Abstract
Three interferon-gamma (IFNG) induced anti-viral pathways have been reported. Involved anti-viral proteins include: Mx, RNaseL/2'-5'-OAS, and PKR. Involvement of OAS and PKR in IFNG-induced anti-HSV-1 pathways has not been previously reported, but IFNG induces OAS and PKR when other viruses invade the nervous system. The aim of the current study was to determine whether the absence of intact OAS and PKR anti-viral pathways affect the anti-viral activity of IFNG during acute HSV-1 infection within trigeminal ganglia (TG). To investigate this, primary TG cultures were established using TGs removed from C57BL/6 (Wt), RNase L knockout, and RNase L/PKR double knockout mice. Each dissociated TG was transduced with an adenoviral vector containing an IFNG transgene or vector alone. Viral titers following HSV-1 infection of primary TG cell cultures were determined. Significant differences in viral titer for Ad:Null versus Ad:IFNG tranduced TGs were found in each genotype. However, the effectiveness of Ad:IFNG was not reduced in the absence of both OAS and PKR pathways or OAS alone. Recombinant IFNG also exhibited anti-HSV-1 activity. The effectiveness of the IFNG transgene was lost in primary TG cells from IFNG receptor knockout mice. The data suggest that novel anti-HSV-1 mechanisms are induced by IFNG.
INTRODUCTION
Interferon gamma (IFNG) is an anti-viral cytokine that is induced by antigenic stimuli, such as the presence of viral antigens.(1) It is released into the extracellular environment by immune system cells including: natural killer cells, CD4+ and CD8+ T cells.(2, 3) Following exocytosis, IFNG binds to an extracellular domain of the interferon gamma receptor 1 subunit (IFNGR1) that is part of a multi-component IFNGR complex.(4, 5) Activation of an IFNGR leads to signal transduction events whose downstream outcomes curtail the replication of neurotropic viruses, such as HSV-1, at the initial cutaneous site of inoculation as well as in associated sensory ganglia.(6, 7) Much detail about the specific mechanism by which IFNG interferes with viral replication within sensory ganglia has not yet been determined.
Following acute HSV-1 infection of mouse cornea, HSV-1 enters sensory nerve endings within the basal aspect of corneal epithelium.(8) From there, HSV-1 undergoes retrograde axonal transport to the trigeminal ganglia (TG).(9) Viral antigens are expressed within TG as soon as 2 days p.i.(10) During acute/lytic HSV-1 infection of TG, IFNG is prominently produced in vivo in response to HSV-1 infection (10) where it has been associated with a reduction in viral replication. Known means of viral inhibition include the ability to prime the complex dsRNA induced anti-viral pathways as well as induction of type I IFN mRNA expression, leading to activation of IFN stimulated gene (ISG) expression.(11)
Indirectly, IFNG is suggested to regulate more than 200 genes. This factor combined with the fact that IFNG immune responses are viral strain(12) and tissue-specific underscores the importance of identifying specific ISGs, which are involved in IFNG anti-HSV activity on a tissue-specific basis. The goal of the current study was to determine whether oligoadenylate synthetases (OAS) and protein kinase R (PKR) anti-viral pathways are involved in the anti-viral activity of IFNG during acute HSV-1 infection of TG. As previously mentioned, IFNG has been shown to be important during acute HSV-1 infection in vivo within TG. However, no studies have examined whether IFNG induces anti-viral pathways in vitro in TG, in the absence of an intact immune system.
PKR and OAS are ISGs with proven anti-viral activity,(11) but cells lacking OAS and PKR can still mount an anti-viral response,(13) indicating that other yet-to-be determined anti-viral pathways are activated by IFNs. Furthermore, the IFNG immune response is not dependent upon an intact type I IFN immune response, so the known ability of type I IFNs to induce OAS and PKR does not necessitate involvement of these anti-viral pathways in the effectiveness of IFNG against acute HSV-1 infection. We did not find evidence that the effectiveness of IFNG is lost in the absence of intact OAS or OAS and PKR pathways in primary TG cultures.
MATERIALS AND METHODS
Animals
Animal treatment was consistent with the National Institute of Health Guidelines on the Care and Use of Laboratory Animals. All procedures were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute institutional animal care and use committees. C57BL/6 (wt) and IFNG receptor deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME). RNase L-null (RL−/−) and RNase L/protein kinase R double knockout mice (RL/PKR −/−) mice were generated with a wt background as previously described.(14, 15) Mice (ages 6-8 wks) were anesthetized by intraperitoneal injection with xylazine (2 mg/ml; 6.6 mg/kg) and ketamine (30 mg/ml; 100 mg/kg and perfused with saline prior to removal of TGs for establishment of dissociated TG cultures.
Establishment of dissociated TG cultures
TGs were aseptically removed following perfusion. Dissociated cell suspensions were prepared by digestion in 1 mg/ml each of collagenase type IV and XI, Sigma-Aldrich, Co., Saint Louis, MO (Sigma) for 20-30 min at 37°C, with trituration every 10 min. Digested cells were diluted in TG medium [RPMI-1640 containing 10 ng/ml nerve growth factor 2.5s (Collaborative Biomedical Products, Bedford, MA), antimycotic/antibiotic solution, and 10% fetal bovine serum (FBS)], as described(16) and subsequently pelleted by centrifugation for 5 min × 200 g. Medium was discarded, and cells were washed again. TG cells were resuspended in TG medium and transferred into wells of a 24-well plate, pre-coated with collagen and laminin, as described.(16)
Transduction and infection of dissociated TG cells
In 3 separate experiments (not shown), it was determined that 1×105 cells were present in each dissociated TG well at 5-7 days after establishing primary TG cultures. At this time, cells were transduced with empty adenovirus vector (Ad:Null) or adenovirus expressing the murine IFN-γ transgene (Ad:IFNG) at 0.1, 0.5, 1.0, or 5.0 transducing units. At 24 h post-transduction, supernatant was collected to determine IFNG levels, using a DuoSet mouse IFNG ELISA kit (R&D Systems, Minneapolis, MN). Medium containing the adenoviral transducing agent was replaced with medium containing HSV-1 at an MOI of 0.1, 0.5, 1.0, or 5.0 and incubated for 1 h. HSV-1 containing medium was then discarded and replaced with fresh medium for an additional 23 h incubation. Cells were subjected to one freeze thaw cycle and the resulting supernatants were clarified by centrifugation (10,000 g × 2 min).
Cells
All cell culture reagents were obtained from Gibco (Grand Island, NY). Vero (African green monkey kidney) cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640, supplemented with 10% FBS, 2% antibiotic/antimycotic, and 0.2% gentamycin. Vero cells were plated in 96-well flat bottom plates (50,000 cells/well), and incubated at 37°C in an atmosphere of 5% CO2 and 95% humidity.
Determination of HSV-1 titer
Serially diluted samples were incubated on Vero cell (ATCC) monolayers for 1 h in 96-well microtiter plates. Supernatants were then discarded and replaced with a 100 μl overlay of RPMI-1640 containing 10% FBS, antibiotic/antimycotic, and 0.5% methylcellulose. Cultures were incubated at 37°C in 5% CO2 and 95% humidity for an additional 31 h. The amount of infectious virus has been reported as mean log plaque forming units (PFU) per TG.
Reverse transcription and real time PCR
Twenty-four h following transduction with an empty adenoviral vector (Ad:Null) or an adenoviral vector containing the INFG transgene (Ad:IFNG), RNA was isolated from dissociated TG cells using an Ultraspec RNA Isolation protocol from Biotecx, Laboratories, Inc. (Houston, TX). Standard reverse transcription was performed on 1 μg of RNA, using an oligo dT primer (Promega, Madison, WI). Real-time PCR was performed using a Bio-Rad iCycler [Bio-Rad Laboratories, Inc. (Bio-Rad), Hercules, CA]. Each reaction was prepared with iQ SYBR Green Super mix (Bio-Rad) at a final concentration of 1x, cDNA template from the reverse transcription diluted 1:10, and 150—200 nM primer in a final volume of 45μl. The conditions for all primers included an initial denaturing step for 3 min at 95°C followed by 40 cycles at 95°C for 30s and annealing/elongation at 60°C for 50s. Gene expression levels of OAS and PKR were determined by the comparative CT method, calculated by normalization to the mean gene expression of two housekeeping genes: β-actin and GAPDH. The following primers were employed: (1). Murine (mu)OAS1a: F: 5' – CTT TGA TGT CCT GGG TCA TGT-3'; R: GCT CCG TGA AGC AGG TAG AG-3'; (2) muPKR: F: GAT GGA AAA TCC CGA ACA AGG AG-3'; R: 5'-AGG CCC AAA GCA AAG ATG TCC AC-3'(3) muGAPDH: F: 5'-CCC CAA TGT GTC CGT CGT G-3'; R: 5'-GCC TGC TTC ACC ACC TTC T-3' (4) mu β-actin: F: 5'- AGA GAG GTA TCC TGA CCC TGA -3'; R: 5'-CAC GCA GCT CAT TGT AGA AGG-3'
Statistics
All statistical analyses were performed with the GBSTAT program (Dynamic Microsystems, Silver Spring, MD). Specific tests that were used to determine statistical significance were Student's t-test (comparing data from two samples) or one-way analysis of variance and Scheffe multiple comparisons (comparing data from more than two samples).
RESULTS
We have previously shown that an adenoviral vector containing a green fluorescent protein construct transduces primary TG cells in a dose-dependent fashion.(17) Furthermore, neurons are the cells most receptive to transduction using transducing units up to 5.0.(17)
Production of IFNG by primary TG cells transduced with Ad:IFNG
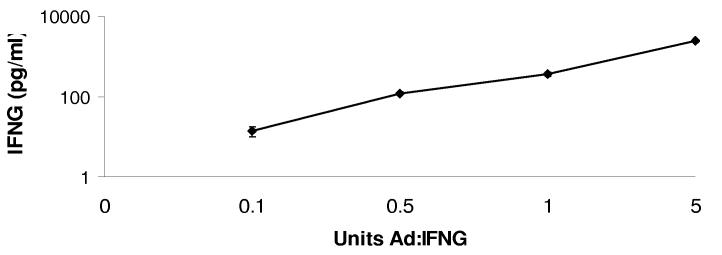
We utilized an adenoviral vector expressing an IFNG transgene to examine the mechanism of IFNG activity against HSV-1 infection in TG. Ad:IFNG transduced TG cells secreted IFNG in a dose-dependent fashion (Fig. 1). IFNG was not detected in supernatants collected from Ad:Null transduced TG cultures (not shown).
FIGURE 1.
Dose-dependent production of IFNG following transduction with Ad:IFNG. Primary TG cell cultures were transduced with 0, 0.1, 0.5, 1.0, and 5.0 U of Ad:IFNG . Twenty four hr post-transduction, the level of IFNG in cell-free culture supernatants was determined by ELISA. The data represent cumulative mean IFNG pg/ml ± SEM from 3-5 experiments. Each sample was collected from one dissociated TG.
Effectiveness of IFNG against acute HSV-1 infection in dissociated TG cells
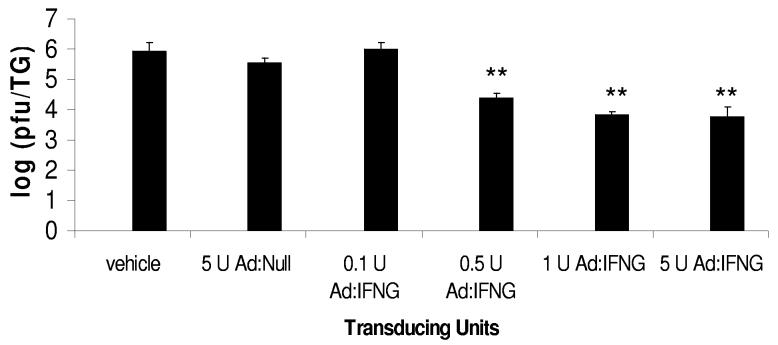
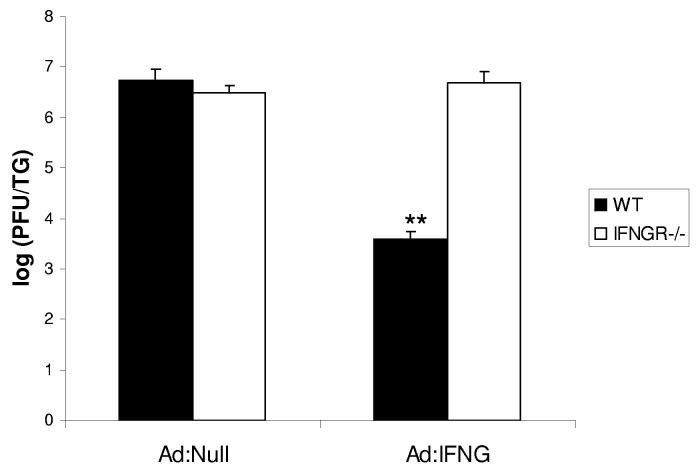
To determine the effectiveness of Ad:IFNG against HSV-1 replication, dissociated TG cells from wt mice were transduced with Ad:IFNG at 24 h prior to infection with HSV-1. Resistance to HSV-1 infection with IFNG production was determined by virus titer. Transduction with 0.5, 1.0, and 5.0 U Ad:IFNG was associated with significant reductions in HSV-1 infection, compared to transduction with 5.0 U Ad:Null or 0.1 U Ad:IFNG (Fig. 2). There was no significant difference in the virus yield in non-transduced cultures and those transduced with Ad:Null (data not shown). Similar to the Ad:IFNG transduction of primary TG cells, pre-exposure of TG cells to recombinant IFNG (1,000-24,000 pg/ml) was found to suppress HSV-1 replication by 3 logs (data not shown). To determine the specificity of the effect, primary TG cells from wt and IFNG receptor deficient mice were prepared and transduced with Ad:Null or Ad:IFNG. In the absence of the IFNG receptor, the resistance to HSV-1 infection by Ad:IFNG transduced primary TG cells was lost (Fig. 3).
FIGURE 2.
Ad:IFNG transduced TG cells are less susceptible to HSV-1 infection. Primary TG cells were transduced with 0.1, 0.5, 1.0, or 5.0 U Ad:IFNG or Ad:Null. Twenty four hr post-transduction, the cells were infected with HSV-1 (MOI = 1.0). Twenty four hr post-infection, viral titers were determined in the supernatant of each culture. The data represent cumulative means ± SEM from 3 separate experiments (n = 7), **p < 0.01) comparing the Ad:IFNG transduced cultures (0.5-5.0 transducing units) to the Ad:Null transduced cultures.
FIGURE 3.
Expression of IFNG receptor is required for resistance to HSV-1 infection following Ad:IFNG transduction. Primary TG cells obtained from wt and IFNG receptor deficient mice were collected and cultures established. The cells were transduced with 1.0 unit of Ad:Null or Ad:IFNG and 24 hr post-transduction, infected with HSV-1 (MOI = 1.0). Viral titers were determined 24 hr post infection. The data represent cumulative means +/− SEM from 2 separate experiments, n=6/group), **p<.01 comparing the Ad:IFNG to Ad:Null for wt TG cell cultures.
Absence of PKR and OAS pathways did impact anti-HSV-1 effectiveness of Ad:IFNG
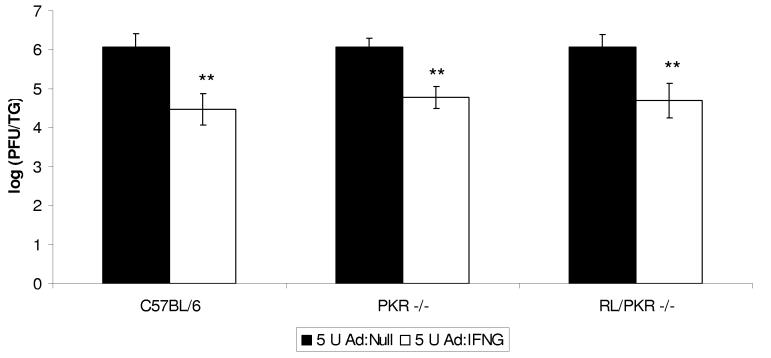
PKR has been found to be involved in IFNG-mediated suppression of coxsackievirus but not varicella-zoster virus replication.(18,19) Therefore, we investigated the potential involvement of both PKR and another IFN-responsive pathway, OAS, in the Ad:IFNG-mediated resistance to HSV-1 infection of primary TG cells. TG cells transduced with 1.0 U Ad;IFNG responded with a 3-4 fold increase in PKR and OAS expression compared to Ad:Null-transduced cells as measured by real time PCR. Based on these results, viral titers were compared in TG cells from wt (control), RL −/−, and RL/PKR −/− mice following transduction with Ad:IFNG. In each genotype, significant differences were observed when TG cells were transduced with 5.0 U Ad:Null versus 5.0 U Ad:IFNG prior to infection with 1.0 MOI HSV-1 (Fig. 4). When mean ratios in titer (5 U Ad:Null to 5 U Ad:IFNG) were compared, similar differences were found in each genotype. In particular, for 2 separate experiments (n = 4-6), mean viral titer ratios for 1 MOI HSV-1 infected C57BL/6, RL −/−, and RL/PKR −/− TG cells were 1.39 ± 0.07, 1.34 ± 0.16; and 1.23 ± 0.02, respectively.
FIGURE 4.
the Absence of a functional OAS or PKR pathway does not attenuate the resistance to HSV-1 infection in Ad:IFNG transduced TG cells. Primary TG cells obtained from wt (C57BL/6), RNase L deficient (RL−/−), and PKR and RNase L deficient (PKR/RL−/−) mice were collected and cultures established. The cells were transduced with 1.0 unit of Ad:Null or Ad:IFNG and 24 hr post transduction, infected with HSV-1 (MOI = 0.1). Viral titers were determined 24 hr post infection. The data represent cumulative means ± SEM from 2 separate experiments (n = 4-6/group), **p < 0.01 comparing the Ad:IFNG to Ad:Null – transduced cultures for each genotype.
Since the sensitivity of the assay could be compromised using a relatively high infectious dose, the same experiments were performed with 1.0 U Ad:Null and 1.0 U Ad:IFNG followed by infection with 0.1 MOI HSV-1. Again, significant differences in titer comparing transductions with 1.0 U Ad:IFNG versus 1.0 U Ad:Null were found for all genotypes . Mean ratios of viral titers, comparing 1 U Ad:Null to 1 U Ad:IFNG, for wt (2.06 ± 0.1), RL −/− (1.97 ± 0.14), and RL/PKR −/− TG cells (2.962 ± 0.24) were again similar.
DISCUSSION
. In the current study, the anti-viral effect of IFNG was not diminished in the absence of a functional OAS or PKR pathway. However, it does not eliminate the possibility that other downstream effects of the OAS pathway may play a role, such as mechanisms associated with protective and anti-proliferative properties including JNK pathway activation and activation of apoptosis.(20, 21) However, RNase L −/− mice have previously been shown to have a reduced capacity for the activation of anti-apoptotic(20) or JNK pathways(21) which would seem to negate the involvement of these pathways in the anti-viral effect elicited by Ad:IFNG transduction. In the present study, the induction of OAS and PKR mRNA was elevated 3-4 fold following Ad:IFNG transduction. By comparison, a previous study reported a 12- to 25-fold induction of PKR and OAS mRNA expression respectively following transduction with an adenovirus vector expressing the murine IFN-β transgene.(22) Therefore, it is likely that the involvement of OAS and PKR the present study is subtle in comparison to other known mediators activated by IFNG such as nitric oxide.(23)
ACKNOWLEDGMENTS
The authors would like to thank Todd Wuest and Lisa Tomanek for their technical help. This work was supported by USPHS grants AI053108 (DJJC), AI34039 (BRGW) and CA44059 (RHS). Additional support included NEI core grant EY12190.
REFERENCES
- 1.SAMUEL CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BACH EA, AGUET M, SCHREIBER RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–91. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 3.FARRAR M, SCHREIBER R. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 4.RASHIDBAIGI A, KUNG HF, PESTKA S. Characterization of receptors for immune interferon in U937 cells with 32P-labeled human recombinant immune interferon. J Biol Chem. 1985;260(14):8514–9. [PubMed] [Google Scholar]
- 5.KOTENKO SV, IZOTOVA LS, POLLACK BP, MARIANO TM, DONNELLY RJ, MUTHUKUMARAN G, COOK JR, GAROTTA G, SILVENNOINEN O, IHLE JN. Interaction between the components of the interferon gamma receptor complex. J Biol Chem. 1995;270(36):20915–21. doi: 10.1074/jbc.270.36.20915. [DOI] [PubMed] [Google Scholar]
- 6.CANTIN EM, HINTON DR, CHEN J, OPENSHAW H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69(8):4898–905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HILL TJ, FIELD HJ, BLYTH WA. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying recurrent disease. J Gen Virol. 1975;28:341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- 8.OHARA PT, CHIN MS, LAVAIL JH. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J Virol. 2000;74(10):4776–86. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BEARER EL, BREAKEFIELD XO, SCHUBACK D, REESE TS, LAVAIL JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci U S A. 2000;97(14):8146–50. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LIU T, TANG Q, HENDRICKS RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70(1):264–71. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COSTA-PEREIRA AP, WILLIAMS TM, STROBL B, WATLING D, BRISCOE J, KERR IM. The antiviral response to gamma interferon. J Virol. 2002;76(18):9060–8. doi: 10.1128/JVI.76.18.9060-9068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CHESLER DA, REISS CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13(6):441–54. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 13.ZHOU A, PARANJAPE JM, DER SD, WILLIAMS BR, SILVERMAN RH. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258(2):435–40. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 14.ZHOU A, PARANJAPE J, BROWN TL, NIE H, NAIK S, DONG B, CHANG A, TRAPP B, FAIRCHILD R, COLMENARES C, SILVERMAN RH. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. Embo J. 1997;16(21):6355–63. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.YANG YL, REIS LF, PAVLOVIC J, AGUZZI A, SCHAFER R, KUMAR A, WILLIAMS BR, AGUET M, WEISSMANN C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. Embo J. 1995;14(24):6095–106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HALFORD WP, GEBHARDT BM, CARR DJ. Mechanisms of herpes simplex virus type 1 reactivation. J Virol. 1996;70(8):5051–60. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CORRIAS MV, GRIBAUDO G, GUARNACCIA F, PONZONI M. Induction of 2.5 OAS gene expression and activity is not sufficient for IFN-gamma-induced neuroblastoma cell differentiation. Int J Cancer. 1995;62(2):223–9. doi: 10.1002/ijc.2910620219. [DOI] [PubMed] [Google Scholar]
- 18.DESLOGES N, RAHAUS M, WOLFF MH. Role of the protein kinase PKR in the inhibition of varicella-zoster virus replication by beta interferon and gamma interferon. J Gen Virol. 2005;86:1–6. doi: 10.1099/vir.0.80466-0. [DOI] [PubMed] [Google Scholar]
- 19.FLODSTROM-TULLBERG M, HULTCRANTZ M, STOTLAND A, MADAY A, TSAI D, FINE C, WILLIAMS B, SILVERMAN R, SARVETNICK RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol. 2005;174:1171–1177. doi: 10.4049/jimmunol.174.3.1171. [DOI] [PubMed] [Google Scholar]
- 20.XIANG Y, WANG Z, MURAKAMI J, PLUMMER S, KLEIN EA, CARPTEN JD, TRENT JM, ISAACS WB, CASEY G, SILVERMAN RH. Effects of RNase L mutations associated with prostate cancer on apoptosis induced by 2′,5′-oligoadenylates. Cancer Res. 2003;63(20):6795–801. [PubMed] [Google Scholar]
- 21.LI G, XIANG Y, SABAPATHY K, SILVERMAN RH. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. J Biol Chem. 2004;279(2):1123–31. doi: 10.1074/jbc.M305893200. [DOI] [PubMed] [Google Scholar]
- 22.AL-KHATIB K, WILLIAMS BRG, SILVERMAN RH, HALFORD WP, CARR DJJ. The murine double-stranded RNA-dependent protein kinase PKR and the murine 2',5'-oligoadenylate synthetase-dependent RNase L are required for IFN-β-mediated resistance against herpes simplex virus type 1 in primary trigeminal ganglion culture. Virology. 2003;313:126–135. doi: 10.1016/s0042-6822(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 23.JARASCH N, MARTIN U, KAMPHAUSEN E, ZELL R, WUTZLER P, HENKE A. Interferon-gamma-induced activation of nitric oxide-mediated antiviral activity of macrophages caused by recombinant coxsachievirus B3. Viral Immunol. 2005;18:355–364. doi: 10.1089/vim.2005.18.355. [DOI] [PubMed] [Google Scholar]