Abstract
Antibody-based assay systems are now accepted by regulatory authorities for detection of the toxins produced by phytoplankton that accumulate in shellfish tissues. However, the generation of suitable antibodies for sensitive assay development remains a major challenge. We have examined the potential of using the chicken immune system to generate high-affinity, high-specificity recombinant antibody fragments against phytotoxins. Following immunization of the chicken with domoic acid-bovine serum albumin, a single-chain antibody variable region (scFv) gene library was generated from single VH and VL genes isolated from the immune cells in the spleen and bone marrow. scFvs reacting with domoic acid were isolated by phage display and affinity matured by light chain shuffling, resulting in an approximate 10-fold increase in sensitivity. The isolated scFvs were effectively expressed in Escherichia coli and readily purified by affinity chromatography. They were then used to develop a convenient and sensitive indirect competitive enzyme-linked immunosorbent assay for domoic acid, with a 50% effective dose of 156 ng/ml, which could be used reliably with shellfish extracts. This study demonstrates that chickens provide a valuable model system for the simplified, rapid generation of high-affinity recombinant antibody fragments with specificity for small toxin molecules.
The accurate quantification of algal toxins in environmental and food samples is critical for consumer protection. The levels of these low-molecular-weight toxins are typically measured using either bioassay or high-performance liquid chromatography methods (16, 23), both of which are time-consuming, relatively low-throughput, and often expensive procedures (11, 13). Immunoassays, by contrast, are more suited to high-throughput screening, while being sensitive and highly specific (30), and recent changes in European Union regulations governing the sale of shellfish (8) indicate that immunoassays are becoming more acceptable for shellfish monitoring programs.
Immunoassay techniques have previously relied on hyperimmune polyclonal sera from rabbits, sheep, or other mammals. These are relatively easy to produce in large quantities but do not offer the consistency required for large-scale application or commercial production. More recently, monoclonal antibodies have been favored because they can be produced in unlimited supply and are more easily standardized. However, monoclonal antibodies have their own set of difficulties, as they are almost exclusively murine in origin (10) and are laborious to screen, and small mammals such as mice do not always provide the high-affinity antibody response to small hapten molecules needed for sensitive assay development (22).
The limitations of traditional techniques have led several research groups to investigate the use of phage display to produce antihapten antibodies. The phage display of recombinant antibody libraries is a robust monoclonal antibody technology that is an increasingly attractive method, as it allows the screening of antibody repertoires from any species in which the immunoglobulin DNA sequences have been characterized (20). These technological advances have led to a number of successful studies using mice (25), rabbits (18), and sheep (6) to produce single-chain antibody fragments (scFv) with high affinities for hapten molecules. scFv provide the most commonly used recombinant antibody format, as they can be rapidly constructed, are typically well expressed in Escherichia coli, and can exhibit high affinity and stability (4).
Birds are increasingly used in biotechnology research as a model animal for polyclonal antibody generation due, in part, to the convenience of yolk antibody (immunoglobulin Y [IgY]) monitoring (31), but also because chickens are cheaply maintained and often respond strongly to mammalian protein antigens (5, 24, 27). More recently, systems have been described for the use of the chicken as an animal model in the generation of antihapten recombinant antibodies (2), but this study did not demonstrate the usefulness of the resulting antibodies in small molecule detection. The chicken is an ideal model for simplified recombinant antibody library generation, since the chicken immunoglobulin repertoire derives from single VH and VL germ line sequences (21). The PCR amplification of chicken V genes is therefore performed with a small primer set (2, 7, 29), which saves time and resources. The small primer set also reduces rare transcript loss due to differences in primer efficiencies, which can be expected when using the complex V-region primer sets designed for mammalian repertoire cloning (4).
This paper describes the isolation and in vitro maturation of a chicken scFv directed against the low-molecular-mass (312-Da) algal toxin domoic acid (DA), its convenient production and purification, and application in the development of a simple competitive enzyme immunoassay for detection of the toxin in shellfish extracts. This is the first description of the use of chicken-derived scFvs in the development of a validated assay for detection of environmental toxins and demonstrates the convenience of this approach for the development of robust antihapten immunoassays.
MATERIALS AND METHODS
Preparation of conjugates.
Domoic acid (Calbiochem) was conjugated to bovine serum albumin (BSA) and ovalbumin (OVA). In both cases, 1 mg of DA was diluted in 50 μl dimethyl sulfoxide (DMSO) before the addition of 20 μl 1-ethyl-3-dimethylaminopropyl carbonimide (25 mg/ml in DMSO) and 30 μl N-hydroxysuccinimide (30 mg/ml in DMSO). This reaction mixture was stirred for 90 min at room temperature before adding 250 μl OVA or BSA (both 20 mg/ml in 0.085 M borate buffer) dropwise and stirring for a further 90 min. The conjugate was then dialyzed 1:5,000 against phosphate-buffered saline (PBS) overnight at 4°C.
Immunizations and serum characterization.
Two adult male Leghorn chickens were immunized subcutaneously, at 21-day intervals, with 50 μg of DA-BSA in a total volume of 400 μl. The first dose was prepared with complete Freund's adjuvant, and three subsequent doses were administered with incomplete Freund's adjuvant. Seven days after the third inoculation, serum was collected to monitor polyclonal antibody response. Serum anti-DA response was evaluated via direct enzyme-linked immunosorbent assay (ELISA) analysis as follows: 96-well immunoassay plates (Maxisorp; Nunc) were coated overnight at 4°C with 50 μl/well DA-OVA at 10 μg/ml. Wells were then blocked for 1 h at 37°C with 250 μl/well of PBS-5% skim milk (PBS-M). Serum samples were serially diluted in PBS-M-0.1% (vol/vol) Tween 20 (PBS-MT) and added to the plates (50 μl/well). Plates were then incubated at 37°C for 1 h, washed 10 times with PBS-0.1% Tween 20 (PBS-T), and probed for 1 h at 37°C with horseradish peroxidase (HRP)-conjugated anti-chicken IgG antibody (Pierce) in PBS-MT. Negative control reactions were performed by coating with native OVA and probing as described above. After washing with PBS-T, the wells were developed with 100 μl/well HRP substrate, tetramethylbenzidine (DAKO), and reactions were stopped with 100 μl/well 1 M H2SO4. All analyses were performed in triplicate.
Antibody library construction.
Once a high-titer DA-specific serum IgG response had been demonstrated for both chickens, the final inoculation was performed. Seven days later, total RNA was isolated from the spleen and bone marrow of one femur from each chicken, as previously described in detail (2). The total RNA samples from both chickens were then pooled, and first-strand cDNA synthesis was performed using 20 μg of total RNA in a 100-μl reaction volume (Superscript 2; Invitrogen). The scFv library (VL-peptide linker sequence-VH) with the peptide linker GGSSRSSSSGGGGSGGGG was constructed from cDNA by overlap extension PCR (14), as previously described (2).
Electroporations and estimation of library size and diversity.
The ligated scFv phagemid libraries were introduced into 300 μl E. coli XL1-Blue cells (Stratagene) by electroporation (Gene-pulser; Bio-Rad). Two electroporations were performed at 2.5 kV, 25 μF, and 200 Ω. Total library sizes were then estimated by antibiotic resistance plate counting on Luria-Bertani agar containing 100 μg/ml carbenicillin (Sigma). The completed library preparations were propagated in E. coli XL1-Blue cells, and expression of phage-scFv was induced by coinfection with VCSM13 helper phage (Stratagene).
Twenty clones were randomly chosen from library-size titration plates, and each clone was examined for scFv insertion by colony pick PCR (94°C for 5 min, then 35 cycles of 94°C for 20 s, 56°C for 30 s, 72°C for 90 s, and final extension at 72°C for 10 min). For initial analysis of sequence diversity, amplification products were restriction digested using the endonuclease BstOI (Promega). The banding patterns of the resulting digest products were examined by electrophoresis in a 4% Tris-acetate-EDTA-agarose gel.
Biopanning of antibody libraries against antigen.
Biopanning of antibody libraries (9) against DA-OVA was performed in Maxisorp immunotubes (Nunc). Briefly, tubes were coated overnight at 4°C with 1 ml of DA-OVA and then blocked with 3% OVA-PBS (wt/vol). Phage-scFv suspended in 1% OVA-PBS was then added (1 ml, or 1011 to 1012 phage particles) and mixed for 1 h at room temperature with slow rotation. Tubes were then washed 15 times with PBS-0.1% Tween and 15 times with PBS, and bound phage was finally eluted with 1 ml 100 mM glycine-HCl (pH 2.2) for 10 min. Eluted phage were neutralized using 2 M Tris base, and propagation of eluted phage and further manipulations were then carried out, as previously described (2). Four rounds of panning were performed, with increasing selection stringency mediated by a progressive reduction in coating antigen concentration (50 μg/ml in the first panning round and 20, 10, and 5 μg/ml in subsequent rounds).
ELISA analysis of phage-scFv preparations.
Phage-scFv suspensions from each round of panning against DA-OVA were tested for specific binding to antigen by direct ELISA analysis. Immunoassay plates were coated and blocked as described above, and threefold dilutions of phage-scFv preparations (in PBS-M) were added to the wells. M13 helper phage (diluted as described above) was included as a negative control. Chicken serum (1:5,000 in PBS-MT) was also used to probe wells as a positive control. After 2 h of incubation at 37°C, plates were washed with PBS-T and probed with HRP-conjugated anti-M13 antibody (Amersham Pharmacia) in PBS-M for 1 h at 37°C. Positive controls were probed with anti-chicken IgG-HRP (Pierce). After extensive washing with distilled water, the wells were developed as described above. All analyses were performed in triplicate.
A 96-well induction of single clones and direct ELISA analysis of scFv production.
Thirty-two random clones in E. coli XL1-Blue cells were chosen from output plates from each of rounds 2, 3, and 4 of panning and inoculated into 2-ml deep-well plates (Nunc) containing 0.5 ml super broth (SB) plus 100 μg/ml carbenicillin, and plates were incubated for 6 h at 37°C, 300 rpm. Isopropyl-β-d-thiogalactoside (IPTG) was then added to each well at a final concentration of 2 mM, and scFv induction was carried on overnight. Cells were then pelleted by centrifugation at 2,500 × g for 15 min at 4°C, and supernatants were removed and stored at 4°C. Periplasmic scFv was liberated by resuspending the cell pellets in 100 μl PBS and two rounds of freeze-thawing at −80°C and 37°C. Plates were then centrifuged again as described above, and supernatants were removed and added to their respective culture supernatant. Direct ELISA analysis of binding to DA-OVA conjugate was then performed as described above, but antihemagglutinin (HA)-HRP conjugate was used to detect scFv binding (Roche).
Small-scale production of soluble scFv.
Bacterial pellets from rounds 3 and 4 of panning were used to perform plasmid purifications (Midi-Prep; QIAGEN). The resulting plasmid preparations were introduced into E. coli strain TOP 10F′ (Invitrogen) by electroporation as described above (but in 50-μl cell volumes). An aliquot from each electroporation was plated on Luria-Bertani-carbenicillin agar to provide single colonies for the examination of single-clone functional properties. Single colonies were cultured overnight in 5 ml SB plus 50 μg/ml carbenicillin (37°C, 250 rpm shaking). Fifty microliters of each clone was then added to 50 ml SB (plus 50 μg/ml carbenicillin and 20 mM MgCl2), and antibody production was induced by overnight culture as described above, with the addition of 2 mM IPTG.
To isolate soluble antibody, the bacterial cells were pelleted by centrifugation (3,000 × g, 4°C, 15 min) and resuspended in equilibration buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0) containing 20 mM phenylmethylsulfonyl fluoride. Periplasmic antibody was then liberated by sonication (2), and soluble scFv antibodies were purified by nickel chelation chromatography (Vivapure MC mini H; Vivascience).
Evaluation of scFv by indirect competitive ELISA.
Ninety-six-well immunoassay plates (Maxisorp; Nunc) were coated overnight at 4°C with DA-OVA at a concentration of 1 μg/ml in PBS (50 μl/well). Wells were then blocked with PBS-M for 1 h at 37°C (250 μl/well). Serial doubling dilutions of DA were performed in PBS-0.1% BSA-0.1% Tween 20 (PBS-BSA-T), and a subsaturating concentration of nickel-purified scFv was added to each dilution and incubated for 1 h at room temperature. Blocked plates were then washed as described above, and 50 μl/well of scFv-DA (at each concentration) was added in triplicate for 1 h at 37°C. Plates were then washed with distilled water, and scFv binding was detected by probing with anti-HA-HRP and developing as described above. The percent binding of the scFv was plotted against the DA concentration, and the 50% effective doses (ED50s; dose of DA causing 50% displacement of the scFv) were determined.
Light chain shuffling and affinity maturation.
To optimize light chain association characteristics, the VH regions of plasmid pools from pannings 3 and 4 were amplified by PCR (100 ng pCOMB3X phagemid was added per reaction mixture) in the following cycles: 94°C for 5 min, then 20 cycles of 94°C for 15 s, 56°C for 15 s, 72°C for 1 min, and final extension at 72°C for 10 min. The VH sequences were gel purified as described above and recombined with the light chains, originally generated from cDNA, by overlap PCR as described above. This light chain shuffled library was then restriction digested, ligated into pCOMB3X, and introduced into E. coli XL1-Blue as for library generation. This secondary library was then subjected to phage display and tailored affinity selection (19), with minor modifications. In pan 1, 10 μg DA-OVA was used to coat the immunotube and phage binding incubation was for 1 h, with washing performed as described above. In pan 2, 2 μg of coating antigen was used, followed by 30 min of phage incubation, and 0.1 μM free DA was then added for 30 min as free antigen challenge. Pan 3 was prepared as with pan 2, but with 2 μM free DA antigen challenge for 3 h, and pan 4 was as with pan 2, but with 1 μM free DA antigen challenge for 24 h. Elution of phage in each panning round was achieved by addition of 1 ml trypsin (Sigma) in PBS (10 mg/ml) and incubation at 37°C for 30 min with vigorous shaking.
Large-scale production of scFv.
Selected scFv clones in E. coli TOP 10F′ were cultured overnight in 5 ml SB (plus 50 μg/ml carbenicillin) at 37°C at 250 rpm. This starter culture was then used to seed 1 liter of SB (plus 50 μg/ml carbenicillin and 20 mM MgCl2), which was cultured for approximately 8 h until the optical density reached between 0.8 and 1.5. Protein production was then induced with 0.01 mM IPTG overnight at 37°C at 250 rpm. Bacteria were harvested by centrifugation (4,000 × g, 4°C, 15 min), resuspended in 10 ml equilibration buffer (as described above), and lysed by sonication. Cell debris was removed by centrifugation (40,000 × g, 4°C, 30 min), and the supernatant was filtered through a 0.2-μm syringe filter. Antibody fragments were purified by metal chelate chromatography using Ni-nitrilotriacetic acid (NTA)-agarose (QIAGEN). Nickel-purified samples were concentrated, rediluted with 5 volumes of PBS, and concentrated again, to a final volume of 1 ml (all performed using 5-kDa cutoff spin filtration tubes [Vivascience]). Protein concentrations on final products were estimated by BCA assay (Pierce). For prolonged storage the scFv concentrates were mixed 50:50 with glycerol and stored at 4°C.
DNA sequencing of single scFv clones.
Clones R4-11c, R4Sh-2, and R4Sh-7 were propagated in E. coli TOP 10F′, and plasmid DNA was isolated from the bacterial pellets (Midiprep system; QIAGEN). Multiple-read sequencing reactions were performed for both strands of the complete scFv inserts (MWG Biotech).
Optimized ELISA procedure and application to shellfish analysis.
An optimized protocol for the indirect competitive ELISA for DA was determined and validated using purified R4Sh-7. Microtiter plate wells were coated overnight at 4°C with 150 μl/well DA-OVA at 1.5 μg/ml and blocked with 250 μl/well of PBS-3% skim milk for 1 h at 37°C. A 1-mg/ml stock solution of DA was prepared in PBS, from which standard solutions ranging from 2,000 to 31.3 ng/ml (6.4 to 0.1 μM) were prepared in PBS-T-0.1% BSA. Standard solutions (20 μl) were added in duplicate to coated wells, followed immediately by 100 μl of the appropriate dilution of scFv in the same buffer. The plate was shaken briefly, incubated for 1 h at 37°C, and washed with 150 mM NaCl, 0.05% Tween 20. Anti-HA-HRP was then added (100 μl of a 1:1,000 dilution per well) and incubated for 1 h at 37°C. Wells were washed, and color was developed as described above.
Scallop tissues—gonads, hepatopancreas, and abductor muscle—were obtained from the Marine Institute, Galway, Ireland, from the institute's routine DA screening program. Extracts of shellfish tissues were prepared following the protocol used for high-performance liquid chromatography analysis (13). Briefly, 4 g of tissue was homogenized (Ultra-Turrax; IKA) for 3 min in 16 ml of extraction buffer (50% methanol, 50% water). The extracts were collected by centrifugation and filtered at 0.2 μm prior to analysis.
RESULTS
Construction and selection of anti-DA scFv library from immunized chickens.
Serum IgY antibody responses were analyzed after the second booster immunization, and both chickens had produced a high-titer (≥1:64,000) response. Serum from one of the chickens gave an ED50 of 1 μg/ml DA in the competitive enzyme immunoassay, showing the response was specific for DA. Therefore, after a final immunization, spleen and femur bone marrow were harvested from each chicken and the C1αDA library was synthesized from the pooled tissue cDNA of both animals. After electroporation of E. coli XL1-Blue, the finished library had a calculated size of 3.1 × 108 total transformants, with a vector self-ligation background of <0.5%. PCR amplification of plasmid inserts from 20 randomly chosen library clones showed 100% carriage of the ∼800-bp expected scFv DNA sequences (data not shown).
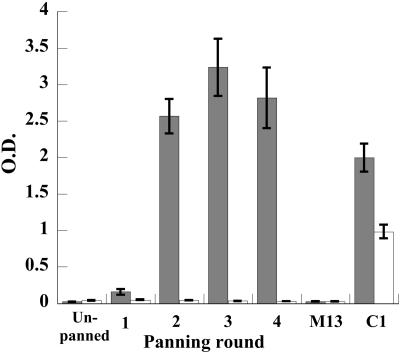
During the first panning process, the C1αDA library exhibited a >100-fold increase in phage output from round 1 to round 3 of selection (8.0 × 104 in round 1, 4.8 × 105 in round 2, and 5.0 × 107 in round 3), with 4 × 1012 to 7 × 1012 input phage in each round, which suggested there was an enrichment of antigen-specific phage-scFv. To confirm enrichment had occurred, we performed a phage-scFv ELISA using the phage pool from each panning against both the DA-OVA conjugate and the carrier molecule, OVA. This analysis demonstrated increasing signal in each round against DA (Fig. 1), with a strong conjugate-specific response observed after the second round (>10 times the background signal). Maximal response was observed in the third round. No concomitant increase in signal was observed against the carrier OVA, and no interactions were observed between the anti-M13 phage antibody and either OVA or DA-OVA conjugate. When soluble antibody expression was induced in 96-well plates from pan 2 clones, 84% of randomly chosen clones exhibited an absorbance signal (at 450 nm) of >1.5 in a direct ELISA against DA-OVA (negative clones all gave a signal of <0.03). In pans 3 and 4, 100% of clones showed similarly high levels of signals.
FIG. 1.
Direct ELISA analysis of phage-scFv binding to DA-OVA (shaded boxes) and OVA (clear boxes) from the unpanned library through four rounds of panning. M13 is VCSM13 helper phage diluted 1:4. C1 is chicken anti-DA antiserum (1:5,000). Error bars indicate standard deviations. O.D., optical density.
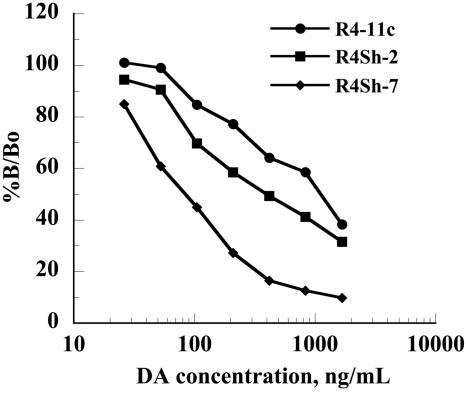
Given that antibodies that recognize hapten-protein conjugate but not free hapten are frequently generated, we ascertained the specificity of the isolated scFv fragments. Single scFv clones (12) from pannings 3 and 4 were induced in small-scale bacterial culture to produce soluble scFv, which were readily purified using spin column Ni-NTA chromatography, as analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Each soluble scFv sample showed specific displacement by DA in the direct competition ELISA, with the most sensitive clone, R4-11c, exhibiting an ED50 of approximately 1 μg/ml (Fig. 2).
FIG. 2.
Indirect competitive ELISA analysis of clones R4-11c, R4Sh-2, and R4Sh-7 against immobilized DA-OVA in the presence of an increasing free DA concentration. All analyses were performed in triplicate on three separate occasions. The binding response is shown as the percent binding (B) divided by the percent binding with no competing DA (Bo).
Affinity maturation by chain shuffling.
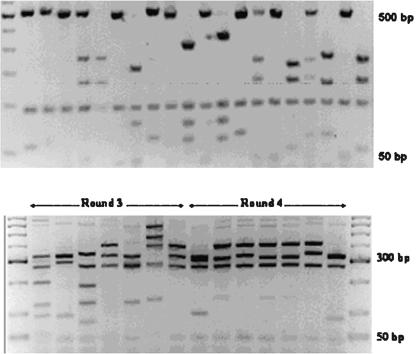
Clonal diversity was then analyzed within 20 initial library insert fragments, as well as among 10 antigen-specific insert sequences from round 4 of panning, by restriction digest using the frequent-cutting endonuclease BstOI. Among the 20 initial fragments, no repetition of banding pattern was observed, suggesting that the C1αDA library is highly diverse (Fig. 3). The clones isolated from round 4 of the first panning series appeared to be considerably less diverse, having only three discernibly different banding patterns (data not shown).
FIG. 3.
Restriction digest analysis of sequence diversity within randomly chosen clones from unselected library (BstOI digest) (top) and rounds 3 and 4 of tailored affinity selection (AluI digest) (bottom).
These data suggested that there was still considerable sequence diversity in the selected antibody populations of rounds 3 and 4. To optimize antibody VH-VL associations and to try to produce a more sensitive scFv, the PCR-amplified VH regions from both rounds were recombined with the original VL pool, derived from chicken cDNA. This VH-biased light chain shuffled library was then reselected using a high-stringency panning technique which involved low concentrations of coating antigen, short binding periods, and challenge of phage binding by free antigen. In this panning series, an overall decrease in output phage was observed (from 1.5 × 107 in round 1 to 2.0 × 105 in round 4), due to the extreme stringency of the panning conditions, as previously observed (19).
Insert diversity among clones from rounds 3 and 4 of high-stringency panning was then analyzed as described above, but using the very-high-frequency cutting endonuclease AluI, as BstOI digest suggested only one clone had been selected in round 4. AluI has been shown previously to be a more efficient enzyme for differentiating between highly related antibody sequences than BstOI (12). Among seven clones from each of rounds 3 and 4 (Fig. 3), there were 10 observably different sequences (six different patterns from round 3 and four high-similarity banding patterns in round 4). These data showed effectively that high-stringency tailored affinity selection leads to the selection of a restricted subset of light chain optimized antibody sequences.
To examine whether the tailored affinity selection process had been effective in raising higher-affinity antibodies for DA, the 14 clones that had been subjected to restriction analysis were induced to produce antibody in small-scale culture and the scFv were purified as described above. In the indirect competitive ELISA, 12 of the scFv exhibited poorer recognition (ED50 ≥ 2.0 μg/ml) of free DA than clone R4-11c. One clone, designated R4Sh-2, showed an ED50 of 400 ng/ml, more than twofold lower than R4-11c (Fig. 2). One further clone, R4Sh-7, appeared to have a considerably increased affinity, exhibiting an ED50 of approximately 100 ng/ml, a 10-fold improvement in affinity for free antigen over R4-11c (Fig. 2).
Sequence analysis of isolated clones.
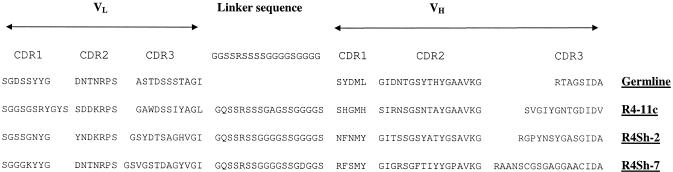
Sequencing analysis of scFv VH and VL regions was performed for clones R4-11c, R4Sh-2, and R4Sh-7. All sequences were confirmed by BLAST search (1) to be consistent with functionally rearranged chicken VH and VL regions. Alignment analyses were then performed for each VH and VL sequence against their respective chicken germ line sequence. All clones exhibited considerable divergence from the germ line in the CDR1 and CDR3 regions, with less variability in the CDR2 region (Fig. 4). These observations suggested that the sequences of the antibodies isolated were all generated in an antigen-driven response, rather than being serendipitously isolated naive IgM-like sequences. Clones R4-11c, R4Sh-2, and R4Sh-7 differ greatly in their respective CDR-encoding sequences, especially in the VH regions (Fig. 4). The lengths of their CDR3s also vary, being 13, 14, and 18 amino acid (aa) residues for the VH of R4-11c, R4Sh-2, and R4Sh-7, respectively. A similar trend of increasing length is observed for the VL CDR3 regions, as affinity increases from R4-11c (11 aa) to R4Sh-2 (12 aa) to higher-affinity R4Sh-7 (13 aa). Several amino acid substitutions were noted in the linker sequence of each scFv (predominantly G-S, G-Q, or S-G) when compared with the original linker design. There was also a change from G to D at position 15 of R4Sh-7. There were no changes in linker length (Fig. 4).
FIG. 4.
Amino acid sequence comparison of CDRs 1 to 3 and linker sequences for clones R4-11c, R4Sh-2, and R4Sh-7 versus the germ line sequence. The uppermost linker sequence represents the putative primer-encoded overlap sequence.
Large-scale production and purification of soluble scFv.
In order to be of use in development of assays for large-scale screening, it must be possible to produce sufficient quantities of the scFv antibodies quickly and conveniently and the scFv must be stable. The three sequenced anti-DA scFv were all efficiently expressed in bacterial culture, repeatedly yielding 3 to 5 mg of nickel-purified protein per liter of culture, with very consistent performance between batches. Purified scFv was stable in glycerol at 4°C for at least 9 months.
ELISA development.
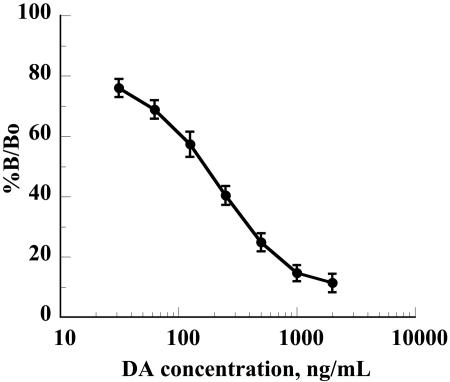
scFv R4Sh-7 was then used to develop a convenient, indirect competitive assay for DA. Following optimization of each step, preincubation of scFv with the standard was no longer necessary to achieve the desired sensitivity. The calibration curve in Fig. 5 represents the mean of six independent analyses, each with duplicate determinations of calibrators. The mean ED50 was 156 ng/ml. A panel of 11 scallop tissue extracts with endogenous DA concentrations of 0 to 609 ng/ml were spiked with 500 ng/ml of standard DA. The mean recovery of added DA was 96.3% (standard deviation, 15.6%). The assay was also shown to measure DA in scallop extracts independently of the volume of sample used over the linear range of the assay. Five extracts with endogenous DA concentrations of 1.5 to 2.0 μg/ml were assayed following dilution at 1/5, 2/5, 3/5, and 4/5. Regression analysis of the correlation between actual and measured values of diluted samples confirmed linearity of the assay, with R2 values ranging from 0.94 to 0.99. Thus, the chicken scFv-based assay is suitable for use in the measurement of DA in shellfish extracts.
FIG. 5.
Standard curve for chicken scFv (R4Sh-7)-based indirect competitive ELISA. The standard curve is derived from results for six sets of standards analyzed in duplicate. Error bars indicate standard deviations. See the legend to Fig. 2 for an explanation of %B/Bo.
DISCUSSION
The rapid monitoring of toxin levels in shellfish samples is highly desirable to provide effective systems which can guarantee seafood quality. Several sensitive and specific immunoassay methods have been developed to date for the analysis of toxin concentration in shellfish, including an assay for DA based on a high-affinity murine monoclonal antibody (17). Despite these advances, it is still not possible to monitor multiple hapten toxins with an automated, high-throughput immunoassay format. To develop such assays, it is important to have a simplified and highly repeatable method to generate high-affinity antibodies which can be synthesized quickly and at minimal expense. Recombinant antibodies fulfill these criteria more readily than monoclonal antibodies, with the chicken scFv system being one of the most simple to exploit (2).
We constructed the C1αDA library (scFv) from two immunized chickens by overlap PCR using an 18-amino-acid peptide linker system, which facilitated the creation of a relatively large library (3.1 × 108 total transformants) with only a single ligation step (2). The use of hyperimmune animals as the source of immunoglobulin cDNA reduced the size of the library required to yield highly specific antibody fragments, in comparison with the larger size required to generate effective naïve antibody libraries (20). The antibody library generated in this study proved to be highly effective, yielding several antibodies which recognize DA, with specific panning responses observed in the phage ELISA after only two rounds of panning selection. This rapid selection of specific antibodies from the C1αDA library is illustrative of the high representation of anti-DA clones within the population, as most naive libraries take four to six rounds of panning to produce high specific signal (9).
One of the main advantages of using phage display for production of antibodies for assay development is the ability to generate antibodies with improved performance from the initial antibody selection. Using the VH gene segments from a DA-specific scFv population resulting from panning of the initial library, a VH-biased light chain shuffled library was generated which, upon further selection, yielded scFv with improved affinity for DA. Restriction digest analysis and DNA sequencing of clones shown to be positive in ELISA analysis showed that a diverse population of scFv had been selected. The observed differences in the CDR sequences between the three highest-affinity clones may have resulted from a diverse in vivo population of DA-specific B cells in each individual animal or possibly from the pooled cDNA of the two animals used to produce the library.
Interestingly, several amino acid changes had occurred in the linker sequences of each of the sequenced fragments. The majority of these were shifts from G to S and vice versa, which presumably did not affect the stability/flexibility of the linker significantly. However, the shift of G to D (glycine to aspartic acid) in clone R4Sh-7 is unusual, with unknown effects. The high frequency of common substitutions in the linker sequences of each scFv is most likely due to primer mis-synthesis, or possibly PCR error (more likely for scFv-specific substitutions). To establish whether or not any of these substitutions is beneficial will require considerable further study. Due to the common occurrence of multimerization, resulting from elevated scFv concentration and/or sample pH (3), any individual scFv sample is likely to contain an unknown proportion of dimeric antibody, which is functionally bivalent and can lead to a profound change in off-rate kinetics profiles. While it is possible that the off-rate selection procedure employed in this study selected clones with higher propensity to dimerize, a recent study has shown that the higher avidity of dimerized scFv can actually reduce the sensitivity of antihapten competition assays (26).
Single scFv clones, induced in bacterial culture, were shown to produce soluble scFv that was readily purified using spin column Ni-NTA chromatography. Without any optimization, the soluble yield was satisfactory and very reproducible, and it is likely that yields could be improved considerably by coexpression of molecular chaperones (28) or the E. coli disulfide isomerase enzyme (15). The resulting soluble scFv gave a reproducible standard curve within the range required for enforcement of the regulatory cutoff level for DA in shellfish (250 mg/kg) (8) and could be used for the detection of DA in shellfish extracts.
Thus, this study has shown that antitoxin scFv can be conveniently generated in chickens and that these scFv can be of suitable affinity and stability for use in screening shellfish samples. Immunization of only two chickens resulted in a number of useful scFv clones in a relatively short time frame compared to traditional hybridoma techniques. Interestingly, single rabbits have been immunized with four separate haptens and shown to yield recombinant antibodies specific for each molecule (18). We therefore hope to exploit this simple chicken system further for the generation of antitoxin scFv with multiple toxin immunizations and multiple screening of the same library for different antitoxin scFv, thereby reducing the number of animals required for such studies.
Acknowledgments
We thank C. F. Barbas at the Scripps Research Institute for providing the phage display vectors used in this study.
W.J.J.F. was funded by a Marie Curie Development Host fellowship from the European Commission. This project was also partly funded by grant ATRP/01/113 from the Enterprise Ireland Science and Technology Development Agency.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Andris-Widhopf, J., C. Rader, P. Steinberger, R. Fuller, and C. F. Barbas. 2000. Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Methods 28:159-181. [DOI] [PubMed] [Google Scholar]
- 3.Arndt, K. M., K. M. Muller, and A. Pluckthun. 1998. Factors influencing the dimer to monomer transition of an antibody single-chain Fv fragment. Biochemistry 37:12918-12926. [DOI] [PubMed] [Google Scholar]
- 4.Barbas, C. F., III, D. R. Burton, J. K. Scott, and G. J. Silverman. 2001. Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 5.Carroll, S. B., and B. D. Stollar. 1983. Antibodies to calf thymus RNA polymerase 2 from egg yolks of immunised hens. J. Biol. Chem. 258:24-26. [PubMed] [Google Scholar]
- 6.Charleton, K. A., S. Moyle, A. J. R. Porter, and W. J. Harris. 2000. Analysis of the diversity of a sheep antibody repertoire as revealed from a bacteriophage display library. J. Immunol. 164:6221-6229. [DOI] [PubMed] [Google Scholar]
- 7.Davies, E. L., J. S. Smith, C. R. Birkett, J. M. Manser, D. V. Anderson-Dear, and J. R. Young. 1995. Selection of specific phage-display antibodies using libraries derived from chicken immunoglobulin genes. J. Immunol. Methods 186:125-135. [DOI] [PubMed] [Google Scholar]
- 8.European Communities. 2002. Decision 2002/225/EC. Off. J. Eur. Communities L75:62-64. [Google Scholar]
- 9.Gao, C., S. Mao, G. Kaufmann, P. Wirsching, R. A. Lerner, and K. D. Janda. 2002. A method for the generation of combinatorial antibody libraries using pIX phage display. Proc. Natl. Acad. Sci. USA 99:12612-12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groves, D. J., and B. A. Morris. 2000. Veterinary sources of nonrodent monoclonal antibodies: interspecific and intraspecific hybridomas. Hybridoma 19:201-214. [DOI] [PubMed] [Google Scholar]
- 11.Hall, J. C., T. D. Van Deynze, J. Struger, and C. H. Chan. 1993. Enzyme immunoassay based survey of precipitation and surface water for the presence of atrazine, metolachlor and 2,4-D. J. Environ. Sci. Health B 28:577-598. [DOI] [PubMed] [Google Scholar]
- 12.Hawlisch, H., A. M. zu Vilsendorf, W. Bautsch, A. Klos, and J. Köhl. 2000. Guinea pig C3 specific rabbit single chain Fv antibodies from bone marrow, spleen and blood derived phage libraries. J. Immunol. Methods 236:117-131. [DOI] [PubMed] [Google Scholar]
- 13.Hess, P., S. Gallacher, L. A. Bates, N. Brown, and M. A. Quilliam. 2001. Determination and confirmation of the amnesic shellfish poisoning toxin, domoic acid, in shellfish from Scotland by liquid chromatography and mass spectrometry. J. AOAC Int. 84:1657-1667. [PubMed] [Google Scholar]
- 14.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 15.Hu, X., R. O'Dwyer, and J. G. Wall. 2005. Cloning, expression and characterisation of a single-chain Fv antibody fragment against domoic acid in Escherichia coli. J. Biotechnol. 120:38-45. [DOI] [PubMed] [Google Scholar]
- 16.Jellett, J. F., L. J. Marks, J. E. Stewart, M. L. Dorey, W. Watson-Wright, and J. F. Lawrence. 1992. Paralytic shellfish poison (saxitoxin family) bioassay: automated end-point determination and standardization of the in vitro tissue culture bioassay, and comparison with the standard mouse bioassay. Toxicon 30:1143-1156. [DOI] [PubMed] [Google Scholar]
- 17.Kawatsu, K., Y. Hamano, and T. Noguchi. 1999. Production and characterization of a monoclonal antibody against domoic acid and its application to enzyme immunoassay. Toxicon 37:1579-1589. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., W. Cockburn, J. B. Kilpatrick, and G. C. Whitelam. 2000. High affinity scFvs from a single rabbit immunized with multiple haptens. Biochem. Biophys. Res. Commun. 268:398-404. [DOI] [PubMed] [Google Scholar]
- 19.Lu, D., J. Shen, M. D. Vil, H. Zhang, X. Jimenez, P. Bohlen, L. Witte, and Z. Zhu. 2003. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J. Biol. Chem. 278:43496-43507. [DOI] [PubMed] [Google Scholar]
- 20.Maynard, J., and G. Georgiou. 2000. Antibody engineering. Annu. Rev. Biomed. Eng. 2:339-376. [DOI] [PubMed] [Google Scholar]
- 21.McCormack, W. T., L. W. Tjoelker, and C. B. Thompson. 1993. Immunoglobulin gene diversification by gene conversion. Prog. Nucleic Acid Res. Mol. Biol. 45:27-45. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor, T., and J. P. Gosling. 1997. The dependence of radioimmunoassay detection limits on antibody affinity. J. Immunol. Methods 208:181-189. [DOI] [PubMed] [Google Scholar]
- 23.Quilliam, M. A., P. G. Sim, A. W. McCulloch, and A. G. McInnes. 1989. High performance liquid chromatography of domoic acid, a marine neurotoxin, with application to shellfish and plankton. Int. J. Environ. Anal. Chem. 36:139-154. [Google Scholar]
- 24.Song, C. S., J. H. Yu, D. H. Bai, P. Y. Hester, and K. H. Kim. 1985. Antibodies to the alpha-subunit of insulin receptor from eggs of immunized hens. J. Immunol. 135:3354-3359. [PubMed] [Google Scholar]
- 25.Tout, N. L., K. Y. F. Yau, J. T. Trevors, H. Lee, and J. C. Hall. 2001. Synthesis of ligand-specific phage-display scFv against the herbicide picloram by direct cloning from hyperimmunized mouse. J. Agric. Food Chem. 49:3628-3637. [DOI] [PubMed] [Google Scholar]
- 26.Townsend, S., W. J. J. Finlay, S. Hearty, and R. O'Kennedy. 2006. Optimizing recombinant antibody function in SPR immunosensing: the influence of antibody structural format and chip surface chemistry on assay sensitivity. Biosens. Bioelectron. [Online.] doi: 10.1016/j/bios.2006.01.010. [DOI] [PubMed]
- 27.Tsen, Y. C., G. Y. Kao, C. L. Chang, F. Y. Lai, C. H. Huang, S. Ouyang, M. H. Yu, C. P. Wang, and Y. N. Chiou. 2003. Evaluation and validation of a duck IgY antibody-based immunoassay for high-sensitivity C-reactive protein: avian antibody application in clinical diagnostics. Clin. Chem. 49:810-813. [DOI] [PubMed] [Google Scholar]
- 28.Wall, J. G., and A. Pluckthun. 1995. Effects of over-expressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol. 6:507-516. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka, H. I., T. Inoue, and O. Ikeda-Tanaka. 1996. Chicken monoclonal antibody isolated by a phage display system. J. Immunol. 157:1156-1162. [PubMed] [Google Scholar]
- 30.Yau, K. Y. F., H. Lee, and J. C. Hall. 2003. Emerging trends in the synthesis and improvement of hapten-specific recombinant antibodies. Biotechnol. Adv. 21:599-637. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, W. W. 2003. The use of gene-specific IgY antibodies for drug target discovery. Drug Discov. Today 8:364-371. [DOI] [PubMed] [Google Scholar]