Abstract
An improved methodology to remove black crusts from stone by using Desulfovibrio vulgaris subsp. vulgaris ATCC 29579, a sulfate-reducing bacterium, is presented. The strain removed 98% of the sulfates of the crust in a 45-h treatment. Precipitation of black iron sulfide was avoided using filtration of a medium devoid of iron. Among three cell carriers, Carbogel proved to be superior to both sepiolite and Hydrobiogel-97, as it allowed an easy application of the bacteria, kept the system in a state where microbial activity was maintained, and allowed easy removal of the cells after the treatment.
The formation of sulfuric acid on stonework causes the chemical transformation of insoluble calcium carbonate (CaCO3) into calcium sulfate dehydrate or gypsum (CaSO4 · 2H2O) (1, 2, 4). During the crystallization of gypsum, airborne pollutants, such as carbonaceous particles, are embedded in the mineral matrix and cause blackening in sheltered areas. None of the available mechanical and chemical treatments devised for the cleaning of stone altered by black crust has proved to be entirely satisfactory (3).
Microorganisms are generally associated with detrimental effects on stone (13, 15). However, it has been recently seen that they can be used for the removal of harmful compounds on artistic objects (5, 12, 14).
Gauri et al. (5) used Desulfovibrio desulfuricans for the cleaning of an old gypsum-encrusted marble statue that was previously consolidated. This was the first time that someone suggested the use of sulfate-reducing bacteria (SRB) for the removal of gypsum. The work of art had to be immersed in a growth medium for 84 h. This method had two drawbacks: firstly, this kind of treatment cannot be applied to large objects, such as buildings, as it necessitates the immersion of the object in a liquid medium; secondly, the consolidation of the statue prior to the treatment becomes mandatory to prevent the statue from undergoing severe damage due to the immersion. In addition, in the study by Gauri et al. (5) it was not fully proved that the treatment removed the gypsum, since its removal was evaluated only by visual observation and not by careful chemical analysis.
Later, Ranalli et al. (12) employed D. desulfuricans and Desulfovibrio vulgaris on two objects, an old marble “horse hoof” and an old marble column. As an improvement over the previous method, the authors used the inorganic material sepiolite as a delivery system. The cells colonized the carrier, allowing a close contact with the surface to be treated and the water that was provided. At the end of treatment, after 36 h, ion-exchange chromatography proved that sulfate removal by noncolonized and colonized sepiolite was 20% and approximately 80%, respectively. However, this method still required a long time (10 to 14 days) for the colonization of the sepiolite, and hydrogen sulfide could react with the iron in the medium, forming iron sulfide precipitates.
In the present work a new method for the removal of black crusts altering stone surfaces is proposed. The method is based on the application of SRB cells entrapped in a Carbogel carrier. In addition, the procedure definitively addresses the concern of iron sulfide formation.
According to the work of Ranalli et al. (12) the three strains D. desulfuricans ATCC 13541 and ATCC 29577 and D. vulgaris subsp. vulgaris ATCC 29579 were efficient for the removal of sulfates from stone. In the present work the strain D. vulgaris subsp. vulgaris ATCC 29579, which is able to reduce sulfates even under low oxygen tension (9), was used. Finally, this microorganism appeared interesting for other applications such as the consolidation of artistic and historical stone (6). Strain ATCC 29579 was maintained in the Desulfovibrio DSMZ 63 medium (K2HPO4, 0.5 g liter−1; NH4Cl, 1.0 g liter−1; Na2SO4, 1.0 g liter−1; CaCl2 · 2H2O, 0.1 g liter−1; MgSO4 · 7H2O, 2.0 g liter−1; dl-sodium lactate, 2.0 g liter−1; yeast extract, 1.0 g liter−1; resazurin, 1.0 mg liter−1; FeSO4 · 7H2O, 0.5 g liter−1; sodium thioglycolate, 0.1 g liter−1; ascorbic acid, 0.1 g liter−1) and incubated at 30°C for 4 days under anaerobic conditions.
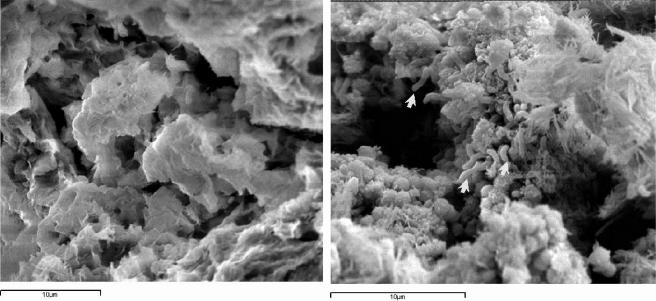
In order to allow easy application of bacteria, to keep a good contact between the cells and the surface to be treated, and to remove the cells after the treatment, the following three delivery systems were tested and compared: the mineral matrix sepiolite (Grupo Tolsa, Tulsa, Spain) and the two organic gels Hydrobiogel-97 (EniTecnologie, San Donato Milanese, Italy) and Carbogel (CST, Vicenza, Italy). Bacterial cells are entrapped in the organic gels in about 10 minutes during gel formation, whereas in the case of the inorganic matrix, they must colonize the matrix by growing and adhering on the surface of the particles, in a process lasting at least 2 days. Among the three carriers, Carbogel was chosen as the best delivery system for the high number of viable bacteria that were retained at the end of the experiment. After 20 h the cell number in Carbogel changed from 108 to 106 cells g−1, while with the other carriers the decrease of cell numbers was much greater, passing from 106 to 101 cells g−1 and 107 to 103 cells g−1 with sepiolite and Hydrobiogel-97, respectively (Table 1). Cell activity in the matrices was measured by ATP content with an enzymatic kit, NRM/Lumit-QM (Lumac B.V., Landgraaf, The Netherlands), in a Biocounter 1500 P luminometer (Lumac B.V.), equipped with a photomultiplier tube set at 7,200 relative luminescence units. ATP contents in the tree matrices measured after 0, 10, 20, and 30 h of incubation were always higher in Carbogel than in the two other matrices, in agreement with the number of cells (Table 1). Observations with a Zeiss DSM 940A LEO scanning electron microscope (Carl Zeiss GmbH, Oberkochen, Germany) indicated that cells with the typical morphology of D. vulgaris subsp. vulgaris ATCC 29579 were well embedded in the gel matrix (Fig. 1). On the basis of these data, Carbogel was selected for further experiments.
TABLE 1.
Cell counts of D. vulgaris subsp. vulgaris ATCC 29579 and total ATP content at different times of incubation of cells in three different delivery systems
| Time (h) | Cell count [log most probable number g−1 (±SD)]
|
ATP content [ng mg−1 (±SD)]
|
||||
|---|---|---|---|---|---|---|
| Carbogel | Hydrobiogel-97 | Sepiolite | Carbogel | Hydrobiogel-97 | Sepiolite | |
| 0a | 7.6 (0.2) | 7.5 (0.6) | 6.4 (0.5) | 2,920.0 (120) | 3,200.0 (140) | 128.0 (9.2) |
| 10 | 6.9 (0.1) | 5.6 (0.4) | 4.6 (0.3) | 970.0 (45.5) | 19.3 (2.2) | 1.50 (0.4) |
| 20 | 5.7 (0.3) | 3.3 (0.3) | 2.4 (0.4) | 60.5 (3.5) | 0.6 (0.1) | 0.22 (0.1) |
| 30 | 5.1 (0.4) | 3.1 (0.5) | 1.3 (0.4) | 25.0 (1.5) | 0.2 (0.05) | 0.01 (0.01) |
Determinations at t = 0 were performed immediately after the application of the carriers.
FIG. 1.
Scanning electron microscopy observations of Carbogel without (left) and with (right) D. vulgaris subsp. vulgaris ATCC 29579 cells. Arrows indicate bacterial cells. Bars = 10 μm.
A second technological problem raised by the application of SRB on a stone surface for the removal of sulfates in the black crusts is the precipitation of the generated sulfide. To overcome this potential problem, we adopted two strategies. The first was the use of a DSMZ 63 medium modified by eliminating any iron source. This was accomplished by omitting the FeSO4 · 7H2O. Strain ATCC 29579 pregrown in the standard DSMZ 63 medium efficiently grew in the modified medium devoid of iron when an inoculum of 8% (vol/vol) was used. Presumably the traces of iron present in the inoculum were sufficient to support the growth of the strain.
The second strategy to avoid sulfide precipitation on the stone was the filtration of the culture on a Rapida A Perfecte cellulose filter (Cartiera di Cordenons, Vicenza, Italy) with an 8-μm particle size range that allowed the bacteria to pass through but trapped the residual iron sulfide precipitates. The filtration step was important for a nonporous material like marble and absolutely necessary for a porous material like Serena stone. In Fig. 2, the filtration treatment can be easily appreciated, since it avoided the presence of undesirable dark stains on the stone following the application of Carbogel and cells. Following filtration, a final cell centrifugation and washing step were used to eliminate by-products, which could cause corrosion and undesirable stains on the stone surface due to bacterial metabolites in the exhausted medium.
FIG. 2.
Comparison of porous Serena stone specimens (10 by 10 by 5 cm) after treatment for 15 h with D. vulgaris subsp. vulgaris ATCC 29579 in nonfiltered DSMZ 63 medium-based Carbogel (left) or with D. vulgaris subsp. vulgaris ATCC 29579 in filtered, iron-free DSMZ 63 medium-based Carbogel (right).
The cell pellet was resuspended in anoxic phosphate buffer at pH 7.0 (KH2PO4, 0.408 g liter−1; K2H PO4, 0.522 g liter−1) added with 0.599 g liter−1 sodium lactate, to reach a cell density of around 108 cells ml−1. This cell concentration was previously shown to be effective for the removal of sulfates on altered stone (12).
Carbogel was hence slowly added to the bacterial suspension in the proportion of 0.13 g ml−1 of cell suspension. Carbogel was stirred for about 15 min under anoxic conditions until it took up the suspension. The final mixture should be stiff enough to remain on the surface of stone.
All the previous steps were conducted in an anaerobic box whereas all the following steps were performed under aerobic conditions. A uniform 0.5- to 1-cm-thick continuous layer of cells mixed into Carbogel was applied on the stone surface by using a sterile spatula. The layer was then covered with tissue paper soaked with phosphate buffer. The entire piece was covered with a layer of cling film to reduce oxygen diffusion and improve water retention in the Carbogel layer. The analysis of sulfate removal was performed during the treatment by removing the Carbogel layer from a small area of the stone with a sterile swab.
The entire procedure was applied to a marble fragment from the cathedral of Milan, Italy, strongly altered by a 2- to 3-mm-thick black crust (35% sulfates). The stone sample was divided into three pieces: one was treated with D. vulgaris subsp. vulgaris ATCC 29579 cells mixed into Carbogel, another was treated with Carbogel without microorganisms, and the last one was left untreated. To work with a high number of viable cells and to have a low amount of microbial metabolism by-products in the carrier, each application lasted 15 h. The treatment ceased only when the black crust removal was visually satisfactory. Three applications (for a total of 45 h) were needed (Fig. 3).
FIG. 3.
Black crust before (left) and after (right) three applications of 15 h each at room temperature of Carbogel entrapping cells of D. vulgaris subsp. vulgaris ATCC 29579. The treated surface is approximately 100 square centimeters.
The removal of the delivery system was performed using hydrophilic cotton and sterile swabs dampened with distilled water. A final microbiological analysis carried out on some powder scraped off from the surface demonstrated that no SRB cells remained on the surface, as no growth was seen in the DSMZ 63 medium and the ATP determinations provided the same values as those of the control.
Two methods were adopted to evaluate the efficiency of the biotreatment, ion-exchange chromatography (IC) and color measurements. For IC 0.1 g of sample was taken by scraping the surface with a scalpel. The sample was finely ground in agate mortar and exsiccated in a stove at 60°C, until weight was constant. Twenty ml of bidistilled deionized water, with conductivity of ≤3 mS cm−1, was added to the sample placed in a vessel with a flat bottom that was then hermetically closed in order to avoid evaporation (7). This solution was agitated for 72 h. A Dionex DX 120 ion-exchange chromatograph equipped with an AS14A 4- by 250-mm anion column plus an AG14A 4- by 50-mm precolumn and a CS12A 4- by 250-mm cation column plus a CG12A 4- by 50-mm precolumn was used. Two solutions (1.0 mM NaHCO3-8.0 mM Na2CO3 and 20 mM methanesulfonic acid), both at a flow rate of 1 ml min−1, were used as eluent for the anion and the cation columns, respectively. The measurements were carried out at 20 (±1)°C.
IC analysis showed that strain ATCC 29579 entrapped in the Carbogel matrix allowed removal of 98% of sulfates from marble, while Carbogel alone removed only 26% (Table 2). This confirmed the active role of the bacterium in the bioremoval process. The treatments efficiently removed chlorine, but this removal was associated with Carbogel only, since no differences in the removal efficiency could be observed between Carbogel with SRB cells and Carbogel without the cells.
TABLE 2.
Percentages of ions of intact Candoglia marble, untreated black crust, and black crust treated with Carbogel without SRB and with Carbogel with SRBa
| Sample | Cl− | NO3− | SO42− | Na+ | Mg2+ | Ca2+ |
|---|---|---|---|---|---|---|
| Sound marble | 0.49 ± 0.01 | 0.00 | 1.84 ± 0.03 | 0.00 | 0.00 | 1.58 ± 0.02 |
| Untreated black crust | 1.78 ± 0.02 | 0.00 | 35.47 ± 0.39 | 0.00 | 0.00 | 18.17 ± 0.24 |
| Black crust treated with Carbogel without SRB | 0.20 ± 0.02 | 0.20 ± 0.01 | 26.10 ± 0.08 | 0.10 ± 0.01 | 0.10 ± 0.01 | 10.50 ± 0.03 |
| Black crust treated with Carbogel plus SRB | 0.24 ± 0.03 | 0.30 ± 0.02 | 0.71 ± 0.03 | 0.05 ± 0.02 | 0.00 | 0.95 ± 0.02 |
Values are the means of three measurements (± standard deviations) and are expressed as percentages of weights of the sample powders.
The treatment with SRB mixed into Carbogel removed calcium ions from the crust, likely calcium bonded in the gypsum, more efficiently than Carbogel alone, with removal efficiencies of 95% and 42%, respectively. It is worth considering that the ratio SO42−:Ca2+ was 2:1 in the untreated black crust and the black crust treated with Carbogel without SRB cells, while in the samples treated with Carbogel with cells it was shown to be 1:1, the same ratio as that of the intact marble. This result suggests that the composition of the bioremediated sample for these ions was restored to that of the intact material. Finally, the iron that was probably present in the Candoglia marble itself (11) was reasonably not available, as no iron sulfide precipitation was observed.
The color was measured with a spectrophotometer (Miniscan with light D65; Hunter Lab, Bergamo, Italy) and observation at 10°. The chromatic coordinates L* (brightness), a* (difference between green and red), and b* (difference between yellow and blue) of stone surfaces were determined according to the CIELAB 1976 system (8). The treatment with SRB mixed into Carbogel led to a remarkable difference between the brightness of the cleaned sample and that of the untreated altered surface. Before and after the treatment the brightness changed respectively from 42.4 to 73.9 (getting closer to the value of the polished stone, which is 89.3), whereas a* changed from 2.0 to 1.5 and b* remained constant, being in both cases 7.7 (polished stone a* and b* are −0.1 and 8.7, respectively). According to the CIELAB 1976 system, the total difference of color between two samples is Δe = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2, where Δe > 5 is perceived by the human eye (10). The comparison of the untreated stone with the polished stone and of the treated stone with the polished stone indicated that both Δa* and Δb* were always less than or equal to 1, whereas ΔL* was, in the first case, 46.9 and, in the latter case, 15.4. In our study Δe was largely due to the variation of L*, Δa* and Δb* being considerably smaller.
The overall data showed that the treatment with Carbogel-entrapped SRB for the removal of black crusts from the altered Candoglia marble from the cathedral of Milan was very successful. However, the feasibility of the treatment should be evaluated on a case-by-case basis. The number of applications and the overall treatment time must be evaluated considering different factors such as the chemical nature and thickness and uniformity of the black crust that can vary greatly depending on the material, location, climate, and atmospheric pollution. In particular, the biotechnological approach proposed here seems to be suitable for precious objects for which traditional cleaning treatments cannot be completely effective.
Acknowledgments
We thank Eric May, University of Portsmouth, for helpful discussion. Marco Realini (Centro Bozza, Istituto per la Conservazione e la Valorizzazione dei Beni Culturali, CNR, Milan, Italy) and the Veneranda Fabbrica del Duomo di Milano (Milan, Italy) are acknowledged for providing Candoglia marble altered by a black crust. EniTecnologie S.p.A. is acknowledged for providing Hydrobiogel-97.
This work is part of the project BIOBRUSH (Bioremediation for Building Restoration of the Urban Stone Heritage in European States, no. EVK4-2001-00055), funded by the European Union. F.C. is a research fellow funded by MIUR in the ambit of the Rientro dei Cervelli program.
REFERENCES
- 1.Ausset, P., R. A. Lefèvre, and M. Del Monte. 2000. Early mechanisms of development of sulfated black crusts on carbonate stone, p. 329-337. In V. Fassina (ed.), Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy. Elsevier Science, Amsterdam, The Netherlands.
- 2.Bugini, R., M. Laurenzi Tabasso, and M. Realini. 2000. Rate of formation of black crusts on marble. A case study. J. Cultural Heritage 1:111-116. [Google Scholar]
- 3.Campanella, C. 1990. La conservazione della “pelle” lapidea: dalle tecniche tradizionali all'impiego del Laser. Recuperare 50:610-613. [Google Scholar]
- 4.Gauri, L. K., A. N. Chowdhury, N. P. Kulshreshtha, and A. R. Punuru. 1989. The sulfation of marble and the treatment of gypsum crusts. Stud. Conserv. 34:201-206. [Google Scholar]
- 5.Gauri, L. K., L. Parks, J. Jaynes, and R. Atlas. 1992. Removal of sulphated-crust from marble using sulphate-reducing bacteria, p. 160-165. In G. M. Robin (ed.), Stone cleaning and the nature, soiling and decay mechanisms of stone. Proceedings of the International Conference, 14 to 16 April 1992. Donhead Publishing Ltd., Webster, Edinburgh, United Kingdom.
- 6.Heselmeyer, K., U. Fischer, W. E. Krumbein, and T. Warscheid. 1991. Application of Desulfovibrio vulgaris for the bioconversion of rock gypsum crusts into calcite. BIOforum 1/2:89. [Google Scholar]
- 7.Istituto Centrale del Restauro and Consiglio Nazionale delle Ricerche. 1994. Dosaggio dei Sali Solubili. NORMAL 13/83 document. ICR-CNR, Rome, Italy.
- 8.Istituto Centrale del Restauro and Consiglio Nazionale delle Ricerche. 1994. Misure colorimetriche di superficie. NORMAL 43/93 document. ICR-CNR, Rome, Italy.
- 9.Johnson, M. S., I. B. Zhulin, M. E. Gapuzan, and B. L. Taylor. 1997. Oxygen-dependent growth of the obligate anaerobe Desulfovibrio vulgaris Hildenborough. J. Bacteriol. 179:5598-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palazzi, S. 1995. Colorimetria. La scienza del colore nell'arte e nella tecnica, p. 91. Nardini Editore, Florence, Italy.
- 11.Pedrazzani, R., I. Alessandri, E. Bontempi, F. Cappitelli, E. Pantos, L. E., L. Toniolo, and L. E. Depero. 2006. Study of sulphatation of Candoglia marble by means of micro X-ray diffraction experiments. Appl. Phys. A Mater. [Online.] doi: 10.1007/s00339-006-3539-7. [DOI]
- 12.Ranalli, G., M. Chiavarini, V. Guidetti, F. Marsala, M. Matteini, E. Zanardini, and C. Sorlini. 1997. The use of microorganisms for the removal of sulphates on artistic stoneworks. Int. Biodeterior. Biodegrad. 40:255-261. [Google Scholar]
- 13.Ranalli, G., E. Zanardini, P. Pasini, and A. Rota. 2003. Rapid biodeteriogen and biocide diagnosis on artworks: a bioluminescent low-light imaging technique. Ann. Microbiol. 54:1-13. [Google Scholar]
- 14.Ranalli, G., G. Alfano, C. Belli, G. Lustrato, M. P. Colombini, I. Bonaduce, E. Zanardini, P. Abbruscato, F. Cappitelli, and C. Sorlini. 2005. Biotechnology applied to cultural heritage: biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 96:73-83. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Moral, S., L. Luque, J. C. Cañaveras, L. Laiz, V. Jurado, B. Hermosin, and C. Saiz-Jimenez. 2004. Bioinduced barium precipitation in St. Callixtus and Domitilla catacombs. Ann. Microbiol. 53:1-12. [Google Scholar]