Abstract
Saccharophagus degradans 2-40 (formerly Microbulbifer degradans 2-40) is a marine gamma-subgroup proteobacterium capable of degrading many complex polysaccharides, such as agar. While several agarolytic systems have been characterized biochemically, the genetics of agarolytic systems have been only partially determined. By use of genomic, proteomic, and genetic approaches, the components of the S. degradans 2-40 agarolytic system were identified. Five agarases were identified in the S. degradans 2-40 genome. Aga50A and Aga50D include GH50 domains. Aga86C and Aga86E contain GH86 domains, whereas Aga16B carries a GH16 domain. Novel family 6 carbohydrate binding modules (CBM6) were identified in Aga16B and Aga86E. Aga86C has an amino-terminal acylation site, suggesting that it is surface associated. Aga16B, Aga86C, and Aga86E were detected by mass spectrometry in agarolytic fractions obtained from culture filtrates of agar-grown cells. Deletion analysis revealed that aga50A and aga86E were essential for the metabolism of agarose. Aga16B was shown to endolytically degrade agarose to release neoagarotetraose, similarly to a β-agarase I, whereas Aga86E was demonstrated to exolytically degrade agarose to form neoagarobiose. The agarolytic system of S. degradans 2-40 is thus predicted to be composed of a secreted endo-acting GH16-dependent depolymerase, a surface-associated GH50-dependent depolymerase, an exo-acting GH86-dependent agarase, and an α-neoagarobiose hydrolase to release galactose from agarose.
Saccharophagus degradans 2-40 (formerly Microbulbifer degradans 2-40) is a rod-shaped, aerobic, marine bacterium isolated from the surface of decomposing saltwater cord grass, Spartina alterniflora, in the lower Chesapeake Bay (3). S. degradans 2-40 is related to a group of marine γ-subgroup proteobacteria capable of degrading complex polysaccharides (CPs) (14, 16), a critical function in the marine food web. S. degradans 2-40 is unique among these bacteria due to its ability to utilize CPs of algal, higher plant, fungal, and animal origins, such as agar, alginate, cellulose, chitin, β-glucan, laminarin, pectin, pullulan, starch, and xylan, as sole carbon and energy sources (3, 15, 20, 23). The mechanism by which this bacterium degrades these normally recalcitrant substrates has been established only for the chitinolytic system (20).
Agar, a cell wall constituent of many red algae (Rhodophyta), exists in nature as a mixture of unsubstituted and substituted agarose polymers that form an agarocolloid gel (10, 12). Agarose is composed of repeating neoagarobiose units (3-6-anhydro-l-galactose-α1-3-d-galactose) joined by β1-4 bonds that form a helix in aqueous environments. The galactose moieties of the repeating neoagarobiose units can be methylated, pyruvated, sulfonated, or glycosylated to form various substituted derivatives with different gelling and solubility characteristics. Up to 70% of the algal cell wall can be agar polymers. The remaining material consists of other galactans and embedded xylan and cellulose microfibrils. There are numerous applications of agar and its enzymatically derived by-products.
Agarolytic organisms are common, but comparatively few agarase systems have been characterized. Agar-degrading organisms were first reported by Gran in 1902 (42). Since then, at least 30 bacteria with this capacity have been identified. The vast majority of these bacteria are marine isolates belonging to the following genera: Agarivorans (28), Alterococcus (37), Alteromonas (34), Cytophaga (45, 47), Microbulbifer (29, 31), Microscilla (50), Pseudoalteromonas (7, 25, 26, 36), Pseudomonas (17, 22), Vibrio (4, 5, 39-41), and Zobellia (2, 6). Agarase activity has also been observed in bacteria isolated from terrestrial environments, such as Paenibacillus spp. (19, 46) and Streptomyces coelicolor (8).
Each of these organisms is thought to degrade agar by using one of two biochemical pathways that employ a variety of secreted agarases. Most known agarolytic bacteria use secreted β-agarases to cleave agarose initially at the β1,4 linkages between neoagarobiose units. In this pathway, β-agarase I is thought to endolytically degrade agarose to neoagarooligosaccharides, with neoagarotetraose as the smallest product (25, 26). These neoagarooligosaccharides appear to be degraded further by a β-agarase II to yield neoagarohexaose, neoagarotetraose, and neoagarobiose (25, 26). This enzyme may have both endolytic and exolytic activities. A neoagarobiose hydrolase then cleaves neoagarobiose to its constituent monosaccharides. A few bacterial species employ an α-agarase pathway in which the α1,3 linkage within neoagarobiose units is cleaved initially. For example, Alteromonas agarlyticus secretes a depolymerizing agarase that yields agarotetraose (34). While the activities of these enzymes have been demonstrated and some have been purified, the nucleotide sequences of comparatively few agarase genes have been determined. GH16, GH50, and GH86 domains have been reported to be present in β-agarases and GH96 domains in α-agarases (http://www.cazy.org/CAZY/). There is one report of a partial amino-terminal sequence of an α-neoagarooligosaccharide hydrolase (38).
S. degradans 2-40 is capable of rapid growth on agarose as the sole carbon source, degrading agar nearly twice as quickly as Pseudoalteromonas atlantica, and appears to produce multiple agarases (48). The mechanism by which S. degradans 2-40 degrades agar is thought to involve a β-agarase system (48). Recently, the genome sequence of S. degradans 2-40 was completed to enable the application of genomic approaches to the characterization of this agarolytic system. In this study, traditional genomic library screens and protein expression coupled with bioinformatic analysis of the genome sequence and proteomics were utilized to identify five agarases encoded by S. degradans 2-40. Several of the agarases were found to have unusual structural features, such as multiple family 6 carbohydrate binding modules (CBM6). Deletion analysis was used to confirm selected components of the system. A model for the agarolytic system of S. degradans 2-40 is proposed.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Saccharophagus degradans 2-40T (ATCC 43961; DSMZ17024) was grown in minimal medium containing (per liter) 2.3% Instant Ocean (Aquarium Systems, Mentor, Ohio), 0.5% ammonium chloride, and 50 mM Tris-HCl, pH 7.6. Carbon sources were added to a final concentration of 0.2%. Agar (1.5%) was added to solid media. Cultures of S. degradans 2-40 were incubated at 28°C. Escherichia coli EPI300, DH5α, and Tuner strains were grown at 37°C in Luria-Bertani (LB) broth or agar supplemented with the appropriate antibiotics. Antibiotics were added to media at the indicated concentrations (in μg/ml): ampicillin, 200; chloramphenicol, 30; and kanamycin (Kan), 50.
Molecular biology protocols.
DNA manipulations were performed using standard procedures (35). Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs (Ipswich, MA). The pETBlue-2 expression vector was purchased from Novagen (Madison, Wis.). All other reagents and substrates were obtained from Sigma-Aldrich (St. Louis, Mo.) unless otherwise noted. PCRs employed either Taq (Invitrogen, Carlsbad, CA) or ProofPro (Continental Lab Products, San Diego, CA) polymerase by use of the manufacturer's recommended conditions. The nucleotide sequences of plasmid DNA or gel-purified PCR products were obtained at the UMBI sequencing facility.
Construction and initial screen of the S. degradans genomic library.
Genomic DNA was isolated from S. degradans 2-40 by using cetyltrimethylammonium bromide (35). The chromosomal DNA was used to construct a genomic library composed of 40-kb fragments cloned into pCC1Fos (Beta version; Epicentre Technologies, Madison, WI) by following the manufacturer's recommendations. The fosmid was packaged into lambda phage and used to transfect E. coli EPI300 (Epicentre). A pitting phenotype on LB agar plates was used initially to screen Cmr transfectants for agarase activity.
Determination of the nucleotide sequences of aga50A and aga16B.
Sau3A fragments of 5 to 10 kb were ligated into BamHI-digested pUC19 and transformed into E. coli DH5α. Random pUC19 derivatives were selected, and a partial DNA sequence of the insert was obtained by using commercially available M13REV and the M13(−21) primers. The nucleotide sequences of candidate agarases were completed by primer walking using synthetic oligonucleotides (see Table S1 in the supplemental material).
Bioinformatic approaches.
Protein modules and domains were identified in deduced products by using the Simple Modular Architecture Tool (SMART; http://smart.embl-heidelberg.de/), the Pfam database (http://www.sanger.ac.uk/Software/Pfam/), and the Carbohydrate-Active enZYme database (CAZy; http://www.cazy.org/CAZY/). Similarity searches were performed using the BLAST algorithm at the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov) or surveys of the S. degradans 2-40 genome (http://genome.jgi-psf.org/cgi-bin/runAlignment?db=micde&advanced=1). Type II secretion signals were identified using the SignalP version 1.1 program (http://www.cbs.dtu.dk/services/SignalP). Molecular masses of polypeptide products were estimated using the peptide mass tool at the ExPASy server of the Swiss Institute of Bioinformatics (http://www.expasy.org). The annotated genomic sequence of S. degradans 2-40 is available at http://genome.jgi-psf.org/finished_microbes/micde/micde.home.html. Sequences were aligned using ClustalW (44) or ClustalX (43). The percent G+C content (%G+C) of whole genes and the %G+C at the third position of synonymous codons (GC3s) were calculated using CodonW (http://codonw.sourceforge.net/). Because a significant number of S. degradans 2-40 genes exhibit high similarity to genes found in diverse taxonomic units, a core set of genes was established to minimize the potential impact of horizontally acquired genes on baseline values. As approximately 20% of S. degradans 2-40 gene models exhibit similarity at the nucleotide level to a gene in a fluorescent pseudomonad (974 genes), most of which are annotated as basic metabolism and housekeeping genes, S. degradans 2-40 genes exhibiting at least 50% identity at the nucleotide level with a Pseudomonas sp. gene were used to calculate baseline %G+C, GC3s, and codon usage patterns. This core gene set included a total of 1,153,863 nucleotides (nt) of sequence data. The core gene set had a %G+C of 46.3, which is slightly higher than the total genome %G+C of 45.8% (16).
Zymograms.
The samples were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in an 8% polyacrylamide gel supplemented with 0.1% agarose. Gels were then washed twice in 20 ml PIPES-Triton buffer {20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 6.8, and 2.5% Triton X-100} for 20 min at room temperature and then incubated in PIPES-Triton buffer overnight at 4°C. Gels were washed twice with 20 ml PIPES buffer and incubated at 42°C for 2 h. Agarase activity of S. degradans 2-40 lysates was maximal at 42°C and was stable at this temperature (48). Zymograms were developed using Gram's iodine solution.
Protein expression and purification.
Genes of interest were amplified by PCR using tailed primers (see Table S1 in the supplemental material). Each fragment was digested with the designed restriction enzyme, ligated into pETBlue-2, and transformed into E. coli Tuner or E. coli DH5α cells. A 50-ml culture of each transformant carrying a clone of interest was grown at 37°C to an optical density at 600 nm of 0.5 and induced with 1 mM (final concentration) isopropyl-β-d-thiogalactopyranoside (IPTG). After growth for 4 h at 37°C, cells were harvested and frozen at −20°C. His fusions were purified from cell lysates by using Ni-nitrilotriacetic acid resin (QIAGEN, Valencia, CA) according to the manufacturer's recommendations.
Assay of agarase activity and identification of reaction products.
Reactions with purified agarase and agarose were performed in 50-μl reaction mixtures containing an aliquot of the purified His-tagged agarase in phosphate-buffered saline and 5 μl of molten 1% agarose. The reaction mixtures were incubated at 42°C and then applied to a Whatman silica gel 60A plate with a 250-μm layer. Neoagarohexaose, neoagarotetraose, neoagarobiose, and d-galactose (5 μg) were used as standards. The plates were developed with 2:1:1 N-butanol:acetic acid:water solution and stained with 2:1 ethanolic sulfuric acid:naphthoresorcinol solution (12). Degradation products were visualized by being baked at 80°C for 10 min.
Mass spectrometry.
Culture filtrates of agarose-grown cells were concentrated ∼25-fold by ultrafiltration, and protein concentrations were determined using a bicinchoninic acid protein assay (Pierce). Proteins were denatured by incubation in 100 mM Tris buffer, pH 8.5, containing 8 M urea and 10 mM dithiothreitol, and the denatured proteins were alkylated in 50 mM iodoacetate. The denatured, reduced, alkylated samples were digested overnight at 37°C by using proteomics-grade trypsin (Promega) at a 1:50 enzyme-to-substrate ratio. Digestions were stopped in 1% formic acid and analyzed by reverse-phase high-pressure liquid chromatography (HPLC)-tandem mass spectrometry (MS) at the UMCP College of Life Sciences Mass Spectrometry facility with a Waters 2960 HPLC system linked to a Finnigan LCQ tandem mass spectrometer. Alternatively, culture supernatants were fractionated on 8% SDS-PAGE gels containing 0.1% agarose and stained with SYPRO Ruby red (Molecular Probes). Regions of interest were excised and were equilibrated with 50% 200 mM ammonium bicarbonate and 50% acetonitrile. The treated samples were submitted to the Stanford University Mass Spectrometry Laboratory for analysis by electrospray ionization-quadrupole time-of-flight MS following trypsin digestion. Identification of peptide fragments employed SEQUEST and MASCOT algorithms and the amino acid sequence translations of all gene models in the S. degradans 2-40 genome as well as the nonredundant mass spectrometry database (ftp://ftp.ncbi.nlm.nih.gov/repository/MSDB/msdb.nam).
Immunoblots.
Proteins were fractionated by SDS-PAGE as described above and electroblotted onto supported nitrocellulose membranes (0.45-mm pore size; Osmotics, Trevose, PA). Membranes were blocked with 1% alkali-soluble casein (Novagen) and incubated with rabbit anti-AgaE antibodies (1:100). Membranes were washed twice and incubated with horseradish peroxidase-conjugated donkey anti-rabbit antibody (Amersham). Immunoreactive proteins were visualized using an ECL detection kit (Amersham Pharmacia Biotech).
Marked chromosomal gene replacements.
The procedures used to generate gene replacement mutants in an Acinetobacter sp. (24) were adapted for use with S. degradans 2-40. Briefly, the 1-kb regions upstream and downstream of the gene of interest were amplified from genomic DNA by PCR employing a standard design primer and a splicing primer containing 5′ tails complementary to the primers used to amplify a Kan resistance cassette. Aliquots of the upstream- and downstream-region amplicons were mixed directly with an amplified Kan cassette in a splicing PCR mixture containing both standard design primers in excess. After amplification for 30 cycles, the complete splicing PCR mixture was added to a culture of S. degradans 2-40 outgrown from stationary phase in minimal medium plus glucose for 4 h and allowed to incubate with shaking at 28°C for 2 h. Cells were harvested, washed once in outgrowth medium, and plated onto selective media containing glucose and 100 μg/ml Kan. After 3 to 4 days, cells from appearing colonies were screened in batches for gene replacements by PCR using one of the standard design primers and the correctly oriented primer specific for the kanamycin cassette. Positive colonies were screened with the opposite primer combination to confirm the insertion.
Nucleotide sequence accession numbers.
The nucleotide sequences for Aga50A, Aga16B, Aga86C, Aga50D, and Aga86E have been reported to the GenBank database under accession no. ZP_00315251, AAT67062, ZP_00315652, ZP_00315360, and ZP_00315657, respectively.
RESULTS
Predicted agarases encoded by S. degradans genome.
Relatively limited genetic tools are available for use in S. degradans 2-40, as stable transformants carrying commonly used narrow- or broad-host-range plasmids could not be isolated and traditional transposon mutagenesis strategies were ineffective. In order to identify agarase-encoding genes, E. coli EPI300 transfectants carrying a genomic library of S. degradans 2-40 created in the copy control fosmid pCC1Fos were screened on solid agar media for a pitting phenotype. Pitting colonies indicative of the hydrolysis of agar were detected at a frequency of 1.7 × 10−3 only when cells were grown under single-copy conditions for the fosmid. This was comparable to the expected frequency for a single gene in this library. A total of nine transfectants that pitted agar were identified, and one, EPI300(pNE10), was chosen for sequence analysis. All Aga+ fosmids were highly unstable in several E. coli strains, with less than 1% of transformants retaining an Aga+ phenotype in repeated plasmid isolation/transformation into fresh hosts. The Aga+ colonies were substantially smaller than those of the Aga− transformants.
Due to the phenotypic instability of the Aga+ fosmids, sequence analysis was used to identify resident agarase genes. The sequence of subcloned fragments of pNE10 revealed two open reading frames that could be involved in the degradation of agar. The first, aga50A, encoded a deduced product of a 776-amino-acid (aa) polypeptide with a predicted molecular mass of 86 kDa that was 45% identical and 62% similar to a putative secreted hydrolase from Streptomyces coelicolor A3 (2) (NP627690) and that also exhibits similarity to a β-agarase identified in Vibrio sp. strain JT0107 (S46651) (Table 1). The second open reading frame, aga16B, was predicted to encode a 593-aa product with a predicted mass of 64 kDa. The amino-terminal 278-aa region of Aga16B is 54% identical and 69% similar to a β-agarase of Pseudomonas sp. strain ND137 (BAD88713) and is 64% identical and 73% similar to a β-agarase from Microbulbifer sp. strain JAMB-A7 (BAC99022). aga50A appeared to be divergently expressed from aga16B by a shared 424-bp promoter region. Immediately downstream of aga16B is the gene for a tRNASer. A PCR screen revealed that aga50A and aga16B were present in all Aga+ E. coli EPI300 transfectants carrying the S. degradans 2-40 genomic library but were specifically absent from spontaneously arising Aga− derivatives of pNE10 (data not shown).
TABLE 1.
Properties of candidate agarases identified in the S. degradans 2-40 genome
| Gene | GenBank accession no. | Size of predicted product (kDa)a | Representative homologb | GenBank accession no. of homolog | % Sc | % Ic |
|---|---|---|---|---|---|---|
| aga50A | ZP_00315251 | 87 | β-Agarase of Vibrio sp. strain JT0107 | S46651 | 62 | 44 |
| aga16B | AAT67062 | 64 | Agarase of Pseudomonas sp. strain ND137 | BAB88713 | 54 | 69 |
| aga86C | ZP_000315652 | 86 | β-Agarase of Pseudoalteromonas atlantica | AAA25696 | 38 | 55 |
| aga50D | ZP_000315360 | 89 | β-Agarase of Vibrio sp. strain JT0107 | S46651 | 43 | 60 |
| aga86E | ZP_000315657 | 146 | β-Agarase of Microbulbifer sp. strain JAMB-A94 | BAD86832 | 60 | 72 |
As estimated by the ExPASy peptide mass tool.
As determined by a BLASTP search of the nonredundant database.
Percent similarity (% S) and percent identity (% I) as calculated by BLASTP alignment.
Upon the release of the S. degradans 2-40 genome sequence by the U.S. Department of Energy Joint Genome Institute (http://www.jgi.doe.gov), genes for three additional candidate agarases were identified by sequence similarity to known agarases. The first, aga86C, encoded a 789-aa, 86-kDa protein with a domain that shares 38% identity to a β-agarase of Pseudoalteromonas atlantica (AAA25696) and 29% identity and 45% similarity to an agarase from Microbulbifer sp. strain JAMB-A94 (BAD86832) (Table 2). Aga86C did not share obvious similarity to either Aga50A or Aga16B. A gene for another candidate agarase, aga50D, produced a 795-aa product with a predicted mass of 88.6 kDa. A portion of Aga50D exhibited 45% identity and 61% similarity to a putative secreted hydrolase from S. coelicolor (NP627690) and 43% identity and 60% similarity to an agarase from Vibrio sp. strain JT0107 (S46651). Aga50D also shared sequence similarity with a domain of the S. degradans 2-40 Aga50A. A third putative agarase gene, aga86E, yielded a 1,335-aa, 146-kDa deduced polypeptide. The carboxy-terminal 679-aa region was 30% identical and 44% similar to the β-agarase of P. atlantica and also exhibited 29% identity and 44% similarity to a domain of the S. degradans 2-40 Aga86C. A homolog to a GH96 domain characteristic of α-agarases was not apparent in the genome.
TABLE 2.
Sequence features for gene models predicted to encode agarases
| Gene(s) | Gene modela | Chromosomal addressb | %G+Cc | GC3sd | No. of amino acids with variant codon usagee |
|---|---|---|---|---|---|
| aga50A | 1176 | 1513741-1516074 | 45.3 | 38.6 | 6f |
| aga16B | 1175 | 1513481-1511685 | 52.4 | 52.6 | 6g |
| aga86C | 2650 | 3352440-3354803 | 46.8 | 44.8 | 8h |
| aga50D | 2644 | 3345735-3343354 | 46.3 | 42.9 | 1i |
| aga86E | 2655 | 3369919-3365912 | 46.1 | 37.3 | 7j |
| Core genesk | 46.3 | 42 |
Gene numbers assigned in the 15 June 2005 annotation of the genome, available at http://genome.ornl.gov/microbial/mdeg/.
Nucleotide address of the apparent coding sequence within the assembled 15 June 2005 version of the S. degradans 2-40 genome. The nucleotide address of the deduced start codon is listed first, followed by the address of the last nucleotide of the apparent stop codon.
%G+C calculated for the indicated predicted coding sequence.
%G+C in the third position of synonymous codons within the deduced coding sequence.
A two-tailed Fisher exact analysis of the codon usage for each amino acid with synonymous codons was performed, comparing codon usage within the predicted coding sequence to that within the core gene set. A P value that indicates the probability that the codon usage is the same as that for the core set was generated. The number of amino acids for which the P value was less than 0.1 is shown.
Variant codon usage was detected for Arg, Cys, Gly, His, Pro, and Ser.
Variant codon usage was detected for Asn, Asp, Gly, Phe, Ser, and Tyr.
Variant codon usage was detected for Ala, Asp, Glu, Gly, Leu, Ser, Tyr, and Val.
Variant codon usage was detected for Asp.
Variant codon usage was detected for Arg, Glu, Gly, Pro, Ser, Thr, and Val.
The core gene set represents the 974 genes with at least 50% similarity to a gene in a Pseudomonas aeruginosa PAO1 genome.
The putative agarase genes were located in two regions of the S. degradans 2-40 genome. aga50A and aga16B clustered as gene models 1176 and 1175, respectively, located between nucleotides 1511685 and 1516074, in the 15 June 2005 annotation of the S. degradans 2-40 genome (http://genome.ornl.gov/microbial/mdeg/) (Table 2). Flanking aga50A is a gene for a candidate β-galactosidase (galA), but the annotations of other nearby genes did not indicate obvious predicted roles in CP metabolism. The gene models for aga86C (gene model 2650), aga50D (gene model 2644), and aga86E (gene model 2655) loosely cluster in a 26,565-nt region beginning at nucleotide 3343354. Most intervening genes do not appear to be associated with degradation of agar or other CPs, but a candidate sugar transporter is present in this region.
Variation in %G+C and codon usage can indicate acquisition from another organism. Using simple %G+C analysis of the coding sequences, only the aga16B coding sequence differed substantially from that of the core gene set (Table 2). In contrast, the GC3s differed from that of the core set by at least 3% in aga50A, aga16B, aga86C, and aga86E, suggestive of acquisition from another organism. These genes also differed in their codon usage patterns relative to the core set of genes. Using a two-tailed Fisher exact test to statistically compare codon usage of the agarase genes to that of the chosen core gene set (33), codon usage for at least 6 of the 18 amino acids with synonymous codons had less than a 10% chance of being the same as that for the core set (Table 2). In contrast, aga50D was similar to the core set in all traits evaluated except codon usage for Asp. A survey of the nonredundant databases for nucleotide sequence similarities revealed only localized sequence similarities, located primarily within conserved domains of the agarases. Because four of five agarases present in the Microscilla sp. strain PRE1 agarolytic plasmid had extensive similarity to S. degradans 2-40 agarases, the S. degradans 2-40 genome was surveyed for other similarities with the Microscilla agarolytic plasmid. No similarities were detected at the nucleotide level between the agarolytic plasmid of Microscilla sp. strain PRE1 and the S. degradans 2-40 genome, but extensive similarities (nt 58656 to 62741) to a 3-kb region internal to the aga50D-aga86C-aga86E cluster that exhibited extensive synteny were detected (data not shown).
Modular structure of the agarases encoded by S. degradans.
Hydrolases typically carry conserved glycoside hydrolase (GH) domains that function in catalysis and sometimes also carry carbohydrate binding modules (CBM) (9). Although commonly used domain recognition algorithms, such as SMART, did not identify conserved GH domains within the predicted S. degradans 2-40 agarases, three distinct GH domains were detected when the localized regions of sequence conservation in Aga16B, Aga50A/Aga50D, and Aga86C/Aga86E were analyzed (Fig. 1). The 120-aa region of Aga16B that shares sequence similarity with seven known β-agarases (aa 31 to 289) contains a GH16 domain (see Fig. S1 in the supplemental material). The signature sequence for GH16 (E-[LIV]-D-[LIVF]-X-E-XX-[GQ]-[KRNF]-X-[PSTA]) is partially conserved in Aga16B, and both critical Glu residues are retained (21). In the agarases, the GH16 domain is located in the amino-terminal region of the polypeptide, immediately adjacent to a signal peptide. It is unclear whether this represents an orthologous relationship or a functional requirement for the domain location. The region in common between Aga50A and Aga50D (aa 350 to 769 and aa 325 to 747, respectively) is similar to the GH50 domains of the Vibrio sp. strain JT0107 agarases (see Fig. S2 in the supplemental material). A partially conserved region extends for at least 375 aa and is located in the carboxy-terminal end of the polypeptides. As mentioned before, it has not been established whether this is a functional requirement for the domain. The region conserved between Aga86C and Aga86E (aa 147 to 787 and aa 633 to 1316, respectively) shows sequence similarity to the GH86 domain of P. atlantica AgrA (see Fig. S3 in the supplemental material). This region, however, is highly divergent, with only localized regions of sequence similarity apparent.
FIG. 1.
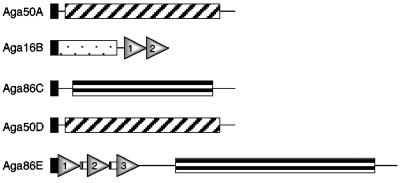
Predicted structural features of S. degradans agarases. The indicated domains were identified by sequence similarity, as shown in Fig. S1, S2, S3, and S4 in the supplemental material. Black box, type II protein secretion signal; white dotted box, GH16 domain; cross-hatched box, GH50 domain; horizontally striped box, GH86 domain; vertically striped box, repeated amino acid linker. Filled triangles indicate family 6 carbohydrate binding modules. Sizes of gene products are listed in Table 1.
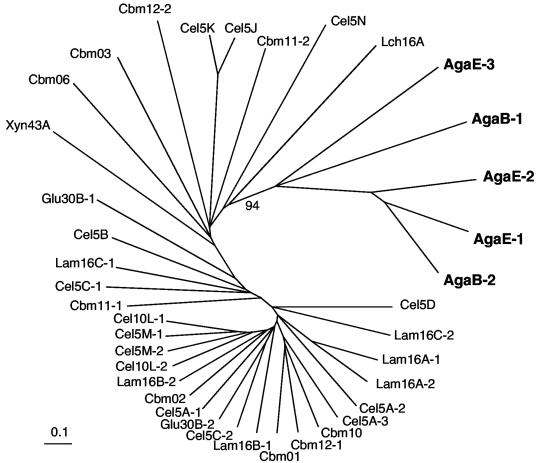
Unusual for agarases, two apparent CBM6 were identified in the carboxy-terminal region of Aga16B (CBM6-B1, aa 322 to 445, and CBM6-B2, aa 455 to 593) and three predicted CBM6 were located in the amino-terminal 650-aa region of Aga86E (CBM6-E1, aa 9 to 148, CBM6-E2, aa 162 to 302, and CBM6-E3, aa 349 to 483) (Fig. 1; also see Fig. S4 in the supplemental material). Phylogenetic analyses indicated that predicted CBM6 of the agarases are distinct from other CBM6 found in the S. degradans 2-40 genome (Fig. 2). CBM6-E1, -E2, and -B2 were most closely related to each other, whereas CBM6-E3 and -B1 formed a second group strongly supported by bootstrap analysis (see Fig. S4 in the supplemental material). A repetitive linker sequence of (P-X)17 separated the first and second CBM6 of Aga86E; the second and third CBM6 were separated by an (E-P)17 repeat. Four thrombospondin type 3 repeats were also identified between aa 511 and 643. The function of these repeats is unknown. As expected for secreted proteins, cleavable type II secretion signals were detected in the amino termini of Aga50A, Aga16B, Aga86C, Aga50D, and Aga86E. A predicted lipoprotein acylation site (11) was identified in the amino terminus of Aga86C.
FIG. 2.
Unrooted phylogenetic tree for CBM6 domains identified in the S. degradans 2-40 genome. The scale bar indicates the number of substitutions per position following alignment with MUSCLE (13) and bootstrap analysis by ClustalW (44). The tree was generated using neighbor joining and displayed with TreeView (32). The bootstrap value (out of a sampling of 100) is shown for the node linking the agarase CBM (bold). Abbreviations: Aga, known agarase; Cbm, protein containing a carbohydrate binding module; Cel, predicted endoglucanase; Glu, predicted β-glucosidase; Lam, candidate laminarinase; Lch, lichenase; Xyn, candidate xylanase.
Activities of the candidate agarases.
To determine whether the candidate agarases have the predicted activities, each was cloned into pETBlue-2 to create carboxy-terminal His6-tagged derivatives. E. coli Tuner(pLacI)(pNEaga16B1) expressing Aga16B-His rapidly pitted agar plates, which was usually apparent after overnight growth, even in the absence of induction. Upon purification of Aga16B-His, the expected 65-kDa product and an 85-kDa derivative were detected in immunoblots probed with anti-His antibodies (data not shown) as well as by mass spectrometry. Anomalous migration of S. degradans 2-40 proteins appears to be common, as several S. degradans 2-40 carbohydrases expressed in E. coli migrate at higher-than-predicted molecular masses for unknown reasons.
In order to determine the biochemical activity of Aga16B, the products of Aga16B-mediated degradation of agarose were characterized by thin-layer chromatography. Digestion of agarose by Aga16B-His released neoagarotetraose and neoagarohexaose (data not shown), but neoagarobiose could not be detected, even after long-term incubation. These results are consistent with endolytic β-agarase I-like activity. In contrast, only d-galactose was detected when agarose was digested with cell-free lysates of S. degradans 2-40, indicating the expression of additional enzymes to degrade neoagarooligosaccharides.
E. coli transformants expressing Aga86E-His also exhibited agarase activity. E. coli Tuner(pLacI)(pNEaga86E1) slowly pitted agar plates, requiring several weeks before the phenotype was evident. Spontaneously forming Aga86E-His amino-terminal truncations with masses of 100 and 86 kDa exhibited agarase activity in zymograms (Fig. 3), but the full-length 146-kDa Aga86E-His lacked activity. It was unclear whether the absence of activity at the expected molecular mass for full-length Aga86E represented a precursor state for the enzyme or the failure to renature the full-length polypeptide under the conditions of the zymogram.
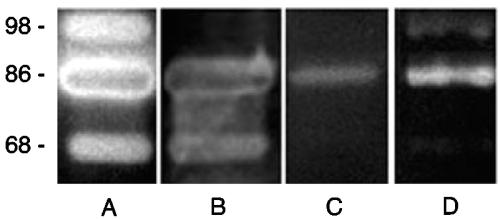
FIG. 3.
Agarase activity detected in culture filtrates of S. degradans and in lysates of E. coli strains expressing Aga16B or Aga86E. Culture filtrates of S. degradans 2-40 (A) were concentrated 50-fold by ultrafiltration and clarified by centrifugation. E. coli EPI300(pNE10) (B), E. coli Tuner(pLacI)(pNEagaB1) (C), and E. coli Tuner(pLacI)(pNEaga86E1) (D) were lysed using a BeadBeater, and lysates were clarified by centrifugation. Protein samples were fractionated in 8% polyacrylamide gels supplemented with 0.1% agarose by otherwise-standard SDS-PAGE. Gels were washed twice in PIPES-Triton buffer and incubated overnight at 4°C in the same buffer. The gels were then transferred to PIPES buffer and, after 2 h at 42°C, stained with iodine. Molecular size markers (in kilodaltons) are noted at the left of the blots.
When the products resulting from the activity of purified Aga86E-His were analyzed by thin-layer chromatography, only neoagarobiose was released from agarose by Aga86E-His digestion after extended incubation (1 to 2 days) (data not shown). This is consistent with the slow-pitting phenotype of the Tuner transfectants expressing Aga86E-His. Because only neoagarobiose appears to be released by Aga86E activity, this enzyme appears to exolytically degrade agarose similarly to a β-agarase II.
It is unusual for agarases to carry CBM6. Like other carbohydrases, the CBM6 of Aga16B and Aga86E do not appear to be required for agarase activity. Truncated His-tagged derivatives of aga16B lacking one or both CBM6 exhibited agarase activity indistinguishable from that of the full-length form (data not shown). Similarly, the spontaneously occurring amino-terminal truncations of Aga86E that are missing one or more of the resident CBM6 also retained agarase activity under laboratory conditions.
For unknown reasons, it was not possible to express Aga50A, Aga86C, and Aga50D in E. coli. The genes could be cloned into the nonexpressing E. coli strain DH5α-Ε, but attempts to transfer these clones into expressing strains, such as E. coli Tuner(DE3)(pLysS), were unsuccessful. Although some codons rarely used in E. coli were present in these genes, the expressible agarases also contained these codons at similar frequencies.
Aga16B, Aga86C, and Aga86E are expressed and secreted by S. degradans during growth on agar.
To determine if any of the demonstrated or predicted agarases are expressed during growth on agarose, clarified and concentrated culture filtrates from agarose-grown cells were surveyed by HPLC-coupled tandem MS. Fragments indicative of Aga16B, Aga86C, and Aga86E were detected in total culture filtrates of agarose-grown S. degradans 2-40 (data not shown), but several predicted cytoplasmic proteins were also detected in these samples, which limited interpretation of the data. Only Aga86E was detected during similar analyses of culture filtrates from glucose-grown cells. When specific size-classed proteins from culture filtrates were analyzed by mass spectrometry, fragments consistent with the presence of Aga86C were detected in the agarase-active 85-kDa sample and Aga86E was the dominant component of the 150-kDa fraction. The data confirm that Aga16B, Aga86C, and Aga86E are expressed and secreted during the degradation of agarose.
Aga50A and Aga86E are required for agar metabolism.
The mosaicism of the S. degradans 2-40 genome and the identification of the components of an apparent competence system within the genome sequence (data not shown) suggested that S. degradans 2-40 may be naturally competent. If so, the mutagenesis procedures developed for an Acinetobacter sp. (24) should be applicable to S. degradans 2-40. This strategy employs linear mutagenic fragments that recombine into the genome by homologous recombination.
Mutagenic constructs for S. degradans 2-40 were assembled by fusing the 1-kb segments flanking each side of a gene of interest to an antibiotic resistance cassette by using splicing PCR (24, 27). Each of the resulting linear mutagenic constructs (5′ flank:nptI:3′ flank) was added to a newly inoculated, exponentially growing culture of S. degradans 2-40, and after 2 h of incubation, potential transformants carrying gene replacements were selected. Kanr colonies appeared at a frequency of 6 × 10−6. Approximately 20% of these apparent transformants appeared to be gene replacements, as indicated by the presence of diagnostic PCR fragments. By this procedure, Δaga50A::nptI (S. degradans NE-A1) and Δaga86E::nptI (S. degradans NE-E1) mutants were created.
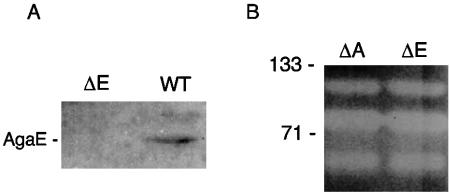
In order to evaluate the role of the demonstrated or predicted agarases in the degradation of agar, the constructed agarase mutants were screened for agarase complement in zymograms of cell lysates and their ability to utilize agar as a sole carbon source. Neither mutant was able to grow on agar as the sole carbon source, whereas a similarly constructed ΔchiA::nptI mutant retained this ability. When grown on media supplemented with glucose, the mutants pitted the medium slowly, but less so than wild-type S. degradans 2-40. The zymograms of the S. degradans NE-Α1 and S. degradans NE-E1 lysates revealed no change in agarase activity relative to that of the wild type (Fig. 4), although immunoblots showed S. degradans NE-E1 to lack Aga86E. These results indicate that the phenotype of the mutants is unlikely to be due to altered expression of other agarases but can be attributed to an essential role for the deleted gene product.
FIG. 4.
Properties of aga50A::nptI and aga86E::nptI mutants. (A) Immunoblot of S. degradans NE-E1 (ΔE) and 2-40 (WT) cell lysates fractionated by SDS-PAGE and probed with anti-AgaE serum (1:100). The cross-reactive 146-kDa polypeptide is labeled AgaE. (B) Agarase activities of Δaga50A::nptI and Δaga86E::nptI. Lysates of NE-A1 and NE-E1 were analyzed for agarase activity as described in the legend for Fig. 3. Molecular size markers (in kilodaltons) are noted at the left of panel B.
The agarolytic system of S. degradans 2-40 requires additional components.
To clarify the essential components of the agarolytic system, the cloned agarases were transformed into E. coli in an attempt to assemble a functional agarolytic system. The Aga+ pNE10 identified during the screen of the S. degradans 2-40 genomic library carries both aga50A and aga16B as well as the β-galactosidase-like galA gene. This plasmid was transformed into Gal+ E coli DH5α or HB101 and Gal− E. coli DH5α-E or TOP10 (Invitrogen) with or without pSHAga86E2 expressing aga86E. None of the resulting strains, however, were able to metabolize agarose as a sole carbon and energy source. Since the Gal+ transformants carrying both plasmids were highly unstable, an alternative test was performed. Gal− Aga+ strains of E. coli carrying either pNE10 or pSHAga86E2 were cross-streaked on several indicator media lacking an added carbon source and then overlaid with a Gal+ indicator strain. Galactose production was not detected in any medium, suggesting that the agarolytic system employs additional components not closely associated with aga50A, aga16B, or aga86E to convert agarose to galactose.
DISCUSSION
Components of agarolytic systems have been identified for other bacteria, but there is limited knowledge of the genetics of complete agarolytic systems. By a combination of conventional library screens and genome sequence analysis, an unusual agarolytic system that is composed of five secreted agarases, designated Aga50A, Aga16B, Aga86C, Aga50D, and Aga86E, was identified for the S. degradans 2-40 genome. Like many carbohydrases and other β-agarases (1, 29-31), these agarases were modular and could be separated into three distinct classes based upon the resident GH domain.
The identification of the S. degradans 2-40 agarases was based upon sequence similarity, conserved structural features, agarase activity of expressed genes in E. coli, and/or the phenotype of gene replacement mutants. For example, although enzymatic activity of Aga50A has yet to be demonstrated directly, there is a strong case to support its identification as an agarase. Aga50A exhibits sequence similarity to several known agarases, and a GH50 domain, which thus far has been found only in agarases (http://www.cazy.org/CAZY/), was identified within the conserved region. The gene is clustered in the S. degradans 2-40 genome with aga16B and is expressed divergently from the aga16B promoter region, suggesting that this gene could be under the same transcriptional control as aga16B. Directed replacement of aga50A by a Kan cassette abolished the ability of the mutant to grow on agar as a carbon source. Taken together, these data are consistent with the identification of Aga50A as agarase.
Aga16B was unequivocally demonstrated to be a secreted β-agarase. All agarase-positive clones identified in the S. degradans 2-40 genomic library included aga16B. The enzyme exhibited sequence similarity to a family of GH16-containing agarases, and the signature sequence for a GH16 catalytic domain was present. When cloned to produce a His-tagged derivative, the purified product had agarase activity in zymograms and endolytically degraded agar similarly to a β-agarase I, producing neoagarotetraose as the smallest product. Analysis of culture filtrates by mass spectrometry indicated that the enzyme is expressed during growth on agar as the sole carbon source. Multiple forms of Aga16B were observed in zymograms of affinity-purified preparations. These forms were similar to the forms observed in culture filtrates of S. degradans 2-40, suggesting that Aga16B is the predominant β-agarase I secreted by the bacterium.
Like the above-discussed agarases, Aga86C exhibits sequence similarity to known agarases, and an apparent GH86 domain is present in the conserved region. Like GH50 domains, GH86 domains have been identified only in agarases thus far (http://www.cazy.org/CAZY/). Although activity could not be demonstrated directly for Aga86C, Aga86C is expressed by agarose-grown cells but not by glucose-grown cells. Aga86C was present in an 85-kDa agarolytic protein fraction obtained after SDS-PAGE of S. degradans 2-40 culture filtrates. While the other agarases appear to be secreted into the medium, Aga86C is predicted to be surface associated. A typical lipobox was present in the amino terminus, and it functions to anchor other carbohydrases, such as Klebsiella pneumoniae pullulanase (11), to the cell surface by acylation. It remains to be determined whether this agarase is an endo- or exo-acting enzyme.
The identification of Aga50D as an agarase is based solely upon conserved sequence features. Extensive sequence similarities to a GH50 domain, apparently unique to agarases, were detected. Whether aga50D is expressed has not been established, nor have the attempts to express or mutate this gene been successful. Thus, Aga50D remains a hypothetical agarase.
There is also a strong case to support the identification of Aga86E as an agarase. Sequence similarity to several other agarases, as well as to a probable GH86 domain found only in agarases (http://www.cazy.org/CAZY/), was detected. When cloned to create a His-tagged derivative, the purified product was active in the degradation of agarose, releasing almost specifically neoagarobiose. This is consistent with exolytic degradation of agarose polymers. The active derivatives of Aga86E appear to be amino-terminal truncations of 100 and 86 kDa. These are most likely proteolytic products lacking one or more of the CBM but formed even in the presence of broad-spectrum protease inhibitors. The Δaga86E::nptI mutant exhibited a diminished ability to grow on agar as a carbon source, indicating a role of Aga86E in agarose metabolism.
Like most other carbohydrases characterized thus far, the S. degradans 2-40 agarases are modular. The catalytic domains found in these agarases include GH16, GH50, or GH86 domains (http://www.cazy.org/CAZY/) (31). GH16 domains are not specific to agarases and have been found in enzymes with other activities, such as β-galactosidases, endoglucanases, lichenases, and carrageenanases (1). Recently, the crystal structure of an agarase-active GH16 domain from Zobellia galactanivorans was characterized (2). This module contains two parallel binding sites that are thought to unwind the helical structure of agarose (1). Since the functional residues are conserved, the endolytic hydrolysis of agarose by Aga16B is likely to be similar. The crystal structures of GH50 and GH86 have not been determined, nor have the residues necessary for catalysis been identified. There are several regions of sequence conservation within each of the predicted GH50 and GH86 domains that may represent conserved active sites and/or binding domains. Like several other GH domains, the GH50 and GH86 domains have been associated with both endolytic and exolytic activities. For example, the GH86 family agarase of a deep-sea Microbulbifer-like strain has been reported to endolytically degrade agarose, but in this study the GH86-containing Aga86E appeared to exolytically degrade agarose to release neoagarobiose.
More unusual are the presence of multiple CBM6 in Aga16B and Aga86E. Aga16B has two homologs of CBM6, whereas Aga86E has three. While CBM are common in other carbohydrases (http://www.cazy.org/CAZY/fam/CBM6.html), a survey for CBM in other agarases revealed only two other agarases with CBM-like domains. The aforementioned GH86-containing β-agarase from a Microbulbifer-like isolate and an agarase of another marine bacterium carry CBM6 homologs (29). The CBM6 found in Aga86E and Aga16B form a distinct subclass within the large CBM6 family. The function of these CBM6 in the activity of these agarases has been difficult to establish. As expected from the modularity of the enzymes, deletion of the CBM6 did not obviously affect the catalytic activity of either enzyme, as the catalytic GH16 and GH86 domains are functional independently of other domains. Most likely, these domains increase the affinity of these enzymes for their substrate or disrupt interactions between adjacent polymers (49). In parallel studies, one of the CBM6 (Aga16B-CBM6-2) has been shown to bind to the nonreducing end of agarose polymers (18). Phylogenetic analyses indicate that this CBM6 is most similar to the first and second CBM6 of the exo-acting Aga86E.
These data can be used to assemble a model for agarose degradation by S. degradans 2-40. Since cell-free lysates of S. degradans 2-40 have the capability to degrade agarose to galactose and several β-agarases were identified, degradation of agarose by S. degradans 2-40 appears to occur by the β-agarase pathway. The major agarose depolymerases of S. degradans 2-40 are Aga16B and likely Aga86C, as the source gene is strongly expressed during growth on agarose (unpublished results). Aga16B is a β-agarase I that endolytically cleaves β1,4 linkages of the polymer to produce neoagarotetraose and other neoagarooligosaccharides. The enzyme appears to be secreted into the medium, as the enzyme is readily detectable in culture filtrates of agar-grown cells. The resident CBM may play a role in attachment of this enzyme to algal cell walls to minimize diffusion of the enzyme or to function to destabilize the cell wall polymers. Aga86C is predicted to be a surface-associated β-agarase. Aga86E appears to occupy an intermediate step in the degradation of agarose by exolytically degrading agarose, most likely from the nonreducing end. This suggests that Aga86E acts on neoagarotetraose and larger agarooligosaccharides to form neoagarobiose similarly to the β-agarase II activity of P. atlantica.
The hydrolysis of neoagarobiose by P. atlantica requires an α-neoagarobiose hydrolase (25, 26). Cell-free lysates of S. degradans 2-40 released d-galactose from polymeric agarose consistent with this activity. The source gene for this activity, however, has been difficult to establish as the sequence of a neoagarobiose hydrolase has yet to be reported. A 23-aa amino-terminal sequence of an α-neoagarooligosaccharide hydrolase of Vibrio sp. strain JT0107 has been reported, but none of the gene models in the S. degradans 2-40 genome exhibited significant similarity to this sequence. Similarly, a homolog of a GH96 domain found in α-agarases was not apparent in the S. degradans 2-40 genome. galA and aga50A are candidate neoagarobiose hydrolases, but E. coli strains carrying pNE10 that includes aga50A and galA did not release galactose from agarase. It may be that S. degradans 2-40 encodes a hydrolase that converts the 3,6-anhydrogalactose to galactose, which is then processed by a β-galactosidase-like enzyme, such as GalA, to release galactose. Since galA is expressed in an apparent operon with aga50A, it would be a strong candidate for this activity.
The agarolytic system of S. degradans 2-40 shares some similarities with β-agarase systems of other bacteria. The β-agarase system of P. atlantica is composed of secreted endolytic GH16- and GH86-containing β-agarase I's, an endolytic/exolytic β-agarase II, and a neoagarobiose hydrolase (7, 25, 26, 36). The agarases of S. degradans 2-40 appear to be structurally distinct from their counterparts of P. atlantica, retaining similarity only in the resident GH16 or GH86 domains, thereby suggesting that these enzymes may not be orthologous. More similar are the agarases present in the agarolytic plasmid of Microscilla sp. strain PRE1 (50). This system was identified by sequence analysis of a plasmid associated with agarolytic activity and is composed of five predicted β-agarases, four of which have homologs in S. degradans 2-40. A portion of the agarolytic plasmid exhibits synteny with the S. degradans 2-40 genome. The Microscilla system, like that of P. atlantica, differs from that of S. degradans 2-40 in that a GH50-containing agarase is not apparent. In contrast, the agarolytic system of Vibrio sp. strain JT0107 consists of two endolytic GH50-containing agarases and the aforementioned α-neoagarooligosaccharide hydrolase (38-41) but GH16- and GH86-containing agarases have yet to be identified in this bacterium.
In conclusion, the analysis of the S. degradans 2-40 genome has enabled the partial characterization of the agarolytic system of S. degradans 2-40. The S. degradans 2-40 agarolytic system appears to have been acquired by horizontal gene transfer and is composed of a secreted and apparently surface-associated β-agarase that appears to bind to and endolytically cleave agarose polymers to form oligosaccharide derivatives that are then exolytically processed to form a disaccharide product. This strategy for the depolymerization and metabolism of agar-associated polymers is common to many CP-degrading systems in S. degradans 2-40. Whether the S. degradans 2-40 system also includes surface-associated enzymes, as seen with other CP-degrading systems and predicted for several S. degradans 2-40 degradative systems, is currently being studied.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Science Foundation (DEB0109869) and from Maryland Sea Grant.
We thank the U.S. Department of Energy Joint Genome Institute for determining the genome sequence of S. degradans 2-40, Oak Ridge National Laboratories (ORNL) for the annotation of the draft genome sequence, and Michael Howard for his thoughtful contributions to this research.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allouch, J., W. Helbert, B. Henrissat, and M. Czjzek. 2004. Parallel substrate binding sites in a beta-agarase suggest a novel mode of action on double-helical agarose. Structure (Cambridge) 12:623-632. [DOI] [PubMed] [Google Scholar]
- 2.Allouch, J., M. Jam, W. Helbert, T. Barbeyron, B. Kloareg, B. Henrissat, and M. Czjzek. 2003. The three-dimensional structures of two beta-agarases. J. Biol. Chem. 278:47171-47180. [DOI] [PubMed] [Google Scholar]
- 3.Andrykovich, G., and I. Marx. 1988. Isolation of a new polysaccharide digesting bacterium from a salt marsh. Appl. Microbiol. Biotechnol. 54:1061-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki, T., T. Araki, and M. Kitamikado. 1990. Purification and characterization of a novel beta-agarase from Vibrio sp. AP-2. Eur. J. Biochem. 187:461-465. [DOI] [PubMed] [Google Scholar]
- 5.Araki, T., M. Hayakawa, Z. Lu, S. Karita, and T. Morishita. 1998. Purification and characterization of agarases from a marine bacterium, Vibrio sp. PO-303. J. Mar. Biotechnol. 6:260-265. [PubMed] [Google Scholar]
- 6.Barbeyron, T., S. L'Haridon, E. Corre, B. Kloareg, and P. Potin. 2001. Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 51:985-997. [DOI] [PubMed] [Google Scholar]
- 7.Belas, R. 1989. Sequence analysis of the agrA gene encoding β-agarase from Pseudomonas atlantica. J. Bacteriol. 171:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibb, M. J., G. H. Jones, R. Joseph, M. J. Buttner, and J. M. Ward. 1987. The agarase gene (dagA) of Streptomyces coelicolor A3(2): affinity purification and characterization of the cloned gene product. J. Gen. Microbiol. 133:2089-2096. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho, P. M., and B. Henrissat. 1999. The modular structure of cellulases and other carbohydrate-active enzymes: an integrated database approach, p. 15-23. In K. Ohmiya, K. Hayashi, K. Sakka, C. Kobayashi, S. Karita, and T. Kimura (ed.), Genetics, biochemistry and ecology of cellulose degradation. Uni Publishers Co., Tokyo, Japan.
- 10.Craigie, J. 1990. Cell walls, p. 221-258. In K. M. Cole and R. G. Sheath (ed.), Biology of the red algae. Cambridge University Press, New York, N.Y.
- 11.d'Enfert, C., I. Reyss, C. Wandersman, and A. P. Pugsley. 1989. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J. Biol. Chem. 264:17462-17468. [PubMed] [Google Scholar]
- 12.Duckworth, M., and W. Yaphe. 1970. Thin-layer chromatographic analysis of enzymic hydrolysates of agar. J. Chromatogr. 49:482-487. [DOI] [PubMed] [Google Scholar]
- 13.Edgar, R. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekborg, N. A., J. M. Gonzalez, M. B. Howard, L. E. Taylor, S. W. Hutcheson, and R. M. Weiner. 2005. Saccharophagus degradans gen. nov., sp. nov., a versatile marine degrader of complex polysaccharides. Int. J. Syst. Evol. Microbiol. 55:1545-1549. [DOI] [PubMed] [Google Scholar]
- 15.Ensor, L., S. Stosz, and R. Weiner. 1999. Expression of multiple complex polysaccharide-degrading enzyme systems by marine bacterium strain 2-40. J. Ind. Microbiol. Biotechnol. 23:123-126. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez, J. M., and R. M. Weiner. 2000. Phylogenetic characterization of marine bacterium strain 2-40, a degrader of complex polysaccharides. Int. J. Syst. Evol. Microbiol. 50:831-834. [DOI] [PubMed] [Google Scholar]
- 17.Ha, J. C., G. T. Kim, S. K. Kim, T. K. Oh, J. H. Yu, and I. S. Kong. 1997. Beta-agarase from Pseudomonas sp. W7: purification of the recombinant enzyme from Escherichia coli and the effects of salt on its activity. Biotechnol. Appl. Biochem. 26:1-6. [PubMed] [Google Scholar]
- 18.Henshaw, J., A. Horne, A. van Bueren, V. Money, D. Bolam, M. Czjzek, N. Ekborg, R. Weiner, S. Hutcheson, G. Davies, A. Boraston, and H. Gilbert. Family 6 carbohydrate binding modules in β-agarases display exquisite selectivity for the non-reducing termini of agarose-chains. J. Biol. Chem., in press. [DOI] [PubMed]
- 19.Hosoda, A., M. Sakai, and S. Kanazawa. 2003. Isolation and characterization of agar-degrading Paenibacillus spp. associated with the rhizosphere of spinach. Biosci. Biotechnol. Biochem. 67:1048-1055. [DOI] [PubMed] [Google Scholar]
- 20.Howard, M. B., N. A. Ekborg, L. E. Taylor, R. M. Weiner, and S. W. Hutcheson. 2003. Genomic analysis and initial characterization of the chitinolytic system of Microbulbifer degradans strain 2-40. J. Bacteriol. 185:3352-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juncosa, M., J. Pons, T. Dot, E. Querol, and A. Planas. 1994. Identification of active site carboxylic residues in Bacillus licheniformis 1,3-1,4-β-d-glucan 4-glucanohydrolase by site-directed mutagenesis. J. Biol. Chem. 269:14530-14535. [PubMed] [Google Scholar]
- 22.Kang, N. Y., Y. L. Choi, Y. S. Cho, B. K. Kim, B. S. Jeon, J. Y. Cha, C. H. Kim, and Y. C. Lee. 2003. Cloning, expression and characterization of a beta-agarase gene from a marine bacterium, Pseudomonas sp. SK38. Biotechnol. Lett. 25:1165-1170. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, S. K., V. Coyne, D. Sledjeski, C. Fuqua, and R. Weiner. 1990. Identification of a tyrosinase from a periphytic marine bacterium. FEMS Microbiol. Lett. 67:275-280. [Google Scholar]
- 24.Metzgar, D., J. Bacher, V. Pezo, J. Reader, V. Doring, P. Schimmel, P. Marliere, and V. de Crecy-Legard. 2004. Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome sequencing. Nucleic Acid Res. 32:5780-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrice, L. M., M. W. McLean, W. F. Long, and F. B. Williamson. 1983. Beta-agarases I and II from Pseudomonas atlantica. Substrate specificities. Eur. J. Biochem. 137:149-154. [DOI] [PubMed] [Google Scholar]
- 26.Morrice, L. M., M. W. McLean, F. B. Williamson, and W. F. Long. 1983. Beta-agarases I and II from Pseudomonas atlantica. Purifications and some properties. Eur. J. Biochem. 135:553-558. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, K. C. 1998. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohta, Y., Y. Hatada, S. Ito, and K. Horikoshi. 2005. High-level expression of a neoagarobiose-producing beta-agarase gene from Agarivorans sp. JAMB-A11 in Bacillus subtilis and enzymic properties of the recombinant enzyme. Biotechnol. Appl. Biochem. 41:183-191. [DOI] [PubMed] [Google Scholar]
- 29.Ohta, Y., Y. Hatada, Y. Nogi, Z. Li, S. Ito, and K. Horikoshi. 2004. Cloning, expression, and characterization of a glycoside hydrolase family 86 beta-agarase from a deep-sea Microbulbifer-like isolate. Appl. Microbiol. Biotechnol. 66:266-275. [DOI] [PubMed] [Google Scholar]
- 30.Ohta, Y., Y. Hatada, Y. Nogi, M. Miyazaki, Z. Li, M. Akita, Y. Hidaka, S. Goda, S. Ito, and K. Horikoshi. 2004. Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from a novel species of deep-sea Microbulbifer. Appl. Microbiol. Biotechnol. 64:505-514. [DOI] [PubMed] [Google Scholar]
- 31.Ohta, Y., Y. Nogi, M. Miyazaki, Z. Li, Y. Hatada, S. Ito, and K. Horikoshi. 2004. Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from the novel marine isolate, JAMB-A94. Biosci. Biotechnol. Biochem. 68:1073-1081. [DOI] [PubMed] [Google Scholar]
- 32.Page, R. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 33.Plotkin, J., H. Robins, and A. Levine. 2004. Tissue-specific codon usage and the expression of human genes. Proc. Natl. Acad. Sci. USA 101:12588-12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potin, P., C. Richard, C. Rochas, and B. Kloareg. 1993. Purification and characterization of the alpha-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. Eur. J. Biochem. 214:599-607. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schroeder, D. C., M. A. Jaffer, and V. E. Coyne. 2003. Investigation of the role of a beta(1-4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 149:2919-2929. [DOI] [PubMed] [Google Scholar]
- 37.Shieh, W. Y., and W. D. Jean. 1998. Alterococcus agarolyticus, gen. nov., sp. nov., a halophilic thermophilic bacterium capable of agar degradation. Can. J. Microbiol. 44:637-645. [DOI] [PubMed] [Google Scholar]
- 38.Sugano, Y., H. Kodama, I. Terada, Y. Yamazaki, and M. Noma. 1994. Purification and characterization of a novel enzyme, α-neoagarooligosaccharide hydrolase (α-NAOS hydrolase) from a marine bacterium, Vibrio sp. strain JT0107. J. Bacteriol. 176:6812-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugano, Y., T. Matsumoto, H. Kodama, and M. Noma. 1993. Cloning and sequencing of agaA, a unique agarase 0107 gene from a marine bacterium, Vibrio sp. strain JT0107. Appl. Environ. Microbiol. 59:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugano, Y., T. Matsumoto, and M. Noma. 1994. Sequence analysis of the agaB gene encoding a new beta-agarase from Vibrio sp. strain JT0107. Biochim. Biophys. Acta 1218:105-108. [DOI] [PubMed] [Google Scholar]
- 41.Sugano, Y., I. Terada, M. Arita, M. Noma, and T. Matsumoto. 1993. Purification and characterization of a new agarase from a marine bacterium, Vibrio sp. strain JT0107. Appl. Environ. Microbiol. 59:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swartz, M. N., and N. Gordon. 1959. Agarase from an agar-digesting bacterium. J. Bacteriol. 77:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, J., T. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, J., D. Higgins, and T. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turvey, J. R., and J. Christison. 1967. The hydrolysis of algal galactans by enzymes from a Cytophaga species. Biochem. J. 105:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uetanabaro, A. P., C. Wahrenburg, W. Hunger, R. Pukall, C. Sproer, E. Stackebrandt, V. P. de Canhos, D. Claus, and D. Fritze. 2003. Paenibacillus agarexedens sp. nov., nom. rev., and Paenibacillus agaridevorans sp. nov. Int. J. Syst. Evol. Microbiol. 53:1051-1057. [DOI] [PubMed] [Google Scholar]
- 47.Van der Meulen, H. J., and W. Harder. 1975. Production and characterization of the agarase of Cytoplaga flevensis. Antonie Leeuwenhoek 41:431-447. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead, L. A., S. K. Stosz, and R. M. Weiner. 2001. Characterization of the agarase system of a multiple carbohydrate degrading marine bacterium. Cytobios 106(Suppl. 1):99-117. [PubMed] [Google Scholar]
- 49.Xu, Q., M. Morrison, K. Nelson, E. Bayer, N. Atamna, and R. Lamed. 2004. A novel family of carbohydrate-binding modules identified with Ruminococcus albus proteins. FEBS Lett. 566:11-16. [DOI] [PubMed] [Google Scholar]
- 50.Zhong, Z., A. Toukdarian, D. Helinski, V. Knauf, S. Sykes, J. E. Wilkinson, C. O'Bryne, T. Shea, C. DeLoughery, and R. Caspi. 2001. Sequence analysis of a 101-kilobase plasmid required for agar degradation by a Microscilla isolate. Appl. Environ. Microbiol. 67:5771-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.