Abstract
While characterizing the intestinal bacterial community of broiler chickens, we detected ɛ-proteobacterial DNA in the ilea of 3-day-old commercial broiler chicks (J. Lu, U. Idris, B. Harmon, C. Hofacre, J. J. Maurer, and M. D. Lee, Appl. Environ. Microbiol. 69:6816-6824, 2003). The sequences exhibited high levels of similarity to Campylobacter jejuni and Campylobacter coli sequences, suggesting that chickens can carry Campylobacter at a very young age. Campylobacter sp. was detected by PCR in all samples collected from the ilea of chicks that were 3 to 49 days old; however, it was detected only in the cecal contents of chickens that were at least 21 days old. In order to determine whether the presence of Campylobacter DNA in young chicks was due to ingestion of the bacteria in food or water, we obtained commercial broiler hatching eggs, which were incubated in a research facility until the chicks hatched. DNA sequencing of the amplicons resulting from Campylobacter-specific 16S PCR performed with the ileal, cecal, and yolk contents of the day-of-hatching chicks revealed that Campylobacter DNA was present before the chicks consumed food or water. The 16S rRNA sequences exhibited 99% similarity to C. jejuni and C. coli sequences and 95 to 98% similarity to sequences of other thermophilic Campylobacter species, such as C. lari and C. upsaliensis. The presence of C. coli DNA was detected by specific PCR in the samples from chicks obtained from a commercial hatchery; however, no Campylobacter was detected by culturing. In order to determine whether the same strains of bacteria were present in multiple levels of the integrator, we cultured Campylobacter sp. from a flock of broiler breeders and their 6-week-old progeny that resided on a commercial broiler farm. The broiler breeders had been given fluoroquinolone antibiotics, and we sought to determine whether the same fluoroquinolone-resistant strain was present in their progeny. The isolates were typed by pulsed-field gel electrophoresis, which confirmed that the parental and progeny flocks contained the same strain of fluoroquinolone-resistant C. coli. These data indicate that resistant C. coli can be present in multiple levels of an integrated poultry system and demonstrated that molecular techniques or more sensitive culture methods may be necessary to detect early colonization by Campylobacter in broiler chicks.
Campylobacter jejuni and C. coli have been recognized as major causes of sporadic food-borne enteritis in humans (40). Recently, workers have focused on this pathogen because of the emergence of antibiotic-resistant Campylobacter isolates that cause disease in humans (38, 42). The Centers for Disease Control and Prevention have estimated that 2 million persons were infected with Campylobacter in the United States from 1996 to 1999 (33). Food-borne illnesses are a major public health concern, and many of these illnesses may be linked to the consumption of poultry products. Raw poultry meat is considered to be an important vector of Campylobacter (13, 33). Some reports indicate that 80% of broiler chickens that are between 5 and 7 weeks old can carry C. jejuni or C. coli in the intestinal tract (29, 32).
Many studies have demonstrated that horizontal transmission is the major route of Campylobacter colonization of poultry because the same strain can be shown to colonize multiple flocks on a farm (29, 32). However, some investigations have detected egg-associated bacteria, suggesting that vertical transmission could occur (1, 6, 11, 27, 36). Fertile chicken eggs can be infected experimentally with C. jejuni (6), and the organism can be recovered from the inner membranes of the eggs and egg contents (35). Chuma et al. detected C. jejuni in the cecal contents of newly hatched chicks using DNA-DNA hybridization (5). Several recent reports have shown that C. jejuni can colonize the oviducts of laying hens and turkeys (3, 4, 7, 11, 17). Campylobacter spp. have also been detected in semen samples of commercial broiler breeder roosters and turkey toms, which provides an opportunity for venereal infection of hens (7, 10, 18).
In a characterization of the bacterial community of the broiler chicken intestine, we detected ɛ-proteobacterial 16S rRNA sequences in the ilea of 3-day-old chicks (23). The majority of the clones in the intestinal 16S rRNA library were most similar to the sequence of C. coli 16S rRNA. In the current study, we detected the same strain of fluoroquinolone-resistant C. coli in a flock of commercial broiler breeders and their progeny and obtained molecular evidence that Campylobacter can be present in day-of-hatching chicks.
MATERIALS AND METHODS
Sample collection.
Freshly voided cecal droppings were collected from a flock house (∼22,000 Ross/Cobb hybrid chickens per house) on each of three commercial broiler farms (farms CF-1, CF-2, and CF-3); two flocks were sampled from each farm. These poultry farms had contracted to raise broiler chickens for the same poultry company. Most houses were sampled when the birds were 3 and 6 weeks old; however, flock 1 on farm CF-2 was also sampled when the chickens were 5 and 7 weeks old, before and after oxytetracycline treatment at 6.5 weeks. In addition, the birds on farm CF-3 were given sarafloxacin when they were 5 weeks old; the approved dosage was 20 ppm in the drinking water for 5 days. Farms CF-2 and CF-3 had an antibiotic usage history of sarafloxacin and oxytetracycline administration in the drinking water during the year before sampling, and farm CF-1 had not used therapeutic antibiotics for more than 1 year. For each sampling, approximately 120 cecal droppings were collected from the top of the chicken litter using sterile cotton-tipped swabs. The 120 swabs were pooled in 30 tubes, each of which contained 1 ml of brain heart infusion broth, and were placed on ice until they were cultured.
Through information obtained by our clinicians at the Poultry Diagnostic and Research Center, we located a commercial broiler breeder (parental) flock that had been treated with a poultry fluoroquinolone (Saraflox; Abbott Laboratories, Abbott Park, IL). The broiler breeder farm had contracted with the same poultry company as our three broiler chicken farms. One week after antibiotic administration, we collected 120 cecal droppings from this flock. Cecal droppings were also collected from a flock of broilers that were hatched from eggs from the breeders. Samples were placed in brain heart infusion broth, cooled on ice, and transported to the laboratory for culture.
Ten embryonating eggs were obtained from the treated breeder flock and incubated in a research hatching cabinet until the chicks emerged. Ten chicks from the same breeder flock were also obtained from the commercial broiler hatchery before they were placed on a farm. None of the chicks had access to food or water before they were sacrificed. The chicks were sacrificed on the day of hatching by CO2 asphyxiation. The surface of the necropsy table and the chick surfaces were disinfected with 70% ethanol, and the ileum, cecum, and yolk sacs were aseptically removed using sterile instruments. A separate set of sterile instruments was used for each chick. Ileum, cecum, and yolk contents from each set of chicks were pooled in sterile tubes for culture and DNA extraction.
Campylobacter culture.
Culturing from the cecal droppings of commercial broiler flocks and the broiler breeder flock was performed as follows. The contents of the 30 tubes containing pooled cecal droppings were pooled into 10 tubes and diluted with saline. Campylobacter spp. were isolated from 10−1 and 10−2 dilutions by membrane filtration (12) by placing sterile cellulose acetate membrane filters (pore size, 0.45 μm; diameter, 25 mm) on the surfaces of blood agar plates. One hundred microliters of a diluted sample was carefully placed on top of a filter, avoiding spillage around the edges of the filter, and the plates were incubated at room temperature for 30 min until the liquid was absorbed by the agar. The filters were removed using sterile tweezers, and the plates were placed in Ziploc bags that were flushed with a microaerophilic gas mixture (10% CO2, 5% O2, 85% N2). The plates were incubated at 37°C and observed for 72 h for growth. Thirty isolated Campylobacter-like colonies (gray, watery) were selected in order to detect resistant phenotypes with a flock prevalence of 5% or greater. The colonies were randomly selected from the 10 plates, streaked for isolation, placed in freezer medium (15% glycerol, 1% peptone), and stored at −80°C. However, in order to enhance detection of a resistant strain of C. coli in the breeder flock, blood agar containing 1 μg/ml of ciprofloxacin was used to culture Campylobacter from the broiler flock.
Pooled samples from chick ileum, cecum, and yolk contents were directly plated on blood agar using the direct filtration method described above in order to avoid the use of selective agar media that may inhibit some Campylobacter species or isolates (12, 37). In addition, Campylobacter culturing from the pooled samples obtained from day-of-hatching chicks was done using enrichment broth containing 0.6% yeast extract or tryptic soy broth containing 0.6% yeast extract as described by Moore (25). Duplicate enrichment tubes were incubated in one of two microaerophilic gas mixtures as described by Engberg et al. (12); one of these gas mixtures was hydrogen enriched (6% CO2, 6% O2, 85%N2, 3% H2), and the other was not (10% CO2, 5% O2, 85% N2). The enrichment broth preparations were loosely capped, placed in a rack that was placed in a Ziploc bag, flushed with gas, and incubated with gentle shaking at 37°C. After incubation for 24 h, 30 μl from each tube was streaked on blood agar plates.
Susceptibility testing for C. jejuni was performed by the agar dilution method as described by the National Committee for Clinical Laboratory Standards (26), using C. jejuni ATCC 33560 as the quality control organism.
Molecular detection of Campylobacter.
Detection of C. jejuni or C. coli in the Campylobacter isolates cultured from the broiler breeder and commercial broiler flocks was performed by species-specific PCR as described by Gonzales et al. (14). Templates from the isolates were prepared as described by Woods et al. (43). PCR detection of Campylobacter in community DNA samples acquired from a previous study (23) was performed using Campylobacter-specific 16S rRNA primers (22). PCR detection of Campylobacter in day-of-hatching chick ileum, cecum, and yolk samples was performed using Campylobacter-specific 16S rRNA primers (22) or species-specific Campylobacter primers (14). C. jejuni ATCC 33560 and a chicken isolate of C. coli (M1-19), whose identity was confirmed by biochemical testing, were used as positive controls. The template from day-of-hatching chicks was isolated as follows. The bacterial fraction was recovered from the enrichment broth by centrifugation (5,000 × g for 6 min) at room temperature. DNA was extracted using a Mo Bio kit (Mo Bio Laboratories Inc., California). In order to determine the distribution of Campylobacter species in the chick ileum, cecum, and yolk samples, the Campylobacter-specific 16S rRNA amplicons were cloned and the DNA was sequenced as previously described (23). The resulting sequences were analyzed by BLAST algorithms (www.ncbi.nlm.nih.gov) and were used to search the GenBank database in order to determine similarities to known species of bacteria. Phylogenetic relatedness among 16S rRNA sequences was evaluated by neighbor-joining analysis of a distance matrix obtained from a multiple-sequence alignment.
Molecular typing.
The Campylobacter isolates were initially typed by the randomly amplified polymorphic DNA (RAPD) method, using primer HWL85 as described by Zimmer et al. (44). Isolates from the broiler breeder flock and commercial flock that produced the same RAPD pattern were further typed using pulsed-field gel electrophoresis (PFGE). PFGE was performed as follows. Isolates were grown as confluent lawns on blood agar under microaerophilic conditions. Bacterial cells were then harvested by scraping each plate with an inoculating loop and suspended in 2 ml of phosphate-buffered saline. Cells were pelleted by centrifugation at 18,000 × g for 2 min and then suspended in 1.2 ml PIV buffer (10 mM Tris [pH 7.4], 1 M NaCl). Subsequent steps were performed as described by Barrett et al. (2). The agarose-embedded bacterial genomic DNA was digested with 30 U of SmaI or KpnI by incubation overnight at 25°C for SmaI and at 37°C for KpnI. DNA fragments were separated by PFGE in a 1.2% agarose gel using a CHEF DR-II electrophoresis unit (Bio-Rad, Hercules, California). Electrophoresis was performed for 25 h at 14°C and 200 V with a linearly ramped pulse time of 5 to 30 s. Saccharomyces cerevisiae DNA was used as the molecular weight marker (Bio Whittaker Molecular Applications, Rockland, ME).
Nucleotide sequence accession numbers.
Representative sequences have been deposited in the GenBank database under accession numbers DQ057348 to DQ057352.
RESULTS
Detection of fluoroquinolone-resistant Campylobacter from untreated commercial broiler flocks.
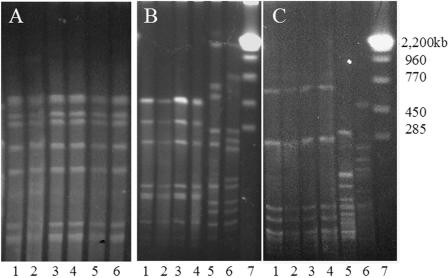
Table 1 shows the distribution of ciprofloxacin susceptibility among Campylobacter isolates cultured from commercial broiler chickens. Sixty percent of the C. jejuni isolates and 23.5% of the C. coli isolates exhibited MICs of ciprofloxacin of ≥4 μg/ml. Since ciprofloxacin breakpoints have not been established for Campylobacter species, we utilized the ≥4-μg/ml level used by the National Antimicrobial Resistance Monitoring System (http://ars.usda.gov/SP2UserFiles/Place/66120508/NARMS/animal_campy/campy2003histogram.pdf; accessed 9 May 2005) to categorize Campylobacter isolates as resistant. Ciprofloxacin-resistant Campylobacter isolates were detected in four of the six flocks, although only one flock had been treated with a fluoroquinolone. Two of the farms had a recent history of fluoroquinolone usage, but on farm CF-1, from which we isolated ciprofloxacin-resistant C. jejuni and C. coli, therapeutic antibiotics had not been used within 1 year of our sampling and fluoroquinolones had not been used in the flock house that was sampled. However, all of the farms had contracts with the same poultry integrator, suggesting that the ciprofloxacin-resistant Campylobacter isolates could have originated from a common source. In order to investigate whether a common Campylobacter strain was present on all three broiler chicken farms, isolates from each sampling were typed using RAPD-PCR. Several Campylobacter isolates from different farms produced similar RAPD fingerprints (data not shown), and these isolates were compared further using PFGE. Figure 1A shows the results of the PFGE typing for the Campylobacter isolates cultured from the second flock from farms CF-1 and CF-3. These Campylobacter isolates produced the same SmaI PFGE pattern, indicating that the same strain may be present on multiple broiler chicken farms. This finding suggests that the Campylobacter strain present on these broiler chicken farms may have come from a common source at the hatchery or in the breeder flock.
TABLE 1.
Fluoroquinolone susceptibility (MIC90) of C. jejuni and C. coli cultured from commercial broiler chicken flocks at various agesa
| Farmb | Ciprofloxacin MIC90 (μg/ml) (no. of isolates)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Flock 1
|
Flock 2
|
|||||||
| 3-5 wks
|
6-7 wks
|
3 wks
|
6 wks
|
|||||
| C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | |
| CF-1 | NDc | ND | 8 (24) | 8 (2) | 0.25 (30) | ND | 2 (25) | 2 (5) |
| CF-2 | 0.25 (30) | ND | 0.5 (58) | 0.5 (2) | 16 (21) | 16 (9) | 16 (30) | ND |
| CF-3 | ND | ND | 4 (7)d | 16 (2)d | 0.125 (22) | 8 (8) | 16 (26) | 16 (4) |
C. jejuni (273 isolates) and C. coli (31 isolates) were cultured from the fresh cecal droppings of two consecutive flocks of commercial broiler chickens located on three geographically separated farms. Fluoroquinolone susceptibility was determined by agar dilution. All of the isolates from the same sampling exhibited the same MIC.
Campylobacter was cultured from most flocks when the birds were 3 and 6 weeks old; the exception was flock 1 on farm CF-2, from which Campylobacter was cultured when the birds were 5, 6, and 7 weeks old.
ND, no bacteria detected by culturing.
The birds were given sarafloxacin (20 ppm for 5 days) in their drinking water when they were 5 weeks old. Campylobacter isolates were cultured after this treatment.
FIG. 1.
Strain typing of C. jejuni isolates by PFGE. (A) Isolates from two different commercial broiler farms. Lanes 1 to 3 contained isolates from farm CF-3, and lanes 4 to 6 contained isolates from farm CF-1. Genomic DNA was digested with SmaI. (B and C) SmaI (B) and KpnI (C) typing of C. coli isolated from a broiler breeder flock and the commercial broiler progeny of this flock. Lanes 1 and 2 contained C. coli isolated from breeder parental chickens, lanes 3 and 4 contained C. coli isolated from their 6-week-old progeny, lanes 5 and 6 contained C. jejuni isolated from the progeny broilers, and lane 7 contained S. cerevisiae molecular weight markers.
Detection of fluoroquinolone-resistant Campylobacter coli in a broiler breeder flock and the commercial broiler chicken progeny of this flock.
We cultured isolates of C. jejuni and C. coli from a flock of broiler breeder chickens that had been treated with a poultry fluoroquinolone antibiotic 1 week prior to sampling. The C. jejuni isolates exhibited ciprofloxacin MICs of <0.25 μg/ml; however, the MIC for the C. coli isolates was 16 μg/ml. The C. coli isolates were typed by RAPD-PCR in order to determine the diversity of strains that were cultured. All of the C. coli isolates produced the same RAPD DNA pattern (data not shown).
In order to investigate whether the ciprofloxacin-resistant C. coli strain had also colonized broiler chicks originating from the parental flock, we sampled a flock of 6-week-old broiler chickens that were hatched from the treated breeder flock. Four C. coli isolates were detected in the commercial broiler flock on plates containing ciprofloxacin. RAPD-PCR typing indicated that the breeder flock and the descendants of this flock were colonized with the same strain of C. coli (data not shown), and definitive confirmation was obtained by PFGE using two restriction enzymes (Fig. 1B and C). The C. coli isolates from the broiler flock produced the same PFGE fingerprints that the isolates from the breeders produced. In addition, antimicrobial susceptibility testing confirmed that the C. coli isolates from the broilers exhibited drug susceptibility patterns identical to those exhibited by isolates from the breeder flock; the ciprofloxacin and sarafloxacin MIC90 was 16 μg/ml, the enrofloxacin MIC90 was 8 μg/ml, and the tetracycline MIC90 was 64 to 128 μg/ml. These findings indicate that the same antibiotic-resistant C. coli strain can be found in both parental and progeny flocks.
Detection of Campylobacter in day-of-hatching chicks.
In order to investigate whether the chicks contained Campylobacter prior to placement on the farm, we received day-of-hatching chicks that were obtained from eggs laid by the fluoroquinolone-treated breeder flock. In addition, to investigate whether hatchery contamination contributed to Campylobacter colonization, we also hatched eggs from this flock in a sanitized research hatching cabinet at our facility. None of these chicks had access to food or water prior to euthanasia and sampling.
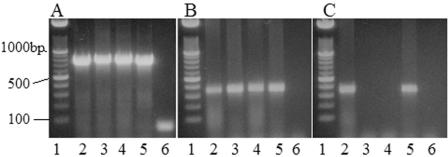
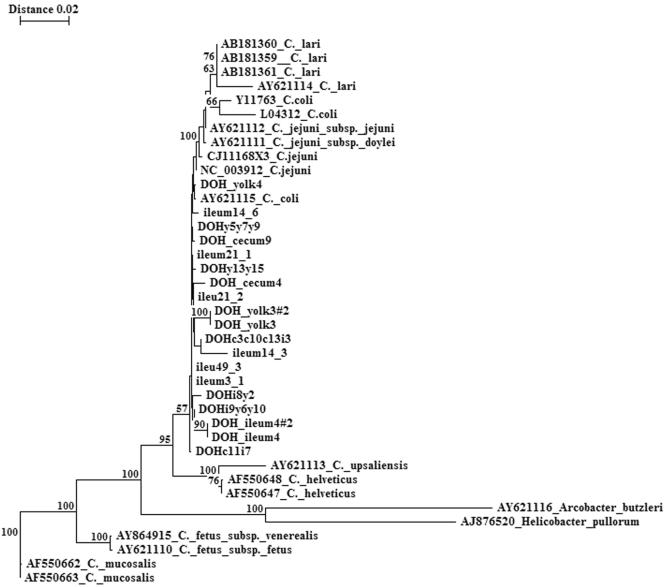
We aseptically removed yolk and intestinal contents from the day-of-hatching chicks for culture and molecular detection of Campylobacter. No campylobacter-like colonies were detected by culturing any of the samples from these chicks. However, PCR demonstrated the presence of Campylobacter DNA in the ileal, cecal, and yolk contents of chicks hatched in the research hatching cabinet (Fig. 2A). C. coli-specific PCR indicated the presence of C. coli (Fig. 2B), but C. jejuni amplicons were not detected (data not shown). Likewise, C. coli DNA was also detected in the yolk of chicks obtained from the commercial hatchery (Fig. 2C). In addition, DNA sequencing of the Campylobacter-specific 16S rRNA libraries that were produced from amplification of DNA extracted from the chick intestinal and yolk contents revealed sequences that exhibited the highest levels of similarity to C. coli, C. jejuni, and C. lari (Fig. 3). These findings indicate that Campylobacter can be present in the intestine of very young chicks, but its presence may not be detected by culturing.
FIG. 2.
PCR detection of Campylobacter species present in the intestinal and yolk contents of chicks on the day of hatching. Ten embryonating eggs were obtained from a sarafloxacin-treated broiler breeder flock and hatched in a research hatching cabinet. In addition, 10 chicks from this flock were obtained from the commercial hatchery. On the day of hatching, the ileal, cecal, and yolk contents were aseptically removed, and the DNA was extracted. PCR targeting Campylobacter was performed with pooled samples, and the amplicons were separated on a 1.5% agarose gel. (A) 16S rRNA PCR results for chicks hatched in the research cabinet. Pooled yolk, ileum, and cecum samples contained Campylobacter DNA. Lane 1 contained DNA molecular weight markers, lane 2 contained C. jejuni ATCC 33560 as the positive control, lane 3 contained ileal contents, lane 4 contained cecal contents, lane 5 contained yolk contents, and lane 6 contained a control (no DNA template). (B and C) PCR results for chicks hatched in the research facility (B) and for chicks obtained from the commercial hatchery (C), obtained by using primers specific for the ceuE gene of C. coli. Pooled yolk, ileum, and cecum samples from chicks hatched in the research facility contained C. coli DNA, while only the pooled yolk samples from the commercial hatchery were positive. Lane 1, DNA molecular weight markers; lane 2, C. coli M1-19 (positive control); lane 3, ileal contents; lane 4, cecal contents; lane 5, yolk contents; lane 6, control containing no DNA template.
FIG. 3.
Phylogenetic tree showing the relatedness of chick intestinal 16S rRNA sequences to sequences of Campylobacter species. The 16S rRNA clone libraries were produced by amplification of DNA extracted from the ileum, cecum, and yolk contents of day-of-hatching chicks and from the ileal and cecal contents of chickens that were 3 to 49 days old (23). The tree was constructed by neighbor-joining analysis of a distance matrix obtained from a multiple-sequence alignment of clone library sequences and GenBank ɛ-proteobacterial 16S rRNA sequences. Bootstrap values (expressed as percentages of 100 replications) are indicated at branch points. Branches labeled with DOH represent sequences obtained from the clone library from day-of-hatching chicks; branches labeled ileum or cecum (but without DOH) represent sequences obtained from clone libraries of older chickens. Branches labeled with a specific organism's name and accession number represent sequences obtained from the GenBank database.
Detection of Campylobacter in the ileal and cecal contents of broiler chickens.
Since we detected C. coli 16S rRNA sequences in the ilea of 3-day-old chicks in a previous study (23), we used PCR to detect Campylobacter in community DNA isolated from birds at different ages. In order to evaluate the diversity of Campylobacter species detected, the amplicons were also cloned, sequenced, and compared to the 16S rRNA sequences acquired from the day-of-hatching chicks (Fig. 3). Campylobacter species were detected in the bacterial community of the ileum at all sample times; however, cecal samples were negative until the birds were 21 days old. These results indicate that Campylobacter may initially colonize the small intestine of broilers and that the colonization niche may expand with maturation of the birds. The results of our molecular ecology studies concurred with this finding and demonstrated that the cecal bacterial community was derived from the ileal community during the first 14 days after the birds hatched (23). Since many studies that investigate the epidemiology of Campylobacter on broiler farms use feces or cecal droppings for culture detection, this finding may explain why commercial flocks are often culture negative before the chickens are 3 weeks old (15, 28, 29, 30, 31).
DISCUSSION
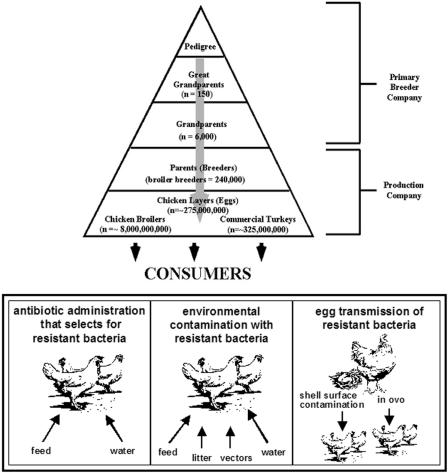
C. jejuni and C. coli are significant food-borne pathogens, and consumption of poultry is a significant risk factor for contracting disease (33, 40). Campylobacter infection can cause symptoms that persist long enough to require antimicrobial therapy; therefore, resistant strains are a significant public health concern (38, 42). Figure 4 shows the potential sources of antibiotic-resistant Campylobacter for poultry. Many studies have shown that chickens become colonized with Campylobacter present in their environment (15, 21, 28, 31), but egg transmission has been hypothesized because some genetic types that colonize broilers cannot be identified in the house environment prior to arrival of the broiler chicks (28, 30, 31). Identical Campylobacter strains have been detected in breeder and broiler flocks (9), although the results of the vast majority of studies have not supported the hypothesis that vertical transmission makes a significant contribution to the dissemination of C. jejuni on poultry farms (15, 19, 20, 21, 28, 29, 31, 32). In this study, we were able to detect C. coli DNA in the ileum, cecum, and yolk contents of chicks on the day of hatching. Since these birds had not received food or water prior to sampling, the bacteria were probably present within or on the surface of the egg at the time of hatching. Hatching eggs vary in the level of surface fecal contamination, and several studies have demonstrated that C. jejuni can be detected in the inner shell and membranes of eggs, revealing a possible route of exposure for hatching chicks (1, 11, 35). Furthermore, multiple studies have shown that C. jejuni can colonize the oviduct of hens (3, 4, 7) and can be cultured from the semen of roosters and toms (7, 10). It is known that a related species of Campylobacter, C. fetus, can be venereally transferred and cause reproductive infections in livestock (34). The infection correlates with expression of an antigenically variable group of proteins that form an S-layer on the surface of the bacterial cell (8, 16). The production of S-layers has not been described for C. jejuni or C. coli, and the published genome sequences of two human isolates of C. jejuni do not contain sag loci that code for an S-layer. However, it is not clear whether the distribution of sag genes has been investigated for animal isolates of either C. jejuni or C. coli.
FIG. 4.
(Top panel) Integrated structure of the commercial poultry production system. Production companies acquire breeder flocks from the pedigree flocks owned by three to five companies worldwide. These breeder flocks produce hatching eggs that become meat birds and layers for human consumption. (Lower panel) Potential sources of antibiotic-resistant bacteria for poultry. While antibiotic use may select for resistant populations (left box), young chicks lacking a stable intestinal bacterial community may be more susceptible to colonization with resistant strains present in the hatchery or on the farm (middle box). Exposure of hatchlings to resistant bacteria present on the surface of the egg can potentially result in dissemination of resistant strains from the breeder flock to progeny (right box).
In our study, a common source of Campylobacter was revealed by culture detection of a ciprofloxacin-resistant C. coli strain that was isolated from both progeny broilers and the parent flock. This strain could have been acquired by horizontal transmission from a common environmental source. However, Pearson et al. (31) postulated that there was low-level vertical transmission of C. jejuni based on detection of the same serotypes in the hatchery and in the broilers examined. These strains could be masked by the more prevalent strains already present in the broiler house environment. It was through a fortuitous opportunity that we had a selectable marker, ciprofloxacin resistance, that may have enhanced our ability to isolate the C. coli described in this study. Most epidemiology studies utilize culturing as the primary mechanism of detection of C. jejuni. However, many selective agar media used for isolation may not consistently detect all Campylobacter isolates (37). In our study, we used several enrichment techniques, including selective filtration rather than selective agar, to isolate Campylobacter from young chicks. This approach was not successful for detecting viable Campylobacter in the intestine or yolk of day-of-hatching chicks. Our inability to isolate Campylobacter colonies might have been due to low numbers present in the chicks, although the medium and incubation conditions may not have been appropriate for culturing from these environments. Campylobacter can exist in a viable but nonculturable form in water (41), which can be detected by molecular methods (24). Stern et al. (39) demonstrated that viable but nonculturable cells colonize chickens. Molecular detection in chicks, supported by culture detection later in the life of the flock, allowed us to demonstrate that C. coli could be present in the chicks at placement. Furthermore, these findings indicate that integrator sources must be considered when workers evaluate management practices in order to reduce Campylobacter colonization of broiler chickens and, specifically, that antibiotic usage in the upper levels of the poultry production pyramid might affect the prevalence and dissemination of drug-resistant Campylobacter among meat birds. It may be necessary to examine poultry production at all levels to better understand the impact of antibiotic use on the emergence of drug-resistant pathogens like Salmonella and Campylobacter. Further investigations to identify critical control points for limiting the spread of drug-resistant food-borne pathogens in this important food source are warranted.
Acknowledgments
We thank the poultry integrators and operators who participated in this study.
This work was funded by a contract from the Food and Drug Administration.
REFERENCES
- 1.Allen, K. J., and M. W. Griffiths. 2001. Use of luminescent Campylobacter jejuni ATCC 33291 to assess eggshell colonization and penetration in fresh and retail eggs. J. Food Prot. 64:2058-2062. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, T. J., H. Loir, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhr, R. J., N. A. Cox, N. J. Stern, M. T. Musgrove, J. L. Wilson, and K. L. Hiett. 2002. Recovery of Campylobacter from segments of the reproductive tract of broiler breeder hens. Avian Dis. 46:919-924. [DOI] [PubMed] [Google Scholar]
- 4.Camarda, A., D. G. Newel, R. Nasti, and G. Diamond. 2000. Genotypying Campylobacter jejuni strains isolated from the gut and oviduct of laying hens. Avian Dis. 44:907-912. [PubMed] [Google Scholar]
- 5.Chuma, T., T. Yamada, K. Yano, K. Okamoto, and H. Yugi. 1994. A survey of Campylobacter jejuni in broilers from assignment to slaughter using DNA-DNA hybridization. J. Vet. Med. Sci. 56:697-700. [DOI] [PubMed] [Google Scholar]
- 6.Clark, A. G., and D. H. Bueschkens. 1985. Laboratory infection of chicken eggs with Campylobacter jejuni by using temperature or pressure differentials. Appl. Environ. Microbiol. 49:1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, K., A. M. Donoghue, P. J. Blore, and D. J. Donoghue. 2004. Isolation and prevalence of Campylobacter in the reproductive tracts and semen of commercial turkeys. Avian Dis. 48:625-630. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil, L. B., G. G. Schurig, P. J. Bier, and A. J. Winter. 1975. Bovine venereal vibriosis: antigenic variation of the bacterium during infection. Infect. Immun. 11:240-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, N. A., N. J. Stern, K. L. Hiett, and M. E. Berrang. 2002. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 64:535-541. [DOI] [PubMed] [Google Scholar]
- 10.Cox, N. A., N. J. Stern, J. L Wilson, M. T. Musgrove, R. J. Buhr, and K. L. Hiett. 2002. Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Dis. 46:717-720. [DOI] [PubMed] [Google Scholar]
- 11.Doyle, M. P. 1984. Association of Campylobacter jejuni with laying hens and eggs. Appl. Environ. Microbiol. 47:533-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engberg, J., L. W. Stephen, C. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genigeorgis, C., M. Hassunch, and P. Collins. 1986. Campylobacter jejuni infection on poultry farms and its effect on poultry meat contamination during slaughtering. J. Food Prot. 49:895-903. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales, I., K. A. Grant, P. T. Richardson, S. F. Park, and M. D. Collins. 1997. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J. Clin. Microbiol. 35:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory, E., H. Barnhart, D. W Dreesen, N. J. Stern, and J. L. Corn. 1997. Epidemiological study of Campylobacter species in broilers: source, time of colonization and prevalence. Avian Dis. 41:890-898. [PubMed] [Google Scholar]
- 16.Grogono-Thomas, R., J. Dworkin, M. J. Blaser, and D. G. Newell. 2000. Roles of the surface layer proteins of Campylobacter fetus subsp. fetus in ovine abortion. Infect. Immun. 68:1687-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiett, K. L., N. A. Cox, R. J. Buhr, and N. J. Stern. 2002. Genotype analyses of Campylobacter isolated from distinct segments of the reproductive tracts of broiler breeder hens. Curr. Microbiol. 45:400-404. [DOI] [PubMed] [Google Scholar]
- 18.Hiett, K. L., G. R. Siragusa, N. A. Cox, R. J. Buhr, M. T. Musgrove, N. J. Stern, and J. L. Wilson. 2003. Genotype analyses of Campylobacter isolated from the gastrointestinal tracts and the reproductive tracts of broiler breeder roosters. Avian Dis. 47:406-414. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs-Reitsma, W. 1995. Campylobacter in breeder flocks. Avian Dis. 39:355-359. [PubMed] [Google Scholar]
- 20.Jacobs-Reitsma, W., C. Becht, T. De Vries, J. Van der Plas, B. Duim, and J. Wagenaar. 2001. No evidence for vertical transmission of Campylobacter in a study on Dutch breeder and broiler farms. Int. J. Med. Microbiol. 291:39. [Google Scholar]
- 21.Jones, F. T., R. C. Axtell, D. V. Rives, S. E. Schneideler, F. R Tarver, Jr., L. R. Walker, and M. J. Wineland. 1991. A survey of Campylobacter jejuni contamination in modern broiler production system. J. Food Prot. 54:259-262. [DOI] [PubMed] [Google Scholar]
- 22.Linton, D., R. J. Owen, and J. Stanley. 1996. Rapid identification by PCR of the genus Campylobacter and five Campylobacter species enteropathogenic for man and animal. Res. Microbiol. 147:707-718. [DOI] [PubMed] [Google Scholar]
- 23.Lu, J., U. Idris, B. Harmon, C. Hofacre, J. J. Maurer, and M. D. Lee. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 69:6816-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, J., P. Caldwell, and B. Millar. 2001. Molecular detection of Campylobacter spp. in drinking, recreational and environmental water supplies. Int. J. Hyg. Environ. Health 204:185-189. [DOI] [PubMed] [Google Scholar]
- 25.Moore, J. E. 2000. Comparison of basal broth media for the optimal laboratory recovery of Campylobacter jejuni and Campylobacter coli. Isr. J. Med. Sci. 169:187-189. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., approved standard. NCCLS document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.Neill, S., J. Campbell, and J. O'Brien. 1985. Egg penetration by Campylobacter jejuni. Avian Dis. 14:313-320. [DOI] [PubMed] [Google Scholar]
- 28.Nesbit, E. G., P. Gibbs, D. W. Dreesen, and M. D. Lee. 2001. Epidemiologic features of Campylobacter jejuni isolated from poultry broiler houses and surrounding environments determined by use of molecular strain typing. Am. J. Vet. Res. 62:190-194. [DOI] [PubMed] [Google Scholar]
- 29.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne, R. E., M. D. Lee, D. W. Dreesen, and H. M. Barnhart. 1999. Molecular epidemiology of Campylobacter jejuni in broiler flocks using randomly amplified polymorphic DNA-PCR and 23S rRNA-PCR and role of litter in its transmission. Appl. Environ. Microbiol. 65:260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson, A. D., M. Greenwood, R. Kevin, A. Feltham, T. D. Healing, J. Donaldson, D. M. Jones, and R. R. Colwell. 1996. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl. Environ. Microbiol. 62:4614-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin, O., T. Y. Morishita, and Q. Zhang. 2002. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 3:95-105. [DOI] [PubMed] [Google Scholar]
- 33.Samuel, M. C., D. J. Vugia, S. Shallow, R. Marcus, S. Segler, T. McGivern, H. Kassenborg, K. Reilly, M. Kennedy, F. Angulo, R. V. Tauxe, and the Emerging Infections Program FoodNet Working Group. 2004. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999. Clin. Infect. Dis. 38:S165-S174. [DOI] [PubMed] [Google Scholar]
- 34.Scott, P. R. 1994. Control of venereal campylobacteriosis in a beef herd. Vet. Rec. 135:162-163. [DOI] [PubMed] [Google Scholar]
- 35.Shane, S. M., D. H. Gilford, and K. Yogasundram. 1986. Campylobacter jejuni contamination of eggs. Vet. Res. Commun. 10:487-492. [DOI] [PubMed] [Google Scholar]
- 36.Shanker, S., A. Lee, and T. C. Sorrell. 1986. Campylobacter jejuni in broilers: the role of vertical transmission. J. Hyg. 96:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silley, P. 2003. Campylobacter and fluoroquinolones: a biased data set? Environ Microbiol. 5:219-230. [DOI] [PubMed] [Google Scholar]
- 38.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, and M. T. Osterholm. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1531. [DOI] [PubMed] [Google Scholar]
- 39.Stern, N. J., D. M. Jones, L. V. Wedley, and D. M. Rollins. 1994. Colonization of chicks by non-culturable Campylobacter spp. Lett. Appl. Microbiol. 18:333-336. [Google Scholar]
- 40.Tauxe, R. 1992. Epidemiology of Campylobacter jejuni infection in the United States and other industrialized nations, p. 1-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 41.Tholozan, J. L., J. M. Cappelier, J. P. Tissier, G. Delattre, and M. Federighi. 1999. Physiological characterization of viable but nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travers, K., and M. Barza. 2002. Morbidity of infections caused by antimicrobial-resistant bacteria. Clin. Infect. Dis. 34:S131-S134. [DOI] [PubMed] [Google Scholar]
- 43.Woods, C. R., J. Versalovic, T. Koeuth, and J. R. Lupski. 1993. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J. Clin. Microbiol. 31:1927-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmer, M., H. Barnhart, U. Idris, and M. D. Lee. 2003. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 47:101-107. [DOI] [PubMed] [Google Scholar]