Abstract
Intestinal tracts of broilers and turkeys from 10 conventional broiler farms and 10 conventional turkey farms, where antimicrobials were routinely used, and from 5 organic broiler farms and 5 organic turkey farms, where antimicrobials had never been used, were collected and cultured for Campylobacter species. A total of 694 Campylobacter isolates from the conventional and organic poultry operations were tested for antimicrobial resistance to nine antimicrobial agents by the agar dilution method. Although Campylobacter species were highly prevalent in both the conventional and organic poultry operations, the antimicrobial resistance rates were significantly different between the organic operations and the conventional operations. Less than 2% of Campylobacter strains isolated from organically raised poultry were resistant to fluoroquinolones, while 46% and 67% of Campylobacter isolates from conventionally raised broilers and conventionally raised turkeys, respectively, were resistant to these antimicrobials. In addition, a high frequency of resistance to erythromycin (80%), clindamycin (64%), kanamycin (76%), and ampicillin (31%) was observed among Campylobacter isolates from conventionally raised turkeys. None of the Campylobacter isolates obtained in this study was resistant to gentamicin, while a large number of the isolates from both conventional and organic poultry operations were resistant to tetracycline. Multidrug resistance was observed mainly among Campylobacter strains isolated from the conventional turkey operation (81%). Findings from this study clearly indicate the influence of conventional and organic poultry production practices on antimicrobial resistance of Campylobacter on poultry farms.
Food-borne campylobacteriosis, a major public health concern in the United States and many countries worldwide, is caused mainly by Campylobacter jejuni (23). It is estimated that more than 2 million cases of food-borne bacterial diarrhea that occur each year in the United States are caused by Campylobacter (3). In other industrialized countries, the numbers of Campylobacter infections exceeded those of Salmonella, Shigella, and Escherichia coli O157:H7 infections combined (2). Campylobacter jejuni not only is an important cause of bacterial gastroenteritis in humans but also has been associated with Guillain-Barré syndrome, an acute immune-mediated demyelinating disorder of the peripheral nervous system (7, 24). Although most Campylobacter infections in humans are associated with ingestion of contaminated or improperly handled/cooked foods as well as milk or dairy products, consumption of undercooked poultry and/or other foods that are cross-contaminated with raw poultry meat during food preparation is considered a major risk factor for food-borne campylobacteriosis (4, 7). Since thermophilic Campylobacter spp., including C. jejuni and Campylobacter coli, are highly prevalent in chickens and turkeys (29, 34), contamination of poultry carcasses by Campylobacter during processing in slaughter houses occurs frequently, resulting in the potential transmission of Campylobacter from contaminated poultry meats to consumers.
Over the last decade, the emergence of antimicrobial resistance in Campylobacter strains isolated from humans and animals in many countries around the world has increased dramatically (12, 17, 25, 39, 43). In the United States, the prevalence of fluoroquinolone resistance among Campylobacter isolates increased significantly from 1.3% in 1992 to 8% to 13% during 1996 to 1998, and this resistance trend has increased steadily since 1998 (15, 26, 37). In 2001, the National Antimicrobial Resistance Monitoring System (NARMS) and Nachamkin et al. found that about 19% to 40% of Campylobacter strains isolated from humans in the United States were resistant to ciprofloxacin (15, 26). The emergence of antimicrobial resistance, particularly among food-borne pathogens, is in part because of the widespread use of antimicrobial agents in both humans and animals (17, 22, 39, 40, 43).
In conventional production practice, antimicrobial agents can be used for treatment, control, and prevention of the diseases as well as for improvement of growth and feed efficiency of the animals (17, 22, 39, 40). Organic production practice, on the other hand, has restricted the use of antimicrobial substances on the farms (11). In addition to being subjected to the strict rules regarding the use of antimicrobial substances, the organic birds must be fed only on organically produced feed and supplements. Moreover, these organic birds must be provided with uncrowded living areas, and they also need to have access to fresh air, sunlight, and the outside environment (11). Although many studies of antimicrobial resistance in conventional poultry operations have been done, relatively little is known about antimicrobial-resistant Campylobacter in organic poultry operations. Since no antimicrobials have been used in the organic poultry operations and the demand for organic animal produce has been increasing considerably over the last several years (10), the difference in antimicrobial resistance of Campylobacter isolates from conventional and organic poultry operations is of interest. In addition, despite the recent advances in understanding the epidemiology of antimicrobial-resistant Campylobacter, relatively little is known about the impact of conventional and organic animal production practices on the prevalence of antimicrobial-resistant Campylobacter. Therefore, the purpose of this study was to determine the prevalence and antimicrobial resistance of Campylobacter isolates from both conventionally raised and organically raised broilers and turkeys.
MATERIALS AND METHODS
Sample collection.
This study focused on the prevalence of antimicrobial-resistant Campylobacter in slaughter-age birds. A total of 345 broiler and 360 turkey intestinal tracts originating from 10 conventional broiler farms and 10 conventional turkey farms were collected from processing plants, which process approximately 200,000 to 300,000 broilers and 17,000 to 21,000 turkeys per week. Since there are only a limited number of large-scale certified organic broiler and turkey farms in Ohio, only five organic broiler farms and five organic turkey farms were included in this study. A total of 355 intestinal tracts of organic broilers and 230 intestinal tracts of organic turkeys were collected from a state-inspected organic processing plant. In general, the whole intestinal tract of conventionally raised turkeys, organically raised broilers, and organically raised turkeys was manually taken out from each carcass by an employee of the processing plant, while the whole intestinal tract of conventionally raised broilers was taken out from each carcass by automated equipment. The samples in this study were collected from August 2000 to November 2002.
Antimicrobial usage data.
According to direct interviews with farmers, no antimicrobial agents were used in organic broiler or turkey operations from which the samples were collected. In contrast, antimicrobial agents were used in almost every conventional poultry farm according to direct interviews with farmers or production supervisors. For conventionally raised broilers, gentamicin was the most commonly used antimicrobial. This antimicrobial agent was given to the birds at the hatchery to prevent early mortality due to E. coli infections. In addition to gentamicin, lincomycin was also used in some conventional broiler farms to prevent as well as to treat necrotic enteritis in conventionally raised broilers, at a dosage of 2 to 4 g/ton feed for prevention or 64 mg/gallon water for 5 to 10 days for treatment. If these conventional broiler flocks had coccidiosis, they were treated with amprolium at 0.004% in feed continuously or at 0.024% in water for 3 to 5 days. In addition, bacitracin and virginiamycin, which were supplemented in broiler feed at subtherapeutic levels in order to promote growth and improve feed efficiency as well as to prevent and control necrotic enteritis, were also used in these conventional broiler farms. If bacitracin was used for prevention of necrotic enteritis, it was given to the birds at a dosage of 100 mg/gallon water. But, if it was used for control of the disease, this antimicrobial agent was used at a dosage of 200 to 400 mg/gallon water. Virginiamycin was used to prevent necrotic enteritis in these conventionally raised broilers at a dosage of 5 to 15 g/ton feed. For the conventional broiler flocks surveyed in this study, the birds were not exposed to treatments with fluoroquinolones during the production period, according to information obtained from the producers; however, fluoroquinolones were used in the previous flocks of these conventional broiler farms. For conventionally raised turkeys, enrofloxacin was the drug routinely used for flocks with respiratory disease due to E. coli infections, while chlortetracycline was used only for the farms that had a high prevalence of fowl cholera. As with the conventionally raised broilers, bacitracin was also used as a feed additive and used to control necrotic enteritis in conventionally raised turkeys at a dosage of 400 mg/gallon water for 5 to 7 days.
Bacterial isolation and identification.
The intestinal tracts were placed on ice and brought back to the laboratory within 3 h of collection and cultured for Campylobacter species. Each cecum was aseptically opened, and cecal contents were streaked onto Campy CVA agar containing cefoperazone, vancomycin, and amphotericin B as selective supplements (BBL Becton Dickinson Microbiology Systems, Cockeysville, MD) with a sterile cotton swab. The inoculated plates were then incubated at 42°C for 48 h in a microaerophilic environment (approximately 5% O2, 10% CO2, and 85% N2) in an anaerobic system jar with gas-generating system envelopes (BBL Becton Dickinson Microbiology Systems, Sparks, MD). Suspect Campylobacter colonies were identified by colony morphology characteristics, Gram stain, an oxidase test, a catalase test, and a Campylobacter culture plate latex agglutination confirmation test (INDX-Campy [jcl]; PanBio InDx, Inc., Baltimore, MD). The hippurate hydrolysis test was performed to differentiate C. jejuni from C. coli and other Campylobacter species. From each Campylobacter-positive sample, a single colony was used for an antimicrobial susceptibility test. All Campylobacter isolates were stored in sterile cryovial tubes containing skim milk and 30% glycerol at −85°C prior to the antimicrobial susceptibility test.
Antimicrobial susceptibility testing.
A total of 694 Campylobacter isolates from conventional and organic poultry farms were tested for antimicrobial resistance to nine antimicrobial agents, including ampicillin, tetracycline, gentamicin, kanamycin, clindamycin, erythromycin, ciprofloxacin, norfloxacin, and nalidixic acid, by the agar dilution method (27). All antimicrobial agents were obtained from Sigma Chemical Co., St. Louis, MO, except ciprofloxacin (Serologicals Proteins, Inc., Kankakee, IL). The concentrations of most antimicrobial agents tested in this study ranged from 0.06 to 128 μg/ml except for ciprofloxacin (0.008 to 128 μg/ml) and for kanamycin and nalidixic acid (0.25 to 128 μg/ml) (Table 1). Briefly, Campylobacter isolates grown on blood agar plates for 48 h were inoculated onto Mueller-Hinton broth and then adjusted to a turbidity equivalent to a 0.5 McFarland standard by a colorimeter. A multipoint inoculator (a Cathra replicator system) with 1-mm pins (Oxoid, Inc., Ogdensburg, NY) was used to inoculate approximately 104 CFU of samples onto Mueller-Hinton agar containing a twofold concentration series of antimicrobials and supplemented with 5% defibrinated sheep blood. Campylobacter jejuni ATCC 33560 was used as the quality control organism (27). While quality control ranges are not currently available for ampicillin, kanamycin, clindamycin, and norfloxacin, the MIC results for these drugs with C. jejuni ATCC 33560 were consistent, falling within a three-dilution range throughout the study. The inoculated plates were incubated in a CO2 incubator (Thermo Electron Corporation, Marietta, OH) at 42°C for 24 h in a microaerophilic atmosphere of 5% O2, 10% CO2, and 85% N2. The MIC was defined as the lowest concentration of antimicrobial agent that completely inhibited the visible growth on the plates. The resistance breakpoints for the antimicrobial agents were as follows: ≥4 μg/ml for ciprofloxacin and clindamycin, ≥8 μg/ml for erythromycin, ≥16 μg/ml for tetracycline, gentamicin, and norfloxacin, ≥32 μg/ml for ampicillin and nalidixic acid, and ≥64 μg/ml for kanamycin (Table 1) (8, 28). If an isolate was resistant to three or more classes of antimicrobials, it was defined as multidrug resistant.
TABLE 1.
Antimicrobial test ranges, MIC quality control ranges, and MIC breakpoints used for antimicrobial susceptibility testing
| Antimicrobial agent | Agar dilution test range (μg/ml) | MIC quality control range of C. jejuni ATCC 33560 (μg/ml)b | MIC breakpoint (μg/ml)a
|
||
|---|---|---|---|---|---|
| S | I | R | |||
| Ampicillin | 0.06-128 | N/Ac | ≤8 | 16 | ≥32 |
| Tetracycline | 0.06-128 | 1-4 | ≤4 | 8 | ≥16 |
| Gentamicin | 0.06-128 | 0.5-4 | ≤4 | 8 | ≥16 |
| Kanamycin | 0.25-128 | N/A | ≤16 | 32 | ≥64 |
| Clindamycin | 0.06-128 | N/A | ≤0.5 | 1-2 | ≥4 |
| Erythromycin | 0.06-128 | 1-8 | ≤0.5 | 1-4 | ≥8 |
| Ciprofloxacin | 0.008-128 | 0.06-0.5 | ≤1 | 2 | ≥4 |
| Norfloxacin | 0.06-128 | N/A | ≤4 | 8 | ≥16 |
| Nalidixic acid | 0.25-128 | 8-32 | ≤16 | ≥32 | |
MIC breakpoints for enteric bacteria for all agents except norfloxacin were used by the NARMS. MIC breakpoints for Enterobacteriaceae for norfloxacin were recommended by the CLSI (formerly NCCLS). S, susceptible strains; I, intermediate strains; R, resistant strains.
Tentative agar dilution quality control ranges of C. jejuni ATCC 33560 were approved by the CLSI.
N/A, no data available.
Statistical analysis.
A chi-square test at a P significance level of <0.05 (two tailed), with Yates' correction for continuity, was used for comparing the prevalence and antimicrobial resistance rates of Campylobacter isolates between conventional and organic operations and between broilers and turkeys.
RESULTS
Prevalence of Campylobacter.
The prevalence of C. jejuni and C. coli plus other Campylobacter species in conventionally raised broilers was 66%, while the prevalence of these organisms in conventionally raised turkeys was 83%. In terms of the organic poultry production systems, the prevalences of Campylobacter spp. in organically raised broilers and organically raised turkeys were 89% and 87%, respectively (Table 2). On the basis of the hippurate hydrolysis test, C. jejuni was the predominant Campylobacter species in conventionally raised broilers, organically raised broilers, and organically raised turkeys, whereas C. coli and other Campylobacter species were the predominant species in conventionally raised turkeys (Table 2). In this study, Campylobacter spp. could be isolated from every conventional and organic broiler and turkey farm. The prevalence of Campylobacter spp. in conventional broiler farms ranged from 44% to 80%, while the prevalence of these organisms in conventional turkey farms ranged from 63% to 98%. Likewise, the prevalence of Campylobacter spp. ranged from 70% to 100% in organic broiler farms and 6% to 100% in organic turkey farms (Table 2).
TABLE 2.
Prevalence and antimicrobial resistance of C. jejuni and C. coli plus other Campylobacter species in conventional and organic broiler and turkey farms
| Farma | No. (%) of positive samples/total no. of samplesb | No. (%) of positive samples
|
Major antimicrobial resistance patternd | |
|---|---|---|---|---|
| C. jejuni | C. colic | |||
| CB-1 | 20 (66.67)/30 | 20 (100) | 0 (0) | TET or FQ |
| CB-2 | 14 (56.00)/25 | 14 (100) | 0 (0) | TET-KAN-FQ |
| CB-3 | 23 (76.63)/30 | 23 (100) | 0 (0) | TET |
| CB-4 | 22 (73.33)/30 | 22 (100) | 0 (0) | TET |
| CB-5 | 30 (66.67)/45 | 23 (76.67) | 7 (23.33) | TET-FQ |
| CB-6 | 27 (67.50)/40 | 27 (100) | 0 (0) | TET |
| CB-7 | 24 (60.00)/40 | 24 (100) | 0 (0) | TET-FQ |
| CB-8 | 16 (53.33)/30 | 16 (100) | 0 (0) | TET or TET-FQ |
| CB-9 | 11 (44.00)/25 | 11 (100) | 0 (0) | TET or KAN |
| CB-10 | 40 (80.00)/50 | 40 (100) | 0 (0) | TET or TET-FQ |
| Total | 227 (65.80)/345 | 220 (96.92) | 7 (3.08) | |
| OB-1 | 85 (91.40)/93 | 53 (62.35) | 32 (37.65) | TET |
| OB-2 | 82 (88.17)/93 | 61 (74.39) | 21 (25.61) | TET or TET-KAN |
| OB-3 | 22 (81.48)/27 | 13 (59.09) | 9 (40.91) | KAN or TET-KAN |
| OB-4 | 96 (100)/96 | 70 (72.92) | 26 (27.08) | No resistancee |
| OB-5 | 32 (69.57)/46 | 32 (100) | 0 (0) | TET |
| Total | 317 (89.30)/355 | 229 (72.24) | 88 (27.76) | |
| CT-1 | 20 (66.67)/30 | 8 (40) | 12 (60) | TET-KAN-CLI-ERYf |
| CT-2 | 39 (86.67)/45 | 7 (17.95) | 32 (82.05) | TET-KAN-CLI-ERY-FQ |
| CT-3 | 44 (97.78)/45 | 17 (38.64) | 27 (61.36) | TET-KAN-CLI-ERY-FQg |
| CT-4 | 24 (80.00)/30 | 16 (66.67) | 8 (33.33) | TET-KAN-CLI-ERY-FQ |
| CT-5 | 19 (63.33)/30 | 11 (57.89) | 8 (42.11) | TET-KAN-CLI-ERY-FQ |
| CT-6 | 40 (88.89)/45 | 26 (65) | 14 (35) | TET-KAN-CLI-ERY-FQg |
| CT-7 | 21 (70.00)/30 | 11 (52.38) | 10 (47.62) | TET |
| CT-8 | 29 (96.67)/30 | 16 (55.17) | 13 (44.83) | KAN-ERYh |
| CT-9 | 21 (70.00)/30 | 12 (57.14) | 9 (42.86) | No specific patterni |
| CT-10 | 42 (93.33)/45 | 13 (30.95) | 29 (69.05) | TET-KAN-CLI-ERY-FQg |
| Total | 299 (83.06)/360 | 137 (45.82) | 162 (54.18) | |
| OT-1 | 40 (93.02)/43 | 20 (50) | 20 (50) | TET |
| OT-2 | 42 (100)/42 | 33 (78.57) | 9 (21.43) | TET or TET-KAN |
| OT-3 | 88 (93.62)/94 | 49 (55.68) | 39 (44.32) | KAN or TET-KAN |
| OT-4 | 1 (5.56)/18 | 1 (100) | 0 (0) | TET |
| OT-5 | 30 (90.91)/33 | 30 (100) | 0 (0) | TET-KANj |
| Total | 201 (87.39)/230 | 133 (66.17) | 68 (33.83) | |
CB, conventional broiler farm; OB, organic broiler farm; CT, conventional turkey farm; OT, organic turkey farm.
Number (%) of intestines positive for Campylobacter species/number of intestines isolated for Campylobacter species.
Number (%) of intestines positive for C. coli and other Campylobacter species.
CLI, clindamycin; ERY, erythromycin; FQ, fluoroquinolones; KAN, kanamycin; TET, tetracycline.
None of the Campylobacter isolates was resistant to antimicrobial agents tested in this study.
Some isolates were also resistant to fluoroquinolones and ampicillin.
Some isolates were also resistant to ampicillin.
Some isolates were also resistant to fluoroquinolones and tetracycline.
No major antimicrobial resistance pattern was observed.
Only one isolate from this organic turkey farm was resistant to tetracycline and kanamycin, while the rest of the isolates were susceptible to all antimicrobial agents.
Antimicrobial resistance patterns.
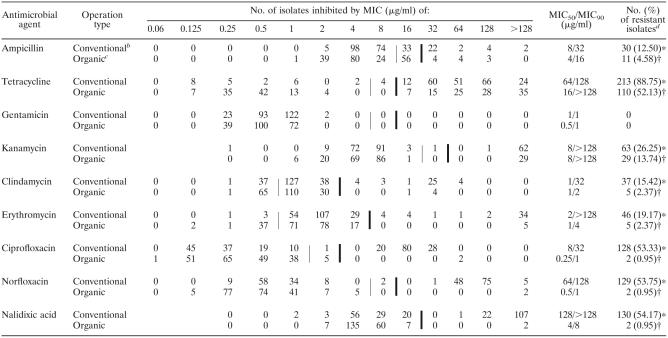
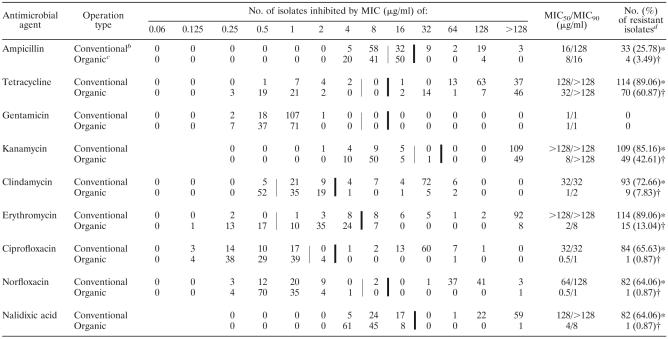
The MIC distributions and the MICs at which 50% and 90% of C. jejuni and C. coli plus other Campylobacter species were inhibited are summarized in Tables 3 and 4. In general, a wider range of MICs of most antimicrobials was observed mainly among Campylobacter isolates from conventional poultry farms than among the isolates from organic poultry farms, except the MIC of gentamicin, for which the lowest concentrations of this antimicrobial agent against Campylobacter strains isolated from both operation types were comparable. When the MIC90 and the resistance breakpoint of each antimicrobial agent were compared, the MIC90 values of ampicillin, clindamycin, erythromycin, ciprofloxacin, norfloxacin, and nalidixic acid for Campylobacter isolates from conventionally raised broilers and turkeys were higher than their resistance breakpoints, while the MIC90 values of these antimicrobials for the isolates from organically raised broilers and turkeys were lower than the resistance breakpoints. Overall, the MIC90 values of these antimicrobial agents for Campylobacter strains isolated from conventional poultry farms were higher than those for the strains isolated from organic poultry farms (Tables 3 and 4). Although Campylobacter strains isolated from both conventional and organic poultry operations in this study were uniformly susceptible to gentamicin, with an MIC90 of ≤1 μg/ml, these Campylobacter isolates were highly resistant to tetracycline, with an MIC90 of ≥128 μg/ml.
TABLE 3.
MIC distributions and resistance rates of C. jejuni isolated from conventional and organic poultry farmsa
Thin vertical lines indicate the breakpoint between susceptible and intermediate strains. Thick vertical lines indicate the breakpoint between intermediate and resistant strains (except for nalidixic acid, for which it indicates the breakpoint between susceptible and resistant strains).
C. jejuni isolates from conventional poultry farms (n = 240).
C. jejuni isolates from organic poultry farms (n = 211).
Different symbols between operation types (conventional and organic) indicate a significant difference (P < 0.05) by a chi-square test with Yates' correction for continuity.
TABLE 4.
MIC distributions and resistance rates of C. coli and other Campylobacter species isolated from conventional and organic poultry farmsa
Thin vertical lines indicate the breakpoint between susceptible and intermediate strains. Thick vertical lines indicate the breakpoint between intermediate and resistant strains (except for nalidixic acid, for which it indicates the breakpoint between susceptible and resistant strains).
Campylobacter isolates from conventional poultry farms (n = 128).
Campylobacter isolates from organic poultry farms (n = 115).
Different symbols between operation types (conventional and organic) indicate a significant difference (P < 0.05) by a chi-square test with Yates' correction for continuity.
One of the most striking findings in this study was the difference in quinolone and fluoroquinolone resistance between Campylobacter strains isolated from conventional poultry farms and organic poultry farms. Approximately 46% of Campylobacter strains isolated from conventionally raised broilers and 67% of Campylobacter strains isolated from conventionally raised turkeys were resistant to ciprofloxacin, norfloxacin, and nalidixic acid. In contrast, none of the Campylobacter strains isolated from organically raised broilers and less than 2% of Campylobacter strains isolated from organically raised turkeys were resistant to these antimicrobials (Table 5). Compared to Campylobacter strains isolated from conventionally raised broilers and organically raised broilers and turkeys, the isolates from the conventional turkey operation were significantly more resistant to erythromycin, clindamycin, kanamycin, tetracycline, and ampicillin (P < 0.05) (Table 5). Regardless of the sources of isolation, none of the Campylobacter strains tested in this study were resistant to gentamicin, while more than 80% of Campylobacter strains isolated from conventionally raised broilers and turkeys and 50% to 60% of Campylobacter strains isolated from organically raised broilers and turkeys were resistant to tetracycline (Table 5). In terms of multidrug resistance, the occurrence of multidrug-resistant Campylobacter strains was observed mainly among the isolates from conventionally raised turkeys, with 81% of these isolates showing resistance to three or more classes of antimicrobials (Table 6). Moreover, about 58% of Campylobacter isolates from conventionally raised turkeys were resistant to both erythromycin and ciprofloxacin, whereas none of the Campylobacter strains isolated from conventionally raised broilers and organically raised broilers and turkeys was concomitantly resistant to these antimicrobial agents. When antimicrobial resistance in individual conventional and organic broiler and turkey farms was investigated, tetracycline resistance was the major resistance pattern observed in almost every conventional broiler farm, organic broiler farm, and organic turkey farm (Table 2). Unlike the isolates from conventionally raised broilers and organically raised broilers and turkeys, the majority of Campylobacter isolates from 6 out of 10 conventional turkey farms were multidrug resistant to tetracycline, kanamycin, clindamycin, erythromycin, and fluoroquinolones (Table 2).
TABLE 5.
Resistance rates of Campylobacter strains isolated from different poultry production systems
| Antimicrobial agent | No. (%) of resistant strains isolated froma:
|
|||
|---|---|---|---|---|
| Conventional broiler farms (n = 167) | Organic broiler farms (n = 165) | Conventional turkey farms (n = 201) | Organic turkey farms (n = 161) | |
| Ampicillin | 0 A | 5 (3.03) A | 63 (31.34) B | 10 (6.21) A |
| Tetracycline | 141 (84.43) A | 99 (60) B | 186 (92.54) C | 81 (50.31) B |
| Gentamicin | 0 | 0 | 0 | 0 |
| Kanamycin | 19 (11.38) A | 28 (16.97) A | 153 (76.12) B | 50 (31.06) C |
| Clindamycin | 2 (1.20) A | 9 (5.45) A | 129 (64.18) B | 5 (3.11) A |
| Erythromycin | 0 A | 15 (9.09) B | 160 (79.60) C | 5 (3.11) D |
| Ciprofloxacin | 76 (45.51) A | 0 B | 136 (67.66) C | 3 (1.86) B |
| Norfloxacin | 77 (46.11) A | 0 B | 134 (66.67) C | 3 (1.86) B |
| Nalidixic acid | 77 (46.11) A | 0 B | 135 (67.16) C | 3 (1.86) B |
Antimicrobial resistance rates of Campylobacter isolates from different poultry production systems are compared by a chi-square test with Yates' correction for continuity. Numbers in the same row with different letters are significantly different (P < 0.05), while numbers with the same letters do not differ significantly.
TABLE 6.
Major multidrug resistance patterns of C. jejuni and C. coli plus other Campylobacter species isolated from conventional and organic poultry operations
| Operation type (no. of isolates) | No. (%) of multidrug-resistant strains
|
Resistance patternb | ||
|---|---|---|---|---|
| C. jejuni | C. colia | Total | ||
| Conventional broiler farms (167) | 15 (8.98) | 0 | 15 (8.98) | TET-KAN-CIP-NOR-NAL |
| Organic broiler farms (165) | 4 (2.42) | 7 (4.24) | 11 (6.67) | TET-KAN-CLI-ERY |
| Conventional turkey farms (201) | 59 (29.35) | 104 (51.74) | 163 (81.09)c | TET-KAN-CLI-ERY-CIP-NOR-NAL |
| Organic turkey farms (161) | 4 (2.48) | 4 (2.48) | 8 (4.97) | TET-KAN-CLI-ERY |
C. coli and other Campylobacter species.
AMP, ampicillin; CIP, ciprofloxacin; CLI, clindamycin; ERY, erythromycin; KAN, kanamycin; NAL, nalidixic acid; NOR, norfloxacin; TET, tetracycline.
The prevalence of multidrug-resistant Campylobacter strains in conventionally raised turkeys was significantly higher (P < 0.05) than that of other operation types.
DISCUSSION
In this study, it is clearly shown that thermophilic Campylobacter is highly prevalent in both organic and conventional poultry production systems. However, the antimicrobial resistance rates vary significantly in different production types. In general, conventionally raised broilers and turkeys harbor more antimicrobial-resistant Campylobacter strains than organically raised broilers and turkeys, and the differences are obvious with fluoroquinolones. The highest resistance rates and multidrug resistance to three or more classes of antimicrobials are observed mainly among the isolates from the conventional turkey operation.
Although the prevalences of Campylobacter species in conventionally raised broilers and organically raised broilers were significantly different (P < 0.05), it should be noted that the average ages of the birds at the processing plants were not the same. Since the average market age of these organically raised broilers was about 8 to 12 weeks old, compared to 6 weeks old for conventionally raised broilers, the high prevalence of Campylobacter strains in organically raised broilers in part seems to be associated with the increased age of the birds at slaughter. The prevalences of Campylobacter species in conventionally raised and organically raised turkeys, on the other hand, were not significantly different (P = 0.19). This is likely because conventionally raised turkeys and organically raised turkeys were sent to the processing plants at approximately the same age (18 to 20 weeks). The association between the Campylobacter colonization rate and the age of the birds at the processing plant was also noted by other studies, which indicated that the prevalence of Campylobacter in poultry elevated when the age of the birds at the processing plant increased (6, 13, 29, 30). Besides the market age of the birds, other factors such as environmental exposure, which is seen particularly in organic poultry operations, can also play a role in the prevalence of Campylobacter in poultry (16, 29).
Although Campylobacter spp. could be isolated from every conventional and organic poultry farm, it should be noted that the prevalences of these organisms varied among farms. Among Campylobacter-positive flocks, C. jejuni was the predominant species in both conventional broiler farms and organic broiler farms, although the prevalence of C. jejuni in conventionally raised broilers was significantly higher (P < 0.05) than that in organically raised broilers. The high prevalence of C. jejuni in conventionally raised and organically raised broilers was also reported in other studies (5, 6, 13, 16, 42). In contrast, the predominant Campylobacter species in the conventional turkey operation was different from that in the organic turkey operation. C. coli and other Campylobacter species were the predominant species in conventionally raised turkeys, while C. jejuni was the predominant species in organically raised turkeys. Although C. coli and other Campylobacter species are the predominant Campylobacter strains isolated from conventionally raised turkeys in this study, it should be noted that the distributions of C. jejuni and C. coli plus other Campylobacter species in the conventional turkey operation are remarkably different among studies. As mentioned earlier, about 46% and 54% of Campylobacter isolates from conventionally raised turkeys in this study were identified as C. jejuni and C. coli plus other Campylobacter species, respectively, while Wallace et al. reported that almost 100% of Campylobacter isolates from conventional turkey flocks were C. jejuni (41). In contrast, Smith et al. revealed that 80% to 90% of Campylobacter strains colonizing turkey flocks were C. coli (36).
A significant difference (P < 0.001) in quinolone and fluoroquinolone resistance rates between Campylobacter strains isolated from conventional poultry operations and organic poultry operations was observed in this study. Since fluoroquinolones are used for therapeutic purpose only, it is not unusual that some conventional broiler and turkey flocks in this study were not treated with these antimicrobial agents. Although no fluoroquinolones were used in the conventional broiler flocks from which the samples were collected, they were used in previous flocks. In addition, because certain quinolone-resistant clones were stable and able to persist on the farms during several rotations even though there had been no selective pressure on that farm for a long period of time (31, 32) and because fluoroquinolone-resistant Campylobacter strains could out-compete fluoroquinolone-susceptible Campylobacter strains in the absence of antimicrobial usage (21), it is not surprising that a high fluoroquinolone resistance rate was observed among Campylobacter strains isolated from conventionally raised broilers in this study. This finding is consistent with previous studies by Pedersen and Wedderkopp and Price et al., who also reported that fluoroquinolone-resistant Campylobacter isolates continued to persist in the flocks that did not use these antimicrobial agents (31, 32). Since fluoroquinolones have never been used in organic poultry operations, it is not surprising that there was little or no resistance to this class of antimicrobials in Campylobacter strains isolated from organic poultry farms.
Compared to Campylobacter strains isolated from organic poultry operations, both C. jejuni and C. coli plus other Campylobacter species isolated from conventional poultry operations, particularly the isolates from conventionally raised turkeys, had significantly higher resistance rates (P < 0.05) not only to quinolone and fluoroquinolones but also to erythromycin, clindamycin, kanamycin, tetracycline, and ampicillin than the isolates from organically raised poultry. The high prevalence of multidrug-resistant Campylobacter strains observed in almost every conventional turkey farm in this study is interesting, since not all antimicrobial agents to which Campylobacter isolates from conventionally raised turkeys were resistant were used in those conventional turkey farms. However, due to the persistence and transmission of antimicrobial-resistant Campylobacter isolates, the antimicrobial resistance rate in a particular flock may not be directly correlated with the antimicrobial usage data. The occurrence of multidrug resistance among Campylobacter isolates from turkeys was also reported by Lee et al. (18).
The high prevalence of tetracycline resistance in Campylobacter isolates from organically raised broilers and turkeys observed in this study is quite interesting. Although tetracycline had never been used in those organic poultry farms, tetracycline-resistant Campylobacter strains were present in four out of five organic poultry farms surveyed in this study. The high prevalence of tetracycline resistance in Campylobacter isolates from the organic production system was also reported by other studies (10, 35). Tetracycline-resistant Campylobacter strains were not limited to the isolates from organic broilers and turkeys; these strains were also noticed among Campylobacter isolates from organic dairy cattle (35). Since tetracyclines have been used as feed additives for livestock and poultry for both therapeutic and subtherapeutic purposes for a long period of time (9, 14), it is possible that Campylobacter may have evolutionally become resistant to this class of antimicrobials, leading to the widespread distribution of tetracycline-resistant Campylobacter in animal reservoirs regardless of the production types. As with tetracycline resistance, the occurrence of kanamycin resistance was also observed in Campylobacter strains isolated from organically raised broilers and turkeys. However, these kanamycin-resistant Campylobacter strains were present mainly in only two organic poultry farms.
Interestingly, none of the C. jejuni and C. coli plus other Campylobacter species isolated from both conventionally raised and organically raised broilers and turkeys in our study was resistant to gentamicin. This finding is in agreement with previous studies by other research groups (19, 20), who reported that no gentamicin resistance was observed among Campylobacter isolates from poultry, except for one study from Spain (33), indicating that 25% of Campylobacter strains isolated from broilers were resistant to this antimicrobial. Although gentamicin was the most commonly used antimicrobial in conventionally raised broilers in this study, it was given to the birds at the hatchery by subcutaneous injection in the neck region. Since gentamicin is seldom absorbed in the gut (1) and Campylobacter is rarely present in the intestinal tracts of the birds during the first week of life, it is not surprising that the use of gentamicin has little or no impact on the selection of gentamicin resistance in Campylobacter species.
In this study, the difference in antimicrobial resistance rates between conventional poultry operations and organic poultry operations was observed mainly among C. coli and other Campylobacter species isolates rather than among C. jejuni isolates. Consistent with other findings (5, 19, 33), the high prevalence of antimicrobial resistance, particularly to erythromycin, clindamycin, and kanamycin, in this study was much more common in C. coli and other Campylobacter strains than in C. jejuni. A coresistance between erythromycin and clindamycin among Campylobacter isolates was also observed in this study as well as in other studies (19, 33, 38).
In summary, this study revealed significant differences in antimicrobial-resistant Campylobacter isolates between conventional poultry operations and organic poultry operations. The results suggest that the practice of antimicrobial usage in conventional poultry production systems influences the prevalence of antimicrobial-resistant Campylobacter organisms in conventionally raised broilers and turkeys. However, antimicrobial usage alone may not be solely responsible for the increased antimicrobial resistance in Campylobacter because even in the absence of antimicrobial exposure, a high level of tetracycline resistance was observed in organically raised broilers and turkeys. Similarly, the resistance rates to fluoroquinolones were also high in the surveyed conventional broiler flocks which were not directly exposed to the class of antimicrobials during the entire production period. These observations suggest that antimicrobial-resistant Campylobacter isolates are stable and able to transmit and persist in poultry even in the absence of selection pressure. Together, these findings reveal the complex nature of the occurrence and spread of antimicrobial resistance as well as underscore the difficulty in eliminating antimicrobial-resistant Campylobacter isolates, especially fluoroquinolone-resistant strains, from conventional poultry productions. In addition, this study also further highlights the need for prudent measures to prevent the occurrence and transmission of antimicrobial-resistant Campylobacter in the poultry reservoir.
Acknowledgments
We thank Sonya M. Bodeis at the Center for Veterinary Medicine, Food and Drug Administration, for her technical assistance in this study. We also thank Amna B. El-Tayeb, Elisabeth J. Angrick, and fellow colleagues at the Avian Disease Investigation Laboratory at Ohio State University for their help, advice, and technical support.
This work was supported by National Research Initiative competitive grants 00-51110-9741 and 2003-35212-13316 from the USDA Cooperative State Research, Education, and Extension Service and grant 2003-38640-13225 from the North Central Region program for Sustainable Agriculture Research and Education (NCR-SARE).
REFERENCES
- 1.Allos, B. M. 1998. Campylobacter, p. 1810-1817. In S. L. Gorbach, J. G. Bartlett, and N. R. Blacklow (ed.), Infectious diseases, 2nd ed. W. B. Saunders Company, Philadelphia, Pa.
- 2.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni-an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse, S. F., and L. K. Tollefson. 2003. Human campylobacteriosis: a challenge for the veterinary profession. J. Am. Vet. Med. Assoc. 223:445-452. [DOI] [PubMed] [Google Scholar]
- 5.Avrain, L., F. Humbert, R. L'Hospitalier, P. Sanders, C. Vernozy-Rozand, and I. Kempf. 2003. Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet. Microbiol. 96:267-276. [DOI] [PubMed] [Google Scholar]
- 6.Berndtson, E., U. Emanuelson, A. Engvall, and M. L. Danielsson-Tham. 1996. A 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 26:167-185. [Google Scholar]
- 7.Blaser, M. J. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl. 2):S103-S105. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2003. The National Antimicrobial Resistance Monitoring System-Enteric Bacteria, 2001 annual report. National Antimicrobial Resistance Monitoring System (NARMS), Atlanta, Ga.
- 9.Chopra, I., and M. Roberts. 2001. Tetracycline antimicrobials: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui, S., B. Ge, J. Zheng, and J. Meng. 2005. Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 71:4108-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Shibiny, A., P. L. Connerton, and I. F. Connerton. 2005. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Appl. Environ. Microbiol. 71:1259-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, S. J., and A. R. Sayers. 2000. A longitudinal study of campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 46:209-223. [DOI] [PubMed] [Google Scholar]
- 14.Fallon, R., N. O'Sullivan, M. Maher, and C. Carroll. 2003. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolates from broiler chickens isolated at an Irish poultry processing plant. Lett. Appl. Microbiol. 36:277-281. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, A., J. M. Nelson, T. J. Barrett, R. V. Tauxe, S. P. Rossiter, C. R. Friedman, K. W. Joyce, K. E. Smith, T. F. Jones, M. A. Hawkins, B. Shiferaw, J. L. Beebe, D. J. Vugia, T. Rabatsky-Ehr, J. A. Benson, T. P. Root, and F. J. Angulo. 2004. Antimicrobial resistance among Campylobacter strains, United States, 1997-2001. Emerg. Infect. Dis. 10:1102-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuer, O. E., K. Pedersen, J. S. Andersen, and M. Madsen. 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 33:269-274. [DOI] [PubMed] [Google Scholar]
- 17.Khachatourians, G. G. 1998. Agricultural use of antimicrobials and the evolution and transfer of antimicrobial-resistant bacteria. Can. Med. Assoc. J. 159:1129-1136. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, B. C., N. Reimers, H. J. Barnes, C. D'Lima, D. Carver, and S. Kathariou. 2005. Strain persistence and fluctuation of multiple-antimicrobial resistant Campylobacter coli colonizing turkeys over successive production cycles. Foodborne Pathog. Dis. 2:103-110. [DOI] [PubMed] [Google Scholar]
- 19.Li, C. C., C. H. Chiu, J. L. Wu, Y. C. Huang, and T. Y. Lin. 1998. Antimicrobial susceptibilities of Campylobacter jejuni and coli by using E-test in Taiwan. Scand. J. Infect. Dis. 30:39-42. [DOI] [PubMed] [Google Scholar]
- 20.Luber, P., J. Wagner, H. Hahn, and E. Bartelt. 2003. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli strains isolated in 1991 and 2001-2002 from poultry and humans in Berlin, Germany. Antimicrob. Agents Chemother. 47:3825-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo, N., S. Pereira, O. Sahin, J. Lin, S. Huang, L. Michel, and Q. Zhang. 2005. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antimicrobial selection pressure. Proc. Natl. Acad. Sci. USA 102:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen, S. A., and P. J. Fedorka-Cray. 2002. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 34:S93-S106. [DOI] [PubMed] [Google Scholar]
- 23.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nachamkin, I., B. M. Allos, and T. Ho. 1998. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 11:555-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachamkin, I., J. Engberg, and F. M. Aarestrup. 2000. Diagnosis and antimicrobial susceptibility of Campylobacter species, p. 45-66. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. American Society for Microbiology, Washington, D.C.
- 26.Nachamkin, I., H. Ung, and M. Li. 2002. Increasing fluoroquinolone resistance in Campylobacter jejuni, Pennsylvania, USA, 1982-2001. Emerg. Infect. Dis. 8:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacterial isolated from animals. Approved standard M31-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing; 12th informational supplement. M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northcutt, J. K., M. E. Berrang, J. A. Dickens, D. L. Fletcher, and N. A. Cox. 2003. Effect of broiler age, feed withdrawal, and transportation on levels of coliforms, Campylobacter, Escherichia coli, and Salmonella on carcasses before and after immersion chilling. Poult. Sci. 82:169-173. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen, K., and A. Wedderkopp. 2003. Resistance to quinolones in Campylobacter jejuni and Campylobacter coli from Danish broilers at farm level. J. Appl. Microbiol. 94:111-119. [DOI] [PubMed] [Google Scholar]
- 32.Price, L. B., E. Johnson, R. Vailes, and E. Silbergeld. 2005. Fluoroquinolone-resistant Campylobacter isolates from conventional and antimicrobial-free chicken products. Environ. Health Perspect. 113:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saenz, Y., M. Zarazaga, M. Lantero, M. J. Gastanares, F. Baquero, and C. Torres. 2000. Antimicrobial resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997-1998. Antimicrob. Agents Chemother. 44:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahin, O., T. Y. Morishita, and Q. Zhang. 2002. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 3:95-105. [DOI] [PubMed] [Google Scholar]
- 35.Sato, K., P. C. Bartlett, J. B. Kaneene, and F. P. Downes. 2004. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter spp. isolated from organic and conventional dairy herds in Wisconsin. Appl. Environ. Microbiol. 70:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, K., N. Reimers, H. J. Barnes, B. C. Lee, R. Siletzky, and S. Kathariou. 2004. Campylobacter colonization of sibling turkey flocks reared under different management conditions. J. Food Prot. 67:1463-1468. [DOI] [PubMed] [Google Scholar]
- 37.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, and the Investigation Team. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, D. E., and P. Courvalin. 1988. Mechanisms of antimicrobial resistance in Campylobacter species. Antimicrob. Agents Chemother. 32:1107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Threlfall, E. J., L. R. Ward, J. A. Frost, and G. A. Willshaw. 2000. The emergence and spread of antimicrobial resistance in foodborne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 40.Van den Bogaard, A. E., and E. E. Stobberingh. 1999. Antimicrobial usage in animals: impact on bacterial resistance and public health. Drugs 58:589-607. [DOI] [PubMed] [Google Scholar]
- 41.Wallace, J. S., K. N. Stanley, and K. Jones. 1998. The colonization of turkeys by thermophilic campylobacters. J. Appl. Microbiol. 85:224-230. [DOI] [PubMed] [Google Scholar]
- 42.Wedderkopp, A., E. Rattenborg, and M. Madsen. 2000. National surveillance of Campylobacter in broilers at slaughter in Denmark in 1998. Avian Dis. 44:993-999. [PubMed] [Google Scholar]
- 43.White, D. G., S. Zhao, S. Simjee, D. D. Wagner, and P. F. McDermott. 2002. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 4:405-412. [DOI] [PubMed] [Google Scholar]