvation, but not during carbon starvation, and has a partial effect in stationary phase and nitrogen starvation. IraP interferes with RssB-dependent degradation of σS through a direct protein–protein interaction with RssB. A point mutant of IraP was isolated and found to be defective both for inhibition of σS degradation and interaction with RssB. Our results reveal a novel mechanism of regulation of σS stability through the regulation of RssB activity and identify IraP as a member of a new class of regulators, the anti-adaptor proteins.
Keywords: RpoS, σ38, YaiB, regulated proteolysis, starvation
Bacteria have evolved sophisticated stress response mechanisms that allow them to adapt to a broad range of stressful conditions. Gram-negative bacteria respond to different stresses by the synthesis and/or activation of alternative RNA polymerase σ factors that direct the transcription of regulons whose gene products will deal with the stress (Ishihama 2000). In Escherichia coli, the primary alternative σ factor, σS (also called σ38 or RpoS), is the master transcriptional regulator of stationary phase and the general stress response (Loewen and Hengge-Aronis 1994; Loewen et al. 1998). E. coli cells enter into stationary phase to cope with nutrient limitation and the different external environmental insults the bacteria may encounter during long periods of starvation. σS is required for the proper development of the stationary phase program. It promotes the expression of ∼100 genes that help the cell to respond to starvation (Loewen et al. 1998; Lacour and Landini 2004), hyperosmotic stress (Hengge-Aronis 1996; Checroun and Gutierrez 2004), hypoosmotic stress (Stokes et al. 2003), acid resistance, alkaline resistance, heat shock, cold shock (Kandror et al. 2002), oxidative damage, and DNA damage (Almiron et al. 1992; Volkert et al. 1994; Serafini and Schellhorn 1999; Wolf et al. 1999; Frenkiel-Krispin et al. 2001; for review, see Hengge-Aronis 2002). Thus, σS is crucial to maintain cellular homeostasis under many conditions, reflected in the tight regulation of its cellular concentration and activity at all possible levels: transcription, translation, protein stability, and activity (Pratt and Silhavy 1998; Hengge-Aronis 2002; Bougdour et al. 2004).
σS protein stability is dependent on both an energy-dependent protease, ClpXP, and an adaptor protein, RssB. In exponentially growing cells, σS is maintained at a very low level due to active degradation by the ClpXP protease (Lange and Hengge-Aronis 1994; Schweder et al. 1996). σS stability increases (∼10-fold) during entry into stationary phase or after exposure to certain stresses, allowing the accumulation of σS in the cells (for review, see Hengge-Aronis 2002). Among the known substrates for E. coli cytosolic proteases, σS is exceptional in that it is poorly recognized by the ClpXP protease alone; its degradation requires the adaptor protein RssB (also termed SprE, MviA in Salmonella and ExpM in Erwinia) (Bearson et al. 1996; Muffler et al. 1996; Pratt and Silhavy 1996; Zhou and Gottesman 1998; Andersson et al. 1999). RssB binds directly to σS and targets it to the ClpXP protease (Zhou et al. 2001). RssB belongs to the two-component response regulator family of proteins. The activity of proteins in this family is modulated by phosphorylation of the N-terminal receiver domain on a highly conserved residue, asp58 in the case of RssB (Bouche et al. 1998).
While RssB affinity for σS can be modulated by phosphorylation (Becker et al. 1999; Zhou et al. 2001), its ability to activate σS proteolysis is regulated by a mechanism that is independent of its ability to be phosphorylated. A nonphosphorylatable RssB protein is still active in vivo and, furthermore, can still respond to environmental signals (Peterson et al. 2004). In cells carrying an rssB mutation in the phosphorylation site, σS is stabilized in stationary phase and upon starvation. The intracellular amounts of ClpXP are constant during growth (Damerau and St John 1993; Schweder et al. 1996; Mandel and Silhavy 2005) but, paradoxically, RssB levels increase as the cells enter into stationary phase, at the same time as σS becomes stable (Becker et al. 2000; Gibson and Silhavy 2000; Ruiz et al. 2001; Pruteanu and Hengge-Aronis 2002). Therefore, RssB activity during stationary phase and starvation must be regulated by an undefined mechanism; unlike previously suggested models, this mechanism may not involve a kinase or a phosphatase.
The work described here was undertaken in order to identify new components involved in the regulation of σS proteolysis. An E. coli genomic DNA library was screened for clones that would affect the activity of an rpoS-lacZ translational fusion designed to be regulated solely at the level of protein stability. We report the isolation and the characterization of a new regulator of σS stability encoded by the previously uncharacterized yaiB gene. YaiB modulates the stability of σS in vivo and in vitro by counteracting the activity of RssB, resulting in the stabilization of σS. Because this protein is critically important for stabilization of σS after phosphate starvation, we have renamed the gene iraP for inhibitor of RssB activity during phosphate starvation.
Results
Identification of a novel regulator of σS stability
As discussed above, there are still unidentified components involved in the regulation of σS degradation. We reasoned that the use of a reporter system that would more specifically respond to changes in σS stability might allow the identification of novel cellular regulators of σS degradation. For this purpose, we used a translational fusion PBAD-rpoS990’-‘lacZ (C. Ranquet and S. Gottesman, in prep.) lacking the 5′ untranslated region of the rpoS mRNA containing the well-described translational control signals (for review, see Gottesman 2004). Expression of the reporter was driven by the PBAD promoter to bypass the normal signals influencing rpoS transcription. The strain carrying this fusion, CRB316, gave slightly Lac+ (red) colonies on MacConkey lactose plates without arabinose but became strongly Lac+ in clpP (AB002) or rssB (AB003) mutant derivatives (data not shown).
To identify potential new regulators of σS stability, this strain was transformed with a pBR322-based E. coli genomic DNA library (Ulbrandt et al. 1997) with the expectation that overproduction of either positive or negative regulators of σS degradation from the plasmid would give white (Lac−) or red (Lac+) colonies, respectively, on MacConkey lactose indicator plates. After transformation with the plasmid library, six red and seven white colonies were isolated out of ∼15,000 colonies. To eliminate plasmids affecting the transcription of the fusion rather than the stability of the σS-LacZ fusion protein, plasmids were isolated and introduced into a strain carrying the rpoS750’-‘lacZ translational fusion, which encodes a hybrid protein that is subject to the same transcriptional, translational, and proteolytic regulation as σS itself. Only plasmids that affected the expression of both fusions were studied further. These plasmids were directly assayed for their effects on σS degradation, as described in Materials and Methods. Two plasmids survived these screens and showed an effect on σS degradation. One contained the complete coding sequence of rssB and produced white colonies, indicating increased degradation of the σS-LacZ reporter. This clone was not further studied. The second plasmid, which produced red colonies, diagnostic of decreased degradation of the σS-LacZ reporter, contained the complete coding sequence of yaiB, encoding a previously uncharacterized protein. For the reasons described below, we have renamed yaiB as iraP, and the protein product as IraP.
IraP inhibits intracellular degradation of σS
The insert carried by the clone containing iraP corresponds to the region located between 399,461 and 401,605 base pairs on the E. coli K12 chromosome. This region contains the coding sequence of iraP flanked by intergenic regions and part of the flanking genes ddlA and phoA, encoding, respectively, D-alanine-D-alanine ligase A and alkaline phosphatase. A series of deletions in the plasmid defined the iraP coding sequence as the active DNA sequence within this plasmid (data not shown). This assignment was confirmed by subcloning the ORF of iraP with its upstream region (387 nucleotides [nt] upstream of the iraP start codon) into the vector plasmid used for the library, pHDB3, yielding the pIraP plasmid, as described in Materials and Methods.
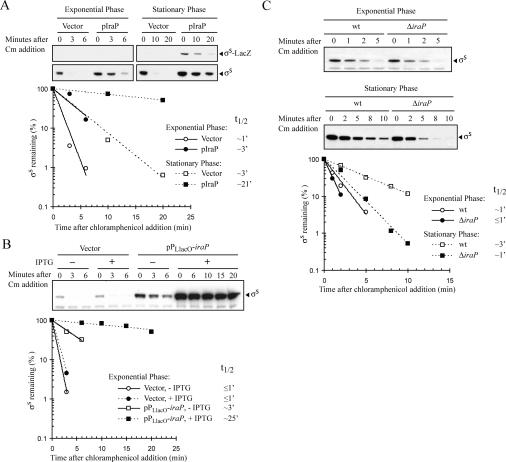
The effect of multicopy iraP (pIraP) on σS stability was measured in cells grown at 37°C in Luria-Bertani broth (LB). As expected, in exponentially growing cells with the vector control (pHDB3), σS was rapidly degraded, whereas in cells carrying pIraP, the σS half-life was increased approximately threefold (Fig. 1A, exponential phase). In stationary phase, the half-life of σS was relatively short, ∼3 min in the presence of the vector control (Fig. 1A). However, in stationary phase cells carrying iraP in multicopy, the σS half-life increased to ∼21 min, a sevenfold increase over the vector control (Fig. 1A). The presence of a multicopy plasmid carrying iraP led to a modest (approximately twofold) increase in the intracellular amount of σS, presumably owing to decreased σS degradation (Fig. 1A, cf. vector and pIraP at 0 min). This decreased degradation permitted the accumulation and, consequently, the detection of σS-LacZ (Fig. 1A, stationary phase), and provides confirmation of the basis for the effect of IraP overproduction on the β-galactosidase activity of the PBAD-rpoS990’-‘lacZ fusion.
Figure 1.
Effects of multicopy iraP and deletion of iraP on σS stability. The half-life of σS was measured in cells grown at 37°C in LB. Protein synthesis was inhibited with chloramphenicol at OD600 of ∼0.3 and ∼3 for the determination of σS half-life in exponential phase and stationary phase, respectively. Samples were removed at specific time points and analyzed by immunoblot with an anti-σS antiserum. (A) σS half-life was determined in the strain CRB316 (PBAD-rpoS990’-‘lacZ chromosomal fusion) containing either pHDB3, as vector control, or pIraP. The accumulation and the degradation of σS-LacZ are shown in the top panel and chromosomally-encoded σS in the bottom panel. Quantification of the σS signals is shown in the graph. The density for samples at time 0 was set to 100% for each of the chase experiments. Half-lives (t½) were calculated by regression analysis of the exponential decay of σS. (B) Effect of iraP expression from a foreign inducible promoter on σS stability in the strain AB009 (ΔiraP∷kn) carrying either the vector control pPLlacO or pPLlacO-iraP. The cells were grown until exponential phase in the presence or absence of 1 mM IPTG, as indicated. (C) Comparison of σS half-lives in the ΔiraP (AB006) and wild-type (MG1655) strains.
To confirm that the iraP ORF, rather than any sequences from the regulatory region, was responsible for the stabilization of σS, iraP was cloned under the control of the inducible promoter PLlacO (described in Materials and Methods). The chromosomal copy of iraP was deleted (see Materials and Methods). σS half-life was monitored in exponential phase in a ΔiraP strain complemented by the pPLlacO-iraP plasmid. σS was dramatically stabilized when iraP expression was induced with isopropyl-β-D-thiogalactopyranoside (IPTG) in comparison to the uninduced cultures or cells with the vector control (Fig. 1B). σS was partially stabilized in strains carrying the uninduced pPLlacO-iraP plasmid, probably reflecting leaky transcription from the repressed PLlacO promoter. Furthermore, iraP induction also influenced the steady-state levels of σS (Fig. 1B, cf. the time 0 min for each set of samples), presumably due to the effect of IraP on σS stability. σS levels in cells containing the uninduced or induced PLlacO-iraP plasmid were increased 10- and 40-fold, respectively, compared with the vector control.
Overexpression of IraP did not affect the activity of either a rpoS’-lacZ transcriptional fusion or a rpoS477’-‘lacZ translational fusion, neither of which is degraded by ClpXP and RssB (data not shown). Thus, IraP does not affect transcription from the rpoS promoter or expression and folding of stable σS fusion proteins. Together with our observations of the effect of multicopy iraP on the PBAD-rpoS990’-‘lacZ fusion, containing a foreign promoter and no upstream sites for translational control, these results strongly suggest that IraP acts at the level of σS protein stability.
IraP is required for σS stabilization during some but not all starvation conditions
To determine whether the iraP gene is required for the stabilization of σS in stationary phase, the half-life of σS was measured in both the wild-type strain and a strain deleted for iraP. In exponentially growing cells, the deletion of iraP had only a modest effect on σS stability. However, in stationary phase cells growing in rich broth, σS half-life was approximately threefold reduced in the ΔiraP strain (Fig. 1C). In cells lacking iraP, σS is almost as unstable in stationary phase as in exponential phase in the wild-type strain; therefore, IraP contributes significantly to stationary phase stabilization of σS.
Expression of a σS-dependent fusion was also examined; as expected from the modest effect on accumulation and stability in LB, there was a slight decrease (twofold or less) in reporter gene expression in the iraP mutant and a slight increase (twofold or less) with iraP on a multicopy plasmid (pIraP) (data not shown).
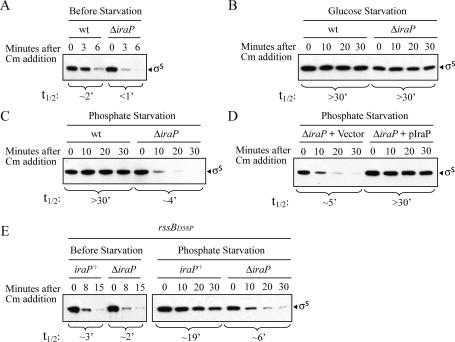
Stationary phase growth in complex media such as LB involves multiple changes in the available nutrients and the environment. Many specific stress responses lead to stabilization of σS, including two well-defined conditions, carbon starvation (Lange and Hengge-Aronis 1994; Zgurskaya et al. 1997) and phosphate starvation (Ruiz and Silhavy 2003; Peterson et al. 2004). To evaluate the role of iraP under these conditions, we assayed σS stability following glucose or phosphate limitation in both wild-type and ΔiraP strains. Exponentially growing cells in minimal medium were harvested and resuspended in fresh medium lacking either phosphate or the carbon source. After 1 h of starvation, chloramphenicol was added and σS half-life was monitored. Prior to nutrient starvation, σS was very unstable, with a half-life of ∼2 min (Fig. 2A). Only a small effect of the ΔiraP mutation on σS degradation was noticed in exponentially growing cells in minimal media, as was seen in LB (Fig. 1C). After 1 h of glucose starvation, σS was dramatically stabilized, as previously reported; σS stabilization during carbon starvation was not significantly affected by the absence of IraP (Fig. 2B).
Figure 2.
Effect of ΔiraP on σS stability during carbon and phosphate starvation. The half-life of σS was measured in cells grown at 37°C in MOPS medium as described in Materials and Methods. σS half-life was determined in exponentially growing cells and after 1 h of glucose or phosphate starvation as indicated. Protein synthesis was inhibited with chloramphenicol at an OD600 ∼0.3. Samples were removed at specific time points and analyzed by immunoblot with an anti-σS antiserum. To obtain the same amount of σS at time 0 of each of the chase experiments, the amount of cell extract used for exponential phase cultures was twice the volume used for starved cells. σS half-lives (t½) were determined from plots of intensity versus time. (A–C) σS degradation was monitored in the wild-type (MG1655) and ΔiraP∷kn (AB006) strains before (A, exponential phase) and after glucose (B) or phosphate (C) starvation. (D) Complementation of ΔiraP by pIraP. σS half-life was determined in phosphate-starved cells with the strain AB006 (ΔiraP∷kn) containing either the vector control pHDB3 or pIraP. (E) The half-life of σS was measured in strains YN868 (rssBD58P) and AB010 (rssBD58P, ΔiraP∷kn) in both exponential phase and after 1 h of phosphate starvation.
Very different results were obtained after phosphate starvation. As previously observed (Peterson et al. 2004), σS became stable when the cells were starved for phosphate. However, in the ΔiraP strain, σS remained unstable in phosphate-starved cells (Fig. 2C). Complementation of ΔiraP with pIraP restored stabilization of σS during phosphate starvation (Fig. 2D), confirming that IraP is required for full σS stabilization during phosphate limitation, although there is also an IraP-independent effect of phosphate starvation (4-min half-life, cf. the 1-min half-life without starvation). These data show that requirements for σS stabilization are different during phosphate starvation and glucose starvation; IraP is required for the former but not the latter.
To test whether IraP affects σS stabilization only in phosphate starvation, we measured the σS half-life in the wild-type and ΔiraP strains during nitrogen starvation, a condition in which σS is also stabilized (Mandel and Silhavy 2005). σS levels increased only modestly and the protein was only partially stabilized (half-life of ∼15 min) after 1 h of nitrogen starvation with the wild-type strain (data not shown), consistent with the data of Mandel and Silhavy (Mandel and Silhavy 2005). In the ΔiraP strain, the σS half-life was reduced from 15 to 4 min, indicating that IraP also participates in σS stabilization during nitrogen starvation (data not shown).
Our results highlight the differences between different starvation conditions in affecting σS stability. IraP is critically important in phosphate starvation and plays a significant role in stationary phase and nitrogen starvation, but has no measurable effect in glucose starvation.
IraP regulates RssB activity by a phosphorylation-independent mechanism
While σS stability is controlled by RssB and ClpXP (Muffler et al. 1996; Pratt and Silhavy 1996; Zhou and Gottesman 1998), it was theoretically possible that another system was responsible for σS degradation in the absence of iraP. If so, σS might be unstable in an iraP mutant host, even in the absence of rssB. This was tested by comparing the σS half-life in an rssB-null strain (AB012) with that in a rssB iraP double mutant (AB013). The σS half-life was longer than 30 min in both strains (data not shown), consistent with IraP acting within the RssB pathway.
If IraP acts directly within the RssB pathway, does it function to change RssB phosphorylation status? To determine this, we measured σS half-life in cells containing a chromosomal rssB allele unable to be phosphorylated. In this strain, the rssB codon for asp58, the location of phosphorylation (Bouche et al. 1998), was mutated to encode proline (rssBD58P, Y. Zhou and S. Gottesman, in prep.), a change previously shown to be partially active for σS degradation (Becker et al. 2000). Peterson et al. (Becker et al. 2000) previously observed that cells carrying a chromosomal copy of rssB in which asp58 was mutated to ala retained the ability to promote σS degradation in exponentially growing cells, and that σS was stabilized after phosphate starvation. Our results agree; σS was degraded in exponential phase, albeit at a slightly slower rate than with the wild-type rssB, in both iraP+ and ΔiraP cells carrying rssBD58P (Fig. 2, cf. A and E, before starvation). After 1 h of phosphate starvation, σS was stabilized in the iraP+ rssBD58P strain (Fig. 2E). Thus, the rssBD58P mutation did not abolish the ability of the cell to down-regulate σS degradation in response to environmental signals, although the stabilization was significantly less than in the rssB+ host.
As in the rssB+ strain, phosphate starvation did not lead to σS stabilization in the ΔiraP derivative of rssBD58P strain (Fig. 2E, phosphate starvation). The effect of IraP overproduction in stabilizing σS (Fig. 1A) and of the loss of IraP (ΔiraP strain) in destabilizing σS (Fig. 1C) in LB cultures could also be detected in the rssBD58P background (data not shown). Thus, IraP still affects σS stability in a strain in which RssB cannot be phosphorylated. These data, taken together, indicate that IraP inhibits σS proteolysis by regulating RssB activity in a manner that does not require the potential to phosphorylate or dephosphorylate the asp58 residue.
Identification and characterization of a mutant of IraP
RssB is the limiting protein governing σS stability; it is generally made at low levels, and increasing the level of RssB increases σS degradation (Pruteanu and Hengge-Aronis 2002). We used a strain, referred to here as rssB+++, carrying the PBAD-rpoS990’-‘lacZ fusion as well as a transposon insertion upstream of rssB (rssA2∷cm) that increases levels of RssB and therefore decreases σS levels below our detection limit (Fig. 3A, vector; Ruiz et al. 2001; Mandel and Silhavy 2005) to develop a simple screen for point mutants in iraP that could be used both in vivo and in vitro as controls to further examine IraP mechanism of action.
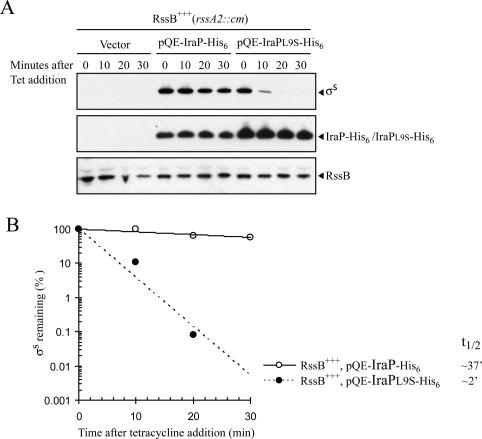
Figure 3.
IraP, but not IraPL9S, overcomes RssB overexpression. (A) The half-life of σS was measured in exponentially growing cells at 37°C in LB after 15 min of induction with 1 mM IPTG. Protein synthesis was inhibited with tetracycline at an OD600 of ∼0.3, and samples were removed at specific time points and analyzed by immunoblot with an anti-σS antiserum. The strain AB011 (rssA2∷cm background) containing the vector control pQE-80L, pQE-IraP-His6, or pQE-IraPL9S-His6 was used. In the absence of IPTG, σS was not detected with any of the plasmids listed above (data not shown). The levels of His-tagged IraP protein expressed were analyzed by immunoblot using an anti-RGS-His4 antibody. RssB levels were determined by immunoblot with anti-RssB antiserum. (B) Quantification of the levels of σS in panel A. σS half-lives (t½) were determined from plots of the log of intensity versus time.
A plasmid carrying iraP-his6 under control of the Lac repressor (pQE-IraP-His6) was introduced into the rssB+++ strain and induced with IPTG for 15 min. The stability of σS was then monitored by addition of tetracycline followed by sampling at various times. Figure 3A shows that after 15 min of induction, σS was highly expressed and was stable for >30 min, demonstrating both that the IraP-His6 protein is active and that it can overcome the somewhat higher RssB levels in the rssB+++ strain. In addition, the somewhat higher RssB levels allowed us to monitor the RssB protein by immunoblot. No change in intracellular levels of RssB was observed in cells overexpressing IraP, and RssB levels remained stable during the tetracycline chase (Fig. 3A). Therefore, IraP does not appear to affect RssB levels or stability.
The effect of IraP-His6 overexpression on σS levels in the rssB+++ strain can easily be monitored on indicator plates with the PBAD-rpoS990’-‘lacZ fusion. A strain carrying this fusion and the rssB+++ mutation is white on MacConkey lactose plates (fusion protein degraded); colonies are dark red on McConkey lactose plates (fusion protein stabilized) when IraP is induced. This change in phenotype allowed us to screen for loss-of-function mutants of iraP using random mutagenesis, as described under Materials and Methods. Several mutants of iraP-his6, each containing a single amino acid substitution and each able to synthesize significant levels of the protein, were obtained; one, iraPL9S-his6, has been studied. IraPL9S-His6 was much less effective at stabilizing σS than was IraP-His6 (Fig. 3).
The levels of the His6-tagged mutant and wild-type proteins were analyzed by immunoblot. Unexpectedly, the IraPL9S-His6 mutant protein was more abundant than was IraP-His6 (Fig. 3A); we do not currently have an explanation for this difference. The levels of IraP (either mutant or wild type) did not change during an antibiotic chase, suggesting that IraP is not itself a substrate for degradation.
IraP interferes directly and specifically with the RssB-mediated degradation of σS
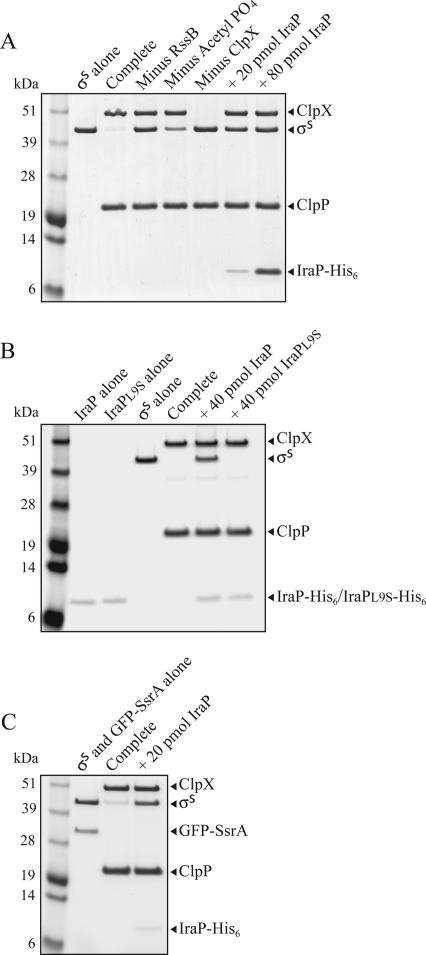
To investigate the mechanism of action of IraP and whether it acts directly to stabilize σS, we assayed σS degradation in vitro with purified proteins. As previously reported (Zhou et al. 2001), σS was degraded in the presence of ClpXP, RssB, and ATP, as measured by SDS-PAGE analysis (Fig. 4A). Acetyl phosphate, which is a known phosphate donor for the phosphorylation of RssB (Bouche et al. 1998), also stimulated σS degradation, as previously described (Zhou et al. 2001). To test whether IraP acts directly to inhibit σS degradation, IraP-His6 was purified under native conditions (see Materials and Methods) and included in the σS degradation reaction mixtures. In the complete reaction mixture, in the absence of IraP, ∼80% of σS was degraded after 30 min of incubation (Fig. 4A). When IraP was present in the reactions, σS was not detectably degraded. Thus, IraP is a direct inhibitor of σS degradation.
Figure 4.
IraP inhibits σS degradation by ClpXP and RssB in vitro. The degradation reactions were carried out as described in Materials and Methods with purified components. Reaction products were separated by SDS-PAGE and stained with Coomassie blue. (A) The reactions were performed using 20 pmol of σS in the presence or absence of IraP-His6. RssB, acetyl phosphate, and ClpX were omitted where indicated. (B) The reactions were performed as above, in the presence or absence of either IraP-His6 or IraPL9S-His6. (C) Ten picomoles of σS was mixed with 10 pmol of GFP-SsrA, in the presence or absence of IraP-His6, and degradation reactions were performed as above.
We then asked whether the L9S mutation in IraP affects its in vitro activity. Figure 4B shows that IraPL9S-His6 did not inhibit σS degradation. Thus, both in vivo and in vitro the L9S mutant protein is inactive. Figure 4B also shows that IraP-His6 or IraPL9S-His6 is not detectably degraded in vitro by ClpXP, confirming that IraP is not itself a substrate of ClpXP.
Given that IraP directly affects σS degradation, IraP might act by interfering with the activity of either the ClpXP protease or the adaptor protein RssB, or by protecting σS from interacting with either of these factors. To test the first possibility, we examined the effect of IraP on the degradation of another ClpXP substrate, GFP-SsrA (Kim et al. 2000; Singh et al. 2000; Farrell et al. 2005). In the absence of IraP-His6, when σS and GFP-SsrA were incubated together with ClpXP and RssB, both were degraded (Fig. 4C). In the presence of IraP-His6, σS was protected from degradation but GFP-SsrA was not (Fig. 4C, last lane). Similarly, degradation of RepAΔ25-SsrA, another known ClpXP substrate (Sharma et al. 2005), was not inhibited by IraP-His6 (data not shown). These results indicate that IraP acts specifically on the σS degradation pathway and, importantly, that IraP does not inactivate ClpXP.
IraP interacts directly with the adaptor protein RssB
The above results showed that IraP controls the half-life of σS and acts within the RssB/ClpXP pathway to protect σS from degradation. One simple way to accomplish this would be a direct IraP interaction with RssB, blocking the RssB interaction with σS. To test whether IraP interacts directly with RssB, purified IraP-His6 was bound to Ni2+-NTA-magnetic beads and incubated with purified RssB. Following elution with imidazole buffer and analysis by immunoblot, ∼25% of the RssB introduced to the IraP-His6-Ni2+-NTA-magnetic beads matrix was retained and coeluted with IraP-His6 (Fig. 5A, cf. lanes 1 [input] and 3 [protein eluted]). RssB did not bind to the Ni2+-NTA-magnetic beads in the absence of IraP-His6 (Fig. 5A, lane 2).
Figure 5.
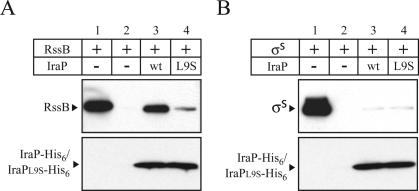
Interaction of IraP and RssB. Purified RssB (A) or σS (B) was incubated with an excess of either IraP-His6-magnetic beads (lane 3) or IraPL9S-His6-magnetic beads (lane 4) or the magnetic beads alone (lane 2), as described in Materials and Methods. The proteins adsorbed to the matrix were washed and eluted with imidazole. RssB, σS, and IraP were separated by SDS-PAGE and visualized by immunoblot. Lane 1 shows the input amount of RssB (A) or σS (B) used per reaction.
We then asked whether the L9S mutant of IraP, which in vivo and in vitro had dramatically diminished activity, had any effect on the interaction between IraP and RssB. With use of the assay described above, significantly less RssB coeluted with the mutant IraP compared with the wild-type IraP protein (Fig. 5A, cf. lanes 3 and 4). Similar results were obtained by using crude extracts as a source of RssB (data not shown).
A similar experiment was performed to probe for association of σS and IraP-His6. We found that σS was not retained and therefore was not coeluted with either IraP-His6 or IraPL9S-His6 attached to the magnetic beads (Fig. 5B).
These results demonstrate a direct and specific interaction between IraP and RssB, and suggest that the L9 residue of IraP is important for this interaction with RssB. We conclude that IraP-dependent stabilization of σS relies on a direct protein–protein interaction between IraP and RssB.
Discussion
IraP belongs to a novel class of regulator: the anti-adaptor proteins
We report here the identification and the characterization of a novel regulator of σS stability, IraP. IraP provides a critical link to the previously unexplained environmental regulation of σS stability. IraP acts in a direct manner to inhibit σS degradation. Deletion of iraP reverses the stabilization of σS after phosphate starvation (Fig. 2C) and partially blocks stabilization in stationary phase (Fig. 1C) and after nitrogen starvation (data not shown). In vivo and in a purified in vitro system, IraP inhibits σS degradation by interfering specifically with RssB-dependent degradation by ClpXP (Figs. 1, 3, 4; data not shown). IraP is not itself subject to degradation by ClpXP or other cellular proteases (Figs. 3A, 4B). Consistent with these observations, in vivo analysis of two classes of ClpXP substrates, represented by Dps and SsrA-tagged proteins, do not accumulate when iraP is overexpressed (data not shown), and in vitro, two SsrA-tagged substrates of ClpXP are not affected by IraP (Fig. 4C; data not shown).
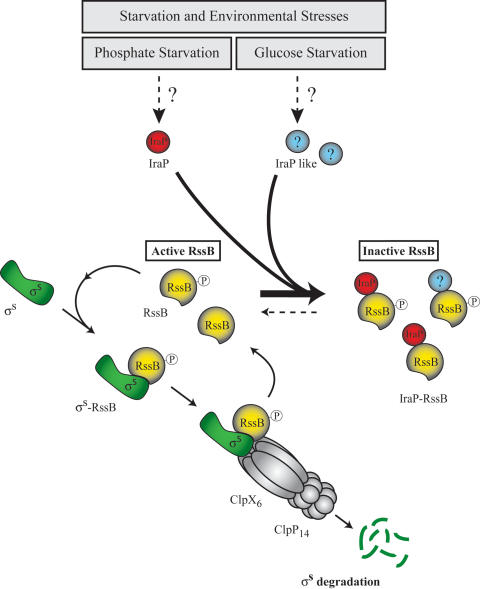
Our model for IraP action is summarized in Figure 6. Under specific stress signals (phosphate starvation, for instance), IraP becomes available, probably because synthesis increases, although this has not yet been demonstrated. IraP directly binds RssB; we suggest that this binding sequesters RssB, thereby preventing it from interacting with σS (Fig. 6). Direct interaction is supported by the association of the wild-type IraP and RssB shown in Figure 5 and the partial loss of that association with the impaired L9S mutant form of IraP. As indicated in the model, we propose that other stress conditions that lead to σS stabilization (e.g., glucose starvation) may do so via other IraP-like proteins made under those specific stress conditions. The availability of a variety of different RssB inhibitors combined with the complex regulation of σS synthesis (Hengge-Aronis 2002) would allow the cell to modulate σS accumulation and activity in response to a large number of different conditions.
Figure 6.
Model of regulation of σS degradation by IraP. In exponentially growing cells, RssB delivers σS to ClpXP for degradation. During the reaction, RssB itself is not consumed; RssB acts catalytically and is able to carry out multiple cycles of σS binding and delivery to ClpXP (Zhou et al. 2001). Phosphorylation of RssB stimulates σS degradation. During phosphate deprivation and under other conditions (i.e., nitrogen starvation), IraP stabilizes σS through a direct interaction with RssB; the model suggests that the RssB–IraP complex is unable to bind and target σS for degradation, although failure to bind σS remains to be shown. The pathway for iraP induction is not known but is independent of the PhoR/PhoB phosphate regulatory pathway. σS is also stabilized under glucose starvation independently of IraP; we suggest that another IraP-like protein acts in that case. Other mechanisms regulate σS synthesis (not shown).
Sequestering RssB with specific anti-adaptors provides an elegant and novel way to block degradation under a variety of environmental conditions. We assume that σS is stabilized because it is needed to activate promoters in response to the stress. Therefore, any modification or interaction with σS itself might compromise its activity. The intracellular levels of RssB are very low (estimated to be only a few molecules per cell) (Becker et al. 2000). If IraP binds RssB stoichiometrically and with high affinity, a low amount of IraP should be sufficient to inactivate RssB. Consistent with this model, increased expression of RssB leads to more rapid degradation of σS but can be overcome by overexpression of IraP (Fig. 3). Because RssB amounts are normally low, even a modest increase in the levels of a protein such as IraP may be sufficient to titrate RssB and totally block σS turnover. Thus, in this model, rapid degradation of σS is the default situation, and only under appropriate environmental conditions is degradation blocked by IraP and/or similar anti-adaptors.
RssB can also act as an anti-σ factor for σS (Zhou and Gottesman 1998; Becker et al. 2000); therefore, even in the absence of degradation, inactivating RssB will free σS to activate its promoters. The sites on RssB that interact with IraP and σS have not yet been identified, but we expect these interactions to be mutually exclusive. Preliminary experiments to examine whether this is so are consistent with our model. RssB attached to beads was able to bind RpoS; RpoS binding was reduced when RssB was preincubated with IraP (data not shown).
RssB shares homology with the response regulator family of proteins, and its affinity for σS is modulated by phosphorylation of the conserved residue asp58 located in the N-terminal domain (Bouche et al. 1998; Zhou et al. 2001). We observed that IraP regulated the stability of σS in cells carrying either wild-type rssB or a mutant allele of rssB that cannot be phosphorylated (D58P) (Fig. 2; data not shown). This is consistent with and provides an explanation for recent work showing that a nonphosphorylable RssB protein retains partial activity and that σS turnover is regulated upon starvation even with the mutant RssB (Peterson et al. 2004). However, some differences in the effectiveness of IraP and phosphate starvation were observed in the rssBD58P mutant. In the rssBD58P background during phosphate starvation, the σS half-life is ∼19 min, which is less than the >30 min observed with the wild-type rssB allele (Fig. 2C,E). Possibly, the D58P mutation somewhat compromises the ability of IraP to interact with RssB and counteract its activity.
These results do not rule out a role for phosphorylation in modulating σS stability; a role for phosphorylation would be consistent with the differences we observed in σS turnover in the rssBD58P strain compared with wild-type rssB. Acetyl-phosphate is known to phosphorylate RssB in vitro, and changing its level of synthesis affects σS turnover in vivo (Bouche et al. 1998). Recently, Mika and Hengge (2005) have shown that the sensor kinase ArcB can also phosphorylate RssB and modulate, to some extent, its activity.
The physiological function of IraP during phosphate starvation
IraP is a small protein (86 amino acids) predicted to be highly helical. The only homologs are found in other Enterobacteria that also contain rpoS and rssB (Escherichia species, Shigella, Salmonella species, and Erwinia species); the N-terminal region where point mutants were identified is particularly well conserved. In E. coli, S. flexneri, and Salmonella, iraP is located upstream of phoA or psiF, genes induced under phosphate starvation. It was this linkage to phosphate-regulated genes that led us to test the effect of iraP mutations after phosphate starvation; our results show that IraP is particularly important under this stress condition (Fig. 2C).
However, while the expression of phoA and other members of the Pho regulon are regulated by the PhoR/PhoB two-component regulatory system in response to phosphate deprivation (Wanner 1996), the IraP-dependent stabilization of σS was not affected by a phoB mutation (data not shown). Therefore, the phosphate starvation must affect IraP expression and/or activity by another mechanism. Our data are consistent with the previous observation that RssB is inactivated upon phosphate starvation, in a PhoBR-independent manner (Ruiz and Silhavy 2003). We do not currently have an explanation for how the phosphate starvation signal is sensed through iraP. Northern blots show a clear but transient induction of iraP transcript accumulation (∼25-fold) upon phosphate starvation, suggesting transcriptional regulation of iraP expression as at least one component of the regulation (data not shown). Both Northern blots and transcriptional fusions show an increase in iraP expression upon entry to stationary phase (data not shown). If increased transcription of iraP is the primary event leading to stabilization upon starvation, it still remains to be determined what happens when cells exit from phosphate starvation, and whether there is an active process to overcome IraP-dependent stabilization.
Regulated degradation of σS is only one of the levels of control of this important protein. It is known, for instance, that σS is also up-regulated at the level of synthesis upon phosphate starvation, most probably by a currently undefined small RNA (Ruiz and Silhavy 2003). Possibly because of these complex additional levels of regulation, the changes in the steady-state level of σS in iraP mutants are modest. This provides a simple explanation for why mutant searches using screens that reflect σS degradation have not previously been successful in identifying targets other that rssB. It is possible, as well, that mutations that impair the stabilization pathway are compensated by an increase in rpoS expression. This increase in synthesis of σS may itself also partially titrate RssB (Zhou and Gottesman 1998; Zhou et al. 2001; Pruteanu and Hengge-Aronis 2002). Such an increase in σS synthesis might be the explanation for the IraP-independent stabilization of σS after phosphate starvation (Fig. 2A,C, cf. exponential phase and phosphate starvation in the iraP mutant), although possibly another anti-adaptor could play a role here as well.
The increase in synthesis and decrease in degradation of σS suggest that its accumulation during phosphate starvation is important, but the function of increased σS under these conditions is not known. Increased σS is found after many stress treatments, including those that induce other, dedicated repair and recovery pathways (such as oxidative stress). σS induction may provide a general stress response, before committing the cell to the full-fledged specific response. In some agreement with this, a recent report suggests that high levels of σS negatively affect most of the phosphate starvation genes, possibly by competition with the primary σ factor, σ70, for core RNA polymerase (Taschner et al. 2004); this paradoxical effect might reflect a need to delay the PhoB-dependent response. σS positively controls the expression of pstS, which encodes the high-affinity inorganic phosphate transporter Pst; transport of phosphate through this system may contribute to the block in induction of the Pho system (Taschner et al. 2004).
IraP-independent control of σS stability
IraP regulates σS turnover during phosphate starvation and participates in the stabilization of σS during nitrogen starvation and stationary phase (Figs. 1C, 2C; data not shown). However, IraP is not needed for σS stabilization during glucose starvation (Fig. 2B). These results indicate that other mechanisms or pathways, independent of IraP, participate in the control of σS turnover.
It is unlikely that RssB activity is titrated by a high amount of σS in glucose-starved cells because the rate of σS synthesis is very low in these conditions (McCann et al. 1993; Lange and Hengge-Aronis 1994; Zgurskaya et al. 1997; Mandel and Silhavy 2005). The simplest explanation would be the existence of another anti-RssB regulator, which would be induced or become active specifically during glucose starvation and would play a similar role to IraP (Fig. 6). Additional evidence for other regulators is suggested by the stabilization of σS in cells carrying an hns mutation (Yamashino et al. 1995; Y. Zhou and S. Gottesman, in prep.), encoding the histone-like protein H-NS, which represses expression of many genes. The stabilization of σS in the hns mutant is not affected by ΔiraP (data not shown), suggesting that at least one other anti-RssB regulator is repressed by H-NS.
The regulation of σS degradation results from the integration of many input signal pathways (Hengge-Aronis 2002). IraP clearly controls σS stabilization, and its overexpression leads to a significant accumulation of σS. Mandel and Silhavy (Hengge-Aronis 2002) pointed out that starvations for different nutrients do not trigger identical responses. We believe that starvation for different nutrients and different stress conditions elicit specific signal transduction pathways converging to regulate σS stability. These pathways may overlap to some extent because we observed that IraP stabilizes σS in more than one condition, although the most pronounced effect was upon phosphate starvation.
Regulating proteolysis in bacteria
Intracellular proteases play a critical role in maintaining cell homeostasis by clearing damaged proteins and controlling the levels of many proteins, including global regulators (Gottesman 2003). Regulated proteolysis allows the cell to adjust to changing conditions by changing the rate of turnover of some of these key regulators. In eukaryotes, the complex ubiquitin ligase machinery provides a target for regulating proteolysis of specific proteins under specific conditions. In bacteria, no ubiquitin tagging exists, and a small number of ATP-dependent proteases are responsible for degradation of all unstable proteins, frequently by directly interacting with the substrate protein. In some cases, adaptors such as RssB provide the link to the protease and the presumed target for environmental regulation, but how RssB was able to respond to many different signals was not understood. Anti-adaptors, of which IraP is a defining example, provide the missing link in understanding this important regulatory circuitry.
Regulated proteolysis of important transcriptional regulators is found in other bacteria, including Bacillus subtilis and Caulobacter crescentus (for review, see Jenal and Hengge-Aronis 2003). A parallel to our findings with IraP and RssB is found in the regulated degradation of ComK, a central regulator of competence genes in the naturally competent bacterium B. subtilis (for review, see Hamoen et al. 2003). ComK is degraded in exponentially growing cells but not in stationary phase; degradation requires the ClpCP protease, related to ClpXP, and the adaptor protein MecA (Turgay et al. 1998; Persuh et al. 1999). A small protein, ComS, is made in stationary phase and required for the development of competence (D’Souza et al. 1994; Hamoen et al. 1995). In vitro, ComS disrupts the ternary complex of MecA–ClpC–ComK through a direct interaction with MecA (Turgay et al. 1997, 1998; Ogura et al. 1999). Presumably disruption is required in vivo to save ComK from degradation, thus allowing the development of competence. Thus, ComS, like IraP, functions as an anti-adaptor, regulating proteolysis under specific environmental conditions.
The examples of ComS and IraP suggest that environmental regulation of proteolysis in bacteria may be mediated in many cases by anti-adaptor proteins. If the small size of these proteins (46 amino acids for ComS, 86 amino acids for IraP) is typical for the anti-adaptors, they may be poor targets for mutagenesis and may, in some cases, not be annotated on genome maps. comS is encoded within the ORF of another gene, making it even harder to predict computationally (Hamoen et al. 1995). Multiple anti-adaptors made under somewhat different conditions may also obscure the detection of strong phenotypes unless precisely the right environmental conditions are tested. The approach taken here of looking for overproduction phenotypes has allowed us to identify IraP and suggest that IraP and ComS are the founding members of a large family of important regulatory molecules.
Materials and methods
Bacterial strains and plasmids
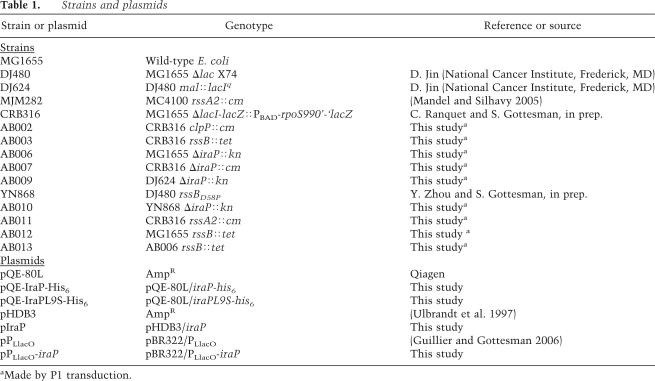
The genotypes of E. coli K12 strains and plasmids used in this work are described in Table 1. All the strains used are derivatives of MG1655, made by P1 transduction as indicated, selecting for the appropriate antibiotic-resistant marker. CRB316, a derivative of MG1655 containing a PBAD-rpoS-lacZ translational fusion integrated into the lac site, was a gift from C. Ranquet (National Institutes of Health, Bethesda, MD) (C. Ranquet and S. Gottesman, in prep.).
Table 1.
Strains and plasmids
aMade by P1 transduction.
To construct the deletion/insertion mutation of iraP, the λ Red recombination system was used as described (Yu et al. 2000). Briefly, a PCR fragment was obtained by amplifying either the kanamycin or chloramphenicol resistance cassettes of the strain MC4100, ybeW∷GBKn cassette, obtained from J.M. Ghigo (Institut Pasteur, Paris, France), and SG30024 (clpP∷cm) respectively. The antibiotic resistance cassettes were amplified by PCR using primers yaiBHRL-catF (5′-aatgttaacttctccatactttggataaggaaataca gacTACCTGTGACGGAAGATCAC-3′) and yaiBHRR-catR (5′ccgtgacaactttatgacagctgttgaaaagattaactttGGGCACCAATAACT GCCTTA-3′) for amplification of the cat cassette and with the primers yaiBHRL-KnF (5′-aatgttaacttctccatactttggataaggaaatac agac AAAGCCACGTTGTGTCTCAA-3′) and yaiBHRL-KnR (5′-ccgtgacaactttatgacagctgttgaaaagattaactttTTAGAAAAACTCATC GAGCA-3′) for amplification of the kn cassette. The capital letters of the primers above are identical to the 5′ and the 3′ ends of the respective antibiotic resistance cassettes. The rest of the primers are homologous to the regions flanking the coding sequence of iraP. The resulting PCR products were recombined into the chromosome of strain NM1100 (N. Majdalani, National Institutes of Health, Bethesda, MD), a Δlac derivative of MG1655 carrying the mini-λ prophage which encodes the λ Red functions (Court et al. 2003). The transformed cells were selected on LB plates containing either 25 μg/mL Cm or 50 μg/mL Kn at 32°C. Recombinant products were verified by sequencing, and the mutations were transferred into MG1655 by P1 transduction.
Plasmid pIraP was constructed by amplifying the iraP region by PCR using the primers yaiBregionF (5′-GATTGTTG CAATCTTCTGCTG-3′) and yaiBregionR (5′-GCTGTTG AAAAGATTAACTT-3′). The resulting PCR fragment, encompassing the iraP chromosomal region with 387 nt upstream of the iraP start codon and 21 nt downstream of the stop codon, was cloned into PCRII-TOPO (Invitrogen). The resulting plasmid was digested with SacI and XbaI, and the resulting linear fragment containing iraP was cloned into pHDB3, cut with the same restriction enzymes. The resulting plasmid was called pIraP.
Plasmid pPLlacO-iraP was constructed by amplifying the coding sequence of iraP by PCR using the primers AatII+1-RBS-ATG-yaiBF (5′-TAAGACGTCAGCGGATAACAATTTCACAC ATAATTCATTAAAGAGGAGAAATTAACTATGAAAAATC TCATTGCTGAGTT-3′) and EcoRI-yaiBregionR (5′-TGGGAA TTCGCTGTTGAAAAGATTAACTT-3′). These primers amplify the entire ORF of iraP, adding the optimal ribosome-binding site (bold) of the expression vector pQE80 (Qiagen) and two restriction sites, AatII and EcoRI. The start codon of IraP is underlined and the expected +1 of transcription is in italics. After digestion with AatII and EcoRI, the PCR product was introduced into the AatII and EcoRI sites of pBR-plac (referred to here as pPLlacO) (Guillier and Gottesman 2006). The resulting plasmid, called pPLlacO-iraP, contains an artificial promoter PLlacO (Lutz and Bujard 1997) driving the transcription of iraP.
To construct the plasmid pQE-IraP-His6, the coding sequence of the iraP gene was cloned in a His6 tag pQE-80L vector (Qiagen), as follows. We PCR-amplified the coding region of iraP using the chromosomal DNA from the MG1655 strain as template and the primers EcoRI-rbs-yaiBF (5′-ATCGGAATTCATTAAAGAGGAGAAATTAACTatgaaaaatctcattgctgagttgttat-3′) and PstI-3*-6hisSGRG-yaiBR (5′-ATAACTGCAGTCAGCTA ATTAAGCTTAGTGATGGTGATGGTGATGCGATCCTCT TCCctgacgaggatgcttcaataact-3′). The sequences corresponding to iraP are indicated in lowercase letters and the sequence coding for GRGSHis6 directly at the C-terminal end of IraP is underlined. The PCR fragment was digested by EcoRI and PstI and cloned into pQE-80L cut by the same enzymes, yielding the pQE-IraP-His6 plasmid.
All plasmid sequences were checked by sequencing.
Media and growth conditions
Cells were grown in LB or morpholinepropanesulfonic acid (MOPS) minimal medium (Teknova) supplemented with 0.4% glucose, 0.2% (NH4)2SO4, 1.32 mM K2HPO4, and 1 μg/mL thiamine. MacConkey agar plates with 1% lactose, containing ampicillin when used with plasmids, were used in analyses of strains carrying the PBAD-rpoS990’-‘lacZ chromosomal fusion. All liquid cultures were grown under aerobic conditions at 37°C, and growth was monitored by measuring the optical density at 600 nm (OD600). Exponential phase and stationary phase correspond to an OD600 of ∼0.3 and ∼3, respectively. Stationary phase cells were obtained 2 h after the break in the exponential growth curve.
For starvation experiments, MOPS was made without K2HPO4 (phosphate starvation medium), without (NH4)2SO4 (nitrogen starvation medium), or without glucose (carbon starvation medium). Cells were first grown overnight in complete MOPS minimal medium, then subcultured into fresh minimal glucose medium at an OD600 of 0.01 (∼1:400 dilution) and grown to mid-logarithmic phase (OD600 of ∼0.3) for the following treatments. Cells were washed twice by filtration with prewarmed (37°C) starvation medium, resuspended in the same volume of starvation medium, and incubation at 37°C was continued for 1 h.
Assay for σS degradation in vivo
Cells were grown in LB or in MOPS media as described above. Exponential phase refers to an OD600 of ∼0.3 and stationary phase to an OD600 of ∼3. Cells were treated with chloramphenicol (200 μg/mL) or tetracycline (100 μg/mL), and 1-mL samples were removed at the indicated time points and treated as described below.
Electrophoresis and immunoblot analysis of proteins
Whole-cell extracts were prepared as follows: Cells grown in LB or MOPS media were assayed for OD600, and 1-mL samples were removed and precipitated with 5% ice-cold tricarboxylic acid (TCA). Precipitated pellets were washed with 500 μL of 80% cold acetone and then resuspended in a volume of sodium dodecyl sulfate (SDS) sample buffer normalized to the OD600. Samples were analyzed using NuPAGE 12% Bis-Tris gels (Invitrogen), transferred, and probed with a 1:4000 dilution of anti-σS antiserum, a 1:1200 dilution of anti-RssB antiserum, or a 1:1000 dilution of anti-RGS-His4 antiserum (Qiagen). The blots were developed with the ECL system (Amersham) and quantified with ImageJ Software (National Institutes of Health).
Library screening
The multicopy plasmid DNA library screen was performed as described previously (Majdalani et al. 2001). Briefly, transformations were performed by electroporation of a pBR322-based library (Ulbrandt et al. 1997) into CRB316, plated on MacConkey lactose plates containing 50 μg/mL ampicillin to give ∼500 colonies per plate, and incubated at 37°C. We screened ∼15,000 colonies and isolated six red colonies and seven white colonies. Plasmid DNA was purified using Qiagen’s Minipreps kit, and insert junctions were sequenced using primers pBRlib.for (5′-CCTGACGTCTAAGAAACCATTATTATC-3′) and pBRlib.rev (5′-AACGACAGGAGCACGATCATGCG-3′).
Isolation of point mutants in iraP by random mutagenesis
Random mutagenesis of iraP was performed at a low mutation rate (three to 16 mutations per kilobase) using the GeneMorph II EZClone Domain Mutagenesis Kit (Stratagene) according to the manufacturer’s specifications. A mutant plasmid library of iraP was generated using pQE-IraP-His6 as template and the primers RMiraP-HisF (5′-CAGAATTCATTAAAGAGGAGA AATTAACTATG-3′) and RMiraP-HisR (5′-GTGATGCGATC CTCTTCC-3′). XL10-Gold ultracompetent cells were transformed with 1.5 μL of EZclone reaction mixture. The transformed cells were cultured in a total volume of 150 mL of LB supplemented with 100 μg/mL ampicillin for 10 h at 37°C. Plasmid library DNA was isolated using HiSpeedPlasmid Maxi Kit (Qiagen) and eluted with 600 μL of 1× TE buffer.
The screen for nonfunctional mutants of iraP was done by electroporation of the iraP mutant plasmid library (see above) into AB011, a strain carrying the chromosomal PBAD-rpoS990’-‘lacZ fusion and a rssA2∷cm mutation, which leads to overexpression of rssB from the chromosome (Ruiz et al. 2001; Mandel and Silhavy 2005). When transformed with pQE-80L, AB011 gave white colonies (Lac−), whereas transformation with pQE-IraP-His6 gave red colonies (Lac+) on MacConkey plates supplemented with 1% lactose and 50 μg/mL ampicillin. We used this phenotype to isolate mutations within the iraP coding sequence. Among ∼10,000 red colonies, 14 white clones were identified. The plasmids isolated were transformed again into AB011, and all gave the expected Lac− phenotype. To discriminate between full-length nonfunctional mutants of IraP and truncated proteins due to the introduction of stop codons, colony-immunoblot against the C-terminal RGS-His6 tag was performed using an anti-RGS-His4 antibody (Qiagen) according to the manufacturer’s instructions. The RGS-His6 tag was detected for five clones out of the 14 white colonies isolated. Sequence analysis showed that four plasmids each contained a single substitution; all were in the 5′ region of the coding sequence of iraP (the most conserved region); three of these, L4P, L9S, and A13D, were in fully conserved residues, and one, A6T, was in a partially conserved residue. Only the L9S substitution, which seemed to be the tightest mutant, was further studied; this mutation changes a TTA codon to TCA.
Proteins
Overproduction and purification of IraP-His6 and IraPL9S-His6 were performed as follows: The DH5α strain (Invitrogen), transformed with pQE-IraP-His6 or pQE-IraPL9S-His6, was grown in LB medium containing 100 μg/mL ampicillin to an OD600 of 0.6. IPTG was added to 1 mM. After 5–6 h of induction, cells were harvested and stored at −80°C until use. Cell pellets were resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole at pH 8.0) and sonicated. The lysates were centrifuged at 10,000 × g for 30 min at 4°C, and the soluble fractions were applied to a Ni2+-NTA column (Invitrogen) equilibrated with lysis buffer. The column was washed with 10 column volumes of buffer A (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole at pH 8.0), followed by elution with 250 mM imidazole, in buffer A. The eluates were desalted using an Amersham Biosciences PD-10 column equilibrated with 50 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, 100 mM KCl, and 0.1 mM DTT. Glycerol was added to a final concentration of 10% (v/v), and the samples were stored at −80°C. Final preparations of IraP-His6 and IraPL9S-His6 were >90% pure as judged by SDS-PAGE.
ClpX (Grimaud et al. 1998), ClpP (Maurizi et al. 1994), and RepA(Δ25)-SsrA (Sharma et al. 2005) were isolated as described. σS was isolated from cells carrying pLHN14 and expressing σS (Nguyen et al. 1993) using a described procedure (Tanaka et al. 1993) with some modifications. RssB was purified as described, with minor modifications (Zhou et al. 2001). His6-GFP-SsrA was isolated by Talon resin (Clontech) column chromatography as described by the manufacturer. All proteins were>90% pure as determined by Coomassie staining following SDS-PAGE. Protein concentrations were determined using the Bradford protein assay kit (Bio-Rad) and bovine serum albumin as a standard. Throughout, proteins are expressed as moles of RssB monomers, ClpX hexamers, ClpP tetradecamers, σS monomers, His6-GFP-SsrA monomers, IraP-His6 monomers, and IraPL9S-His6 monomers.
Assay for σS degradation in vitro
Reaction mixtures were assembled in a 20-μL final volume of buffer (20 mM Tris-HCl at pH 7.5, 10 mM MgCl2, 140 mM KCl, 1 mM DTT, 0.1 mM EDTA, 5% glycerol [v/v], 0.005% Triton X-100 [v/v]) containing 5 mM ATP, 10 mM acetyl phosphate, σS (20 pmol), IraP (20–80 pmol), RssB (1 pmol), ClpX6 (2 pmol), and ClpP14 (2 pmol), unless otherwise indicated. All of the proteins were diluted into the reaction buffer containing 0.05% Triton X-100 before use. The mixtures were incubated at room temperature for 30 min, and the reactions were stopped by the addition of 20 μL of SDS sample buffer. Protein samples were analyzed by SDS-PAGE. SeeBlue Plus2 (Invitrogen) prestained protein standards were used for molecular weight estimation.
Protein–protein interaction assay
Fifty microliters of Ni2+-NTA magnetic agarose beads (Qiagen) was added to 500 μL of interaction buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM MgCl2, 0.005% Tween 20, 20 mM imidazole at pH 7.5) containing 900 pmol of IraP-His6 or IraPL9S-His6. After 1 h at 4°C with agitation, the beads were incubated with bovine serum albumin (30 mg/mL) for 15 min, washed with 600 μL of interaction buffer, and resuspended with 200 μL of the same buffer. RssB (10 pmol) or σS (10 pmol) was added and, after incubation for 1 h at room temperature, the supernatant was removed and the beads were washed with 600 μL of interaction buffer. The proteins were eluted with 50 μL of interaction buffer containing 0.05% Tween 20 and 250 mM imidazole. Fifty microliters of SDS sample buffer was added to the eluted protein, and 20 μL of the mixture was analyzed by immunoblot. RssB, σS, IraP-His6, and IraPL9S-His6 were detected by immunoblot analysis using antiserum against RssB, σS, and RGS-His4, respectively.
Acknowledgments
We thank M. Mandel, N. Ruiz, and T. Silhavy for providing the rssA2∷cm mutation, and N. Majdalani for providing strain NM1100. We are grateful to C. Ranquet for making the PBAD-rpoS-lacZ translational fusion used in this study. We thank Y. Zhou for making the rssBD58P chromosomal mutation. We thank M. Guillier for providing the pPLlacO plasmid. We thank members of the Gottesman laboratory and Michael Maurizi for useful discussions and suggestions, and Carin Vanderpool and Michael Maurizi for their comments on the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/NA
References
- Almiron M., Link A.J., Furlong D., Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes & Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- Andersson R.A., Palva E.T., Pirhonen M. The response regulator expM is essential for the virulence of Erwinia carotovora subsp. carotovora and acts negatively on the σ factor RpoS (σS). Mol. Plant Microbe Interact. 1999;12:575–584. doi: 10.1094/MPMI.1999.12.7.575. [DOI] [PubMed] [Google Scholar]
- Bearson S.M., Benjamin W.H., Jr., Swords W.E., Foster J.W. Acid shock induction of RpoS is mediated by the mouse virulence gene mviA of Salmonella typhimurium. J. Bacteriol. 1996;178:2572–2579. doi: 10.1128/jb.178.9.2572-2579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G., Klauck E., Hengge-Aronis R. Regulation of RpoS proteolysis in Escherichia coli: The response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl. Acad. Sci. 1999;96:6439–6444. doi: 10.1073/pnas.96.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G., Klauck E., Hengge-Aronis R. The response regulator RssB, a recognition factor for σS proteolysis in Escherichia coli, can act like an anti-σS factor. Mol. Microbiol. 2000;35:657–666. doi: 10.1046/j.1365-2958.2000.01736.x. [DOI] [PubMed] [Google Scholar]
- Bouche S., Klauck E., Fischer D., Lucassen M., Jung K., Hengge-Aronis R. Regulation of RssB-dependent proteolysis in Escherichia coli: A role for acetyl phosphate in a response regulator-controlled process. Mol. Microbiol. 1998;27:787–795. doi: 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- Bougdour A., Lelong C., Geiselmann J. Crl, a low temperature-induced protein in Escherichia coli that binds directly to the stationary phase σ subunit of RNA polymerase. J. Biol. Chem. 2004;279:19540–19550. doi: 10.1074/jbc.M314145200. [DOI] [PubMed] [Google Scholar]
- Checroun C., Gutierrez C. σS-Dependent regulation of yehZYXW, which encodes a putative osmoprotectant ABC transporter of Escherichia coli. FEMS Microbiol. Lett. 2004;236:221–226. doi: 10.1016/j.femsle.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Court D.L., Swaminathan S., Yu D., Wilson H., Baker T., Bubunenko M., Sawitzke J., Sharan S.K. Mini-λ: A tractable system for chromosome and BAC engineering. Gene. 2003;315:63–69. doi: 10.1016/s0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Damerau K., St John A.C. Role of Clp protease subunits in degradation of carbon starvation proteins in Escherichia coli. J. Bacteriol. 1993;175:53–63. doi: 10.1128/jb.175.1.53-63.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza C., Nakano M.M., Zuber P. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc. Natl. Acad. Sci. 1994;91:9397–9401. doi: 10.1073/pnas.91.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C.M., Grossman A.D., Sauer R.T. Cytoplasmic degradation of ssrA-tagged proteins. Mol. Microbiol. 2005;57:1750–1761. doi: 10.1111/j.1365-2958.2005.04798.x. [DOI] [PubMed] [Google Scholar]
- Frenkiel-Krispin D., Levin-Zaidman S., Shimoni E., Wolf S.G., Wachtel E.J., Arad T., Finkel S.E., Kolter R., Minsky A. Regulated phase transitions of bacterial chromatin: A non-enzymatic pathway for generic DNA protection. EMBO J. 2001;20:1184–1191. doi: 10.1093/emboj/20.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K.E., Silhavy T.J. SprE levels are growth phase regulated in a σS-dependent manner at the level of translation. J. Bacteriol. 2000;182:4117–4120. doi: 10.1128/jb.182.14.4117-4120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- Gottesman S. The small RNA regulators of Escherichia coli: Roles and mechanisms. Annu. Rev. Microbiol. 2004;58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- Grimaud R., Kessel M., Beuron F., Steven A.C., Maurizi M.R. Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J. Biol. Chem. 1998;273:12476–12481. doi: 10.1074/jbc.273.20.12476. [DOI] [PubMed] [Google Scholar]
- Guillier M., Gottesman S. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol. Microbiol. 2006;59:231–247. doi: 10.1111/j.1365-2958.2005.04929.x. [DOI] [PubMed] [Google Scholar]
- Hamoen L.W., Eshuis H., Jongbloed J., Venema G., van Sinderen D. A small gene, designated comS, located within the coding region of the fourth amino acid-activation domain of srfA, is required for competence development in Bacillus subtilis. Mol. Microbiol. 1995;15:55–63. doi: 10.1111/j.1365-2958.1995.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Hamoen L.W., Venema G., Kuipers O.P. Controlling competence in Bacillus subtilis: Shared use of regulators. Microbiol. 2003;149:9–17. doi: 10.1099/mic.0.26003-0. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Back to log phase: σS as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 2000;54:499–518. doi: 10.1146/annurev.micro.54.1.499. [DOI] [PubMed] [Google Scholar]
- Jenal U., Hengge-Aronis R. Regulation by proteolysis in bacterial cells. Curr. Opin. Microbiol. 2003;6:163–172. doi: 10.1016/s1369-5274(03)00029-8. [DOI] [PubMed] [Google Scholar]
- Kandror O., DeLeon A., Goldberg A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. 2002;99:9727–9732. doi: 10.1073/pnas.142314099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.I., Burton R.E., Burton B.M., Sauer R.T., Baker T.A. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol. Cell. 2000;5:639–648. doi: 10.1016/s1097-2765(00)80243-9. [DOI] [PubMed] [Google Scholar]
- Lacour S., Landini P. σS-Dependent gene expression at the onset of stationary phase in Escherichia coli: Function of σS-dependent genes and identification of their promoter sequences. J. Bacteriol. 2004;186:7186–7195. doi: 10.1128/JB.186.21.7186-7195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. The cellular concentration of the σS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes & Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- Loewen P.C., Hengge-Aronis R. The role of the σ factor σS (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- Loewen P.C., Hu B., Strutinsky J., Sparling R. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 1998;44:707–717. doi: 10.1139/cjm-44-8-707. [DOI] [PubMed] [Google Scholar]
- Lutz R., Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Chen S., Murrow J., St. John K., Gottesman S. Regulation of RpoS by a novel small RNA: The characterization of RprA. Mol. Microbiol. 2001;39:1382–1394. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- Mandel M.J., Silhavy T.J. Starvation for different nutrients in Escherichia coli results in differential modulation of RpoS levels and stability. J. Bacteriol. 2005;187:434–442. doi: 10.1128/JB.187.2.434-442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M.R., Thompson M.W., Singh S.K., Kim S.H. Endopeptidase Clp: ATP-dependent Clp protease from Escherichia coli. Methods Enzymol. 1994;244:314–331. doi: 10.1016/0076-6879(94)44025-5. [DOI] [PubMed] [Google Scholar]
- McCann M.P., Fraley C.D., Matin A. The putative σ factor KatF is regulated posttranscriptionally during carbon starvation. J. Bacteriol. 1993;175:2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika F., Hengge R. A two-component phosphotransfer network involving ArcB, ArcA, and RssB coordinates synthesis and proteolysis of σS (RpoS) in E. coli. Genes & Dev. 2005;19:2770–2781. doi: 10.1101/gad.353705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffler A., Fischer D., Altuvia S., Storz G., Hengge-Aronis R. The response regulator RssB controls stability of the σS subunit of RNA polymerase in Escherichia coli. EMBO J. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.H., Jensen D.B., Thompson N.E., Gentry D.R., Burgess R.R. In vitro functional characterization of overproduced Escherichia coli katF/rpoS gene product. Biochemistry. 1993;32:11112–11117. doi: 10.1021/bi00092a021. [DOI] [PubMed] [Google Scholar]
- Ogura M., Liu L., Lacelle M., Nakano M.M., Zuber P. Mutational analysis of ComS: Evidence for the interaction of ComS and MecA in the regulation of competence development in Bacillus subtilis. Mol. Microbiol. 1999;32:799–812. doi: 10.1046/j.1365-2958.1999.01399.x. [DOI] [PubMed] [Google Scholar]
- Persuh M., Turgay K., Mandic-Mulec I., Dubnau D. The N- and C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol. Microbiol. 1999;33:886–894. doi: 10.1046/j.1365-2958.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- Peterson C.N., Ruiz N., Silhavy T.J. RpoS proteolysis is regulated by a mechanism that does not require the SprE (RssB) response regulator phosphorylation site. J. Bacteriol. 2004;186:7403–7410. doi: 10.1128/JB.186.21.7403-7410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L.A., Silhavy T.J. The response regulator SprE controls the stability of RpoS. Proc. Natl. Acad. Sci. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L.A., Silhavy T.J. Crl stimulates RpoS activity during stationary phase. Mol. Microbiol. 1998;29:1225–1236. doi: 10.1046/j.1365-2958.1998.01007.x. [DOI] [PubMed] [Google Scholar]
- Pruteanu M., Hengge-Aronis R. The cellular level of the recognition factor RssB is rate-limiting for σS proteolysis: Implications for RssB regulation and signal transduction in σS turnover in Escherichia coli. Mol. Microbiol. 2002;45:1701–1713. doi: 10.1046/j.1365-2958.2002.03123.x. [DOI] [PubMed] [Google Scholar]
- Ruiz N., Silhavy T.J. Constitutive activation of the Escherichia coli Pho regulon upregulates rpoS translation in an Hfq-dependent fashion. J. Bacteriol. 2003;185:5984–5992. doi: 10.1128/JB.185.20.5984-5992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N., Peterson C.N., Silhavy T.J. RpoS-dependent transcriptional control of sprE: Regulatory feedback loop. J. Bacteriol. 2001;183:5974–5981. doi: 10.1128/JB.183.20.5974-5981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder T., Lee K.H., Lomovskaya O., Matin A. Regulation of Escherichia coli starvation σ factor (σS) by ClpXP protease. J. Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini D.M., Schellhorn H.E. Endonuclease III and endonuclease IV protect Escherichia coli from the lethal and mutagenic effects of near-UV irradiation. Can. J. Microbiol. 1999;45:632–637. [PubMed] [Google Scholar]
- Sharma S., Hoskins J.R., Wickner S. Binding and degradation of heterodimeric substrates by ClpAP and ClpXP. J. Biol. Chem. 2005;280:5449–5455. doi: 10.1074/jbc.M412411200. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Grimaud R., Hoskins J.R., Wickner S., Maurizi M.R. Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc. Natl. Acad. Sci. 2000;97:8898–8903. doi: 10.1073/pnas.97.16.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes N.R., Murray H.D., Subramaniam C., Gourse R.L., Louis P., Bartlett W., Miller S., Booth I.R. A role for mechanosensitive channels in survival of stationary phase: Regulation of channel expression by RpoS. Proc. Natl. Acad. Sci. 2003;100:15959–15964. doi: 10.1073/pnas.2536607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Takayanagi Y., Fujita N., Ishihama A., Takahashi H. Heterogeneity of the principal σ factor in Escherichia coli: The rpoS gene product, σ 38, is a second principal σ factor of RNA polymerase in stationary-phase Escherichia coli. Proc. Natl. Acad. Sci. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner N.P., Yagil E., Spira B. A differential effect of σS on the expression of the PHO regulon genes of Escherichia coli. Microbiol. 2004;150:2985–2992. doi: 10.1099/mic.0.27124-0. [DOI] [PubMed] [Google Scholar]
- Turgay K., Hamoen L.W., Venema G., Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes & Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- Turgay K., Hahn J., Burghoorn J., Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt N.D., Newitt J.A., Bernstein H.D. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- Volkert M.R., Hajec L.I., Matijasevic Z., Fang F.C., Prince R. Induction of the Escherichia coli aidB gene under oxygen-limiting conditions requires a functional rpoS (katF) gene. J. Bacteriol. 1994;176:7638–7645. doi: 10.1128/jb.176.24.7638-7645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B.L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt R.C.I.F.C., et al., editors. Escherichia coli and Salmonella. American Society for Microbiology Press; Washington, DC: 1996. pp. 1357–1381. [Google Scholar]
- Wolf S.G., Frenkiel D., Arad T., Finkel S.E., Kolter R., Minsky A. DNA protection by stress-induced biocrystallization. Nature. 1999;400:83–85. doi: 10.1038/21918. [DOI] [PubMed] [Google Scholar]
- Yamashino T., Ueguchi C., Mizuno T. Quantitative control of the stationary phase-specific σ factor, σS, in Escherichia coli: Involvement of the nucleoid protein H-NS. EMBO J. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Ellis H.M., Lee E.C., Jenkins N.A., Copeland N.G., Court D.L. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgurskaya H.I., Keyhan M., Matin A. The σS level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol. Microbiol. 1997;24:643–651. doi: 10.1046/j.1365-2958.1997.3961742.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Gottesman S. Regulation of proteolysis of the stationary-phase σ factor RpoS. J. Bacteriol. 1998;180:1154–1158. doi: 10.1128/jb.180.5.1154-1158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Gottesman S., Hoskins J.R., Maurizi M.R., Wickner S. The RssB response regulator directly targets σS for degradation by ClpXP. Genes & Dev. 2001;15:627–637. doi: 10.1101/gad.864401. [DOI] [PMC free article] [PubMed] [Google Scholar]