Abstract
Copper compounds, widely used to control plant-pathogenic bacteria, have traditionally been employed against fire blight, caused by Erwinia amylovora. However, recent studies have shown that some phytopathogenic bacteria enter into the viable-but-nonculturable (VBNC) state in the presence of copper. To determine whether copper kills E. amylovora or induces the VBNC state, a mineral medium without copper or supplemented with 0.005, 0.01, or 0.05 mM Cu2+ was inoculated with 107 CFU/ml of this bacterium and monitored over 9 months. Total and viable cell counts were determined by epifluorescence microscopy using the LIVE/DEAD kit and by flow cytometry with 5-cyano-2,3-ditolyl tetrazolium chloride and SYTO 13. Culturable cells were counted on King's B nonselective solid medium. Changes in the bacterial morphology in the presence of copper were observed by scanning electron microscopy. E. amylovora entered into the VBNC state at all three copper concentrations assayed, much faster when the copper concentration increased. The addition of different agents which complex copper allowed the resuscitation (restoration of culturability) of copper-induced VBNC cells. Finally, copper-induced VBNC cells were virulent only for the first 5 days, while resuscitated cells always regained their pathogenicity on immature fruits over 9 months. These results have shown, for the first time, the induction of the VBNC state in E. amylovora as a survival strategy against copper.
Fire blight, caused by the bacterium Erwinia amylovora (Burrill) Winslow et al. (39) and reported in more than 40 countries around the world, is a very serious and destructive disease of pome fruits and many ornamental plants from the Rosaceae family (34). Copper compounds, widely utilized against fire blight from the beginning of the last century (35), are still employed in many countries, especially in the European Union, where antibiotic utilization is restricted (2). Their use is one of the most common methods for controlling bacterial plant diseases, but it has led many bacteria to develop different strategies against copper ions (31). Until now, very little information on the interaction between E. amylovora and Cu2+ ions has been available (4, 15, 41).
Copper treatments have traditionally been considered as bactericides in agriculture (29, 34, 35), their effectiveness often being measured by the absence of bacterial growth on a solid medium (12, 13). However, recent studies have shown the induction of the viable-but-nonculturable (VBNC) state by copper in several plant-pathogenic bacteria, such as Agrobacterium tumefaciens (1), Xanthomonas campestris pv. campestris (16), and Ralstonia solanacearum (17). This state, in which cells progressively lose their culturability on nonselective solid medium but still remain viable, is considered to be a bacterial survival strategy under adverse environmental conditions (30). Therefore, the failure to produce a visible colony may not necessarily mean that the bacterial cell is dead. Furthermore, it has been reported that VBNC cells can maintain pathogenicity (17, 19). In this respect, it has been suggested that copper-induced VBNC cells of some phytopathogenic bacteria could be related to the persistent nature of infections in copper-treated fields (17). A similar situation could occur with E. amylovora, since fire blight remains difficult to control as a disease (26), which has been related to the ability of this bacterium to survive and spread in different ways (32). Moreover, some bacteria, under favorable environmental conditions, can revert from the VBNC state to a culturable one, in a process usually called resuscitation. This reversion is considered a confirmation that the VBNC state is a bacterial survival strategy (19, 28). The nature of the VBNC state, however, is still the topic of an intense debate in the literature, and some authors argue that this condition may be a physiological state prior to cell death (7, 23).
In spite of previous work concerning the toxic effect of copper ions for E. amylovora (15), many questions on the survival of this bacterium in the presence of this metal remain unanswered. Thus, the aim of this work has been to determine whether copper kills or induces the VBNC state of E. amylovora cells and if such VBNC cells retain their pathogenicity. Furthermore, the possible reversion of this bacterium from the nonculturable state has been studied as well as whether resuscitated cells could regain their pathogenic potential.
MATERIALS AND METHODS
Inoculation of E. amylovora cells in mineral medium with copper.
Containers with 150 ml of AB sterile mineral medium (1) supplemented with CuSO4 (Sigma-Aldrich Chemie) at different concentrations below the MIC of E. amylovora (see Results) (0.005, 0.01, and 0.05 mM Cu2+) and without this metal, as a negative control, were separately inoculated with 107 CFU/ml of two E. amylovora strains. The French reference strain from the Collection Française des Bactéries Phytopathogènes (CFBP1430) and a Spanish strain from the Instituto Valenciano de Investigaciones Agrarias collection (IVIA1892-1) were assayed. The AB medium was chosen because of its very low copper-complexing ability. The containers were kept at 26°C for 9 months, and all the assays were performed at least in duplicate in two independent experiments.
Bacterial cell counts.
Aliquots of 1 ml were taken regularly from all the containers at various times after inoculation (time zero), and bacterial numbers were then determined. Culturable E. amylovora cells were counted by plating on King's B (KB) nonselective solid medium (20). To rule out any growth inhibition effect of copper on this medium, the MIC of copper sulfate for the E. amylovora assayed strains was determined as follows. Forty-eight-hour-old bacterial cultures from KB liquid medium were plated on KB solid medium supplemented with increasing copper concentrations from 0 to 6 mM CuSO4 (at intervals of 0.5 mM). After 48 h at 26°C, the absence of growth was evaluated.
Total and viable cell counts were determined with a Nikon ECLIPSE E800 epifluorescence microscope using the bacterial viability kit BacLight LIVE/DEAD (Molecular Probes Inc., Eugene, Oreg.), based on the permeability of the bacterial cell membrane (8). The manufacturer's instructions were optimized by progressively increasing the incubation time from 15 up to 45 min according to the period that bacterial cells had been in contact with copper.
To check the total and viable counts obtained with the LIVE/DEAD kit, E. amylovora cells were stained with 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) (Polysciences Europe, Eppelheim, Germany) and SYTO 13 (Molecular Probes Inc., Eugene, Oreg.) and counted in a flow cytometer (Becton-Dickinson FACScalibur bench cytometer) with a laser emitting at 488 nm. CTC, an indicator of bacterial respiratory activity (11), was used at a final concentration of 4 mM with a gradual increase in incubation time as described by Créach et al. (9), and stained samples were processed according to the method of Gasol et al. (14). SYTO 13 was used following the manufacturer's instructions.
Culturability restoration or resuscitation of copper-induced VBNC cells.
Before resuscitation assays, the abilities of EDTA (40), citric acid (15), asparagine (4, 15), KB broth, and fresh immature pear juice (24, 40) to bind free copper ions were quantified by the Microquant copper test (Merck, Darmstadt, Germany), widely used to determine the Cu2+ ion concentration in aqueous solutions. The different compounds were added to AB medium at the three copper concentrations studied but without bacteria. EDTA, citric acid, and asparagine were added in stoichiometric amounts in relation to copper, while KB broth and pear juice were diluted 1/10. The samples for measurements of copper bound by the Microquant test were taken after 48 h of shaking incubation at 26°C in liquid medium. The same conditions were used later for assaying the resuscitation of nonculturable cells on solid medium.
To test resuscitation, and with the purpose of complexing the Cu2+ ions from AB medium, the above-indicated compounds were added to 1-ml aliquots from containers at the intermediate copper concentration at different times over 9 months. After incubation, culturability was determined on KB solid medium. The most efficient copper-complexing agents were chosen to assay the restoration of culturability at the other two copper concentrations assayed, as described above.
To demonstrate that the resuscitation was not the result of a regrowth from a few undetected culturable cells, dilution studies were performed according to the method of Whitesides and Oliver (37). Briefly, aliquots of 1 ml from the containers with 4-month-old copper-induced VBNC cells in AB medium at 0.01 mM Cu2+ were 10-fold serially diluted (from 10−1 to 10−7) to reduce the probability (P) of any initial culturable cell down to <0.0000001 CFU/ml. Then, KB broth and fresh immature pear juice were separately added to each dilution and dilutions were incubated as previously described for assaying resuscitation.
SEM of E. amylovora cells in the presence of copper.
Possible changes in bacterial morphology in the presence of copper were analyzed by scanning electron microscopy (SEM). Thus, E. amylovora cells from the copper-free AB medium and AB medium with 0.05 mM Cu2+ were harvested by filtration through 0.2-μm-pore-size polycarbonate filters, fixed, washed, and dehydrated as described by Marco-Noales et al. (22). Then, the bacterial cells were examined with a Hitachi H-4100 scanning electron FE microscope.
Pathogenicity assays of VBNC and resuscitated E. amylovora cells.
Copper-induced VBNC and resuscitated cells of E. amylovora were inoculated at different times over 9 months on immature pear fruits (2- to 3-cm diameter; Pyrus communis cv. Blanquilla, cv. Williams, and cv. Passe Crassane) and immature loquat fruits (2-cm diameter; Eriobotrya japonica cv. Tanaka) as previously described (3, 10). Thus, for the two assayed strains, aliquots of 50 μl from each container with E. amylovora cells under different copper concentrations were inoculated on three pears and four loquats in duplicate in two independent experiments. KB medium-grown bacterial cells and AB medium with copper were used as positive and negative controls, respectively, in all the inoculation assays. After incubation at 26°C, the production of symptoms was examined daily for 2 weeks.
Statistical analysis.
The data (total, viable, and culturable E. amylovora cell counts) are expressed as the means of four determinations (after log transformation) from two independent experiments performed in duplicate (see Fig. 1 in Results). The statistical significance of differences between means was determined by using a three-way factorial analysis of variance (experiment, strain, and copper treatment). Similarly, for each strain, three factors were considered for the analysis: day, experiment, and copper treatment. A P value below 0.05 was considered significant. When no culturable cells were obtained by plating on KB solid medium, these null data were not used for the variance analysis. Trends in the population dynamics between the two E. amylovora strains assayed were also statistically analyzed by analysis of variance.
FIG. 1.
Survival curves of E. amylovora strain CFBP1430 over 9 months in AB mineral medium without copper (white) or with different copper concentrations: 0.005 mM Cu2+ (light gray), 0.01 mM Cu2+ (dark gray), and 0.05 mM Cu2+ (black). (A) Total cell counts (squares); (B) viable cell counts (triangles); (C) culturable cell counts (circles). Total and viable cell counts were determined by epifluorescence using the LIVE/DEAD kit (Molecular Probes), and culturable cell counts were determined by plating on King's B nonselective solid medium. The minimum standard deviation was 0.014, and the maximum was 0.789.
RESULTS
Copper induces the VBNC state in E. amylovora.
The MIC of copper sulfate for the E. amylovora assayed strains in KB solid medium was 3.5 mM Cu 2+, that is, 70 times higher than the highest copper concentration used in the survival experiments, 0.05 mM Cu2+.
Survival curves of E. amylovora over 9 months in the absence and the presence of copper at different concentrations in mineral medium are shown in Fig. 1. As the results were similar for the two assayed strains, and there were no significant differences between their trends at any time, only the reference strain CFBP1430 is represented in the graphs.
Total and viable cell counts remained at high levels (109 to 107 and 108 to 106 cells/ml, respectively) in all the containers, irrespective of the presence of copper, the copper concentration, or the strain assayed (Fig. 1A and B). However, the culturable cell counts decreased at different rates depending on the copper concentration (Fig. 1C). In copper-free AB control medium, the culturability decreased over 1 month from 107 to 104 CFU/ml and remained at this level until the end of the experimental period (Fig. 1C). In contrast, in the presence of copper, the culturability of E. amylovora dropped below the detection limit (1 CFU/ml) and cells became nonculturable on KB medium plates at the three copper concentrations assayed. The time of entry was much sooner when the concentration of this metal was higher: days 36, 1, and 0 for each concentration, respectively (Fig. 1C). This loss of culturability despite the high numbers of viable cells (Fig. 1B) indicates that a considerable fraction of total cells at day 270 (94.5%, 87%, and 80% at 0.005, 0.01, and 0.05 mM Cu2+, respectively) was in the VBNC state. Therefore, most of the bacterial population of the two E. amylovora strains entered into the VBNC state. Significant differences among copper treatments were found only in culturable cell counts, not in the total and viable counts.
The comparison of microscopic counts using the LIVE/DEAD kit with those obtained by flow cytometry using CTC and SYTO 13 showed that total counts by SYTO 13 were half a logarithmic order higher than those by LIVE/DEAD, while viable cell numbers by CTC varied between one-half and 1 logarithmic order lower than those determined by LIVE/DEAD.
Resuscitation of copper-induced VBNC E. amylovora cells.
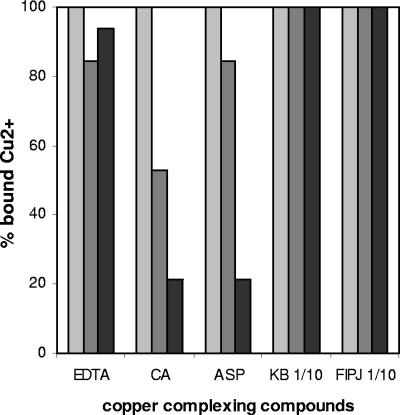
The addition of several compounds was assayed in order to decrease or remove the Cu2+ ions present in the AB medium. The culturability of copper-induced VBNC cells was restored in all cases, but the final efficiency of the compounds in resuscitating such cells varied depending on their copper-complexing ability, the time lapse after the entry of E. amylovora into the VBNC state, and the copper concentration assayed. Regarding copper-complexing activity, KB broth and fresh immature pear juice were the most powerful complexing compounds, since they bound 100% of Cu2+ ions irrespective of the concentration assayed (Fig. 2). They were followed by asparagine and EDTA, which bound more than 80% of copper ions at 0.01 mM, whereas the complexing ability of citric acid was the lowest (Fig. 2).
FIG. 2.
Copper-complexing ability of five chelating compounds in AB medium (without bacteria) supplemented with 0.005 (light gray), 0.01 (dark gray), or 0.05 (black) mM Cu2+. The compounds were EDTA, citric acid (CA), asparagine (ASP), King's B broth diluted 1/10 (KB 1/10), and fresh immature pear juice diluted 1/10 (FIPJ 1/10).
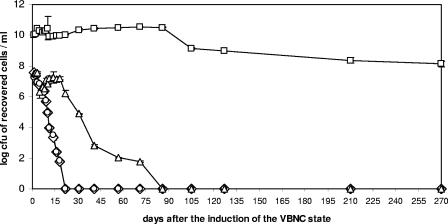
EDTA and citric acid resuscitated VBNC E. amylovora cells induced by 0.01 mM Cu2+ for 18 days after their entry into the VBNC state, while asparagine was effective for up to 75 days (Fig. 3). However, the greatest resuscitation was reached with KB liquid medium, which worked for up to 9 months after the bacterial population entered into the VBNC state (Fig. 3). Results similar to those obtained with KB broth were observed by adding fresh immature pear juice (data not shown). Based on these results, only KB broth and pear juice were used to try to resuscitate VBNC cells induced by the lowest and the highest copper concentrations assayed. Culturable cells were also recovered on KB medium plates for up to 9 months from VBNC cells induced by 0.005 mM Cu2+ (following the same recovery kinetics as the one represented in Fig. 3 for KB broth), while the resuscitation for VBNC cells induced by 0.05 mM Cu2+ was achieved only for 2 days.
FIG. 3.
Culturability restoration curves for 0- to 270-day-old for VBNC E. amylovora cells of strain CFBP1430 induced by 0.01 mM Cu2+ using different copper-complexing compounds: EDTA (diamonds), citric acid (circles), asparagine (triangles), and King's B broth diluted 1/10 (squares).
To check the possible emergence of copper-resistant mutants, the resuscitated cells were plated periodically on KB solid medium supplemented with 3.5 mM Cu2+, the MIC for E. amylovora in this medium. In no case were the resuscitated cells able to grow on this medium, so there was no increase in the MIC and no evidence of copper resistance.
To differentiate between resuscitation and regrowth, dilution studies were performed. Culturable cells were recovered on KB medium plates from all the dilutions when KB broth and pear juice were added to VBNC cells induced by 0.01 mM Cu2+.
SEM of copper-induced VBNC E. amylovora cells.
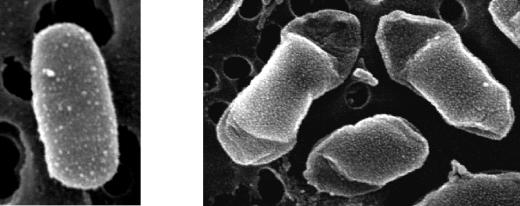
The morphology of bacterial cells in the presence and that in the absence of copper were compared by SEM. After 1 week in the copper-free AB medium, E. amylovora cells had the typical rod shape and a normal size (Fig. 4, left), while at 0.05 mM Cu2+ the cells were slightly bigger, the morphology was altered, and a partial increase in the thickness of the external cell layer was observed (Fig. 4, right).
FIG. 4.
E. amylovora cells of strain CFBP1430 shown by scanning electron microscopy after 7 days in copper-free AB medium (left) and in AB medium with 0.05 mM Cu2+ (right).
Pathogenicity of copper-induced VBNC and resuscitated E. amylovora cells.
About 90% of pear fruits inoculated with the two assayed strains from copper-free AB medium developed symptoms during the whole experimental period, while 40 to 60% of pears inoculated with copper-induced VBNC cells exhibited symptoms for only the first 5 days of being in that state. These results were the same for all the pear cultivars inoculated. Although the VBNC cells caused fire blight symptoms such as necrosis and exudate, they were less serious (necrosis of <10-mm radius and microdrops of exudate) and appeared 1 or 2 days later than in the case of control cells (necrosis of >10-mm radius and more than 4 drops of exudate). The inoculation assays for immature loquat fruits showed similar results.
Resuscitated cells were as pathogenic as the control cells, irrespective of the copper concentration, the time lapse after the induction of the VBNC state, the copper-complexing compound used to restore the culturability, and the pear cultivar. At the two lowest copper concentrations, cells resuscitated by KB broth or fresh immature pear juice produced fire blight symptoms even after being in the VBNC state 9 months. A delay of 2 to 3 days in symptom production was observed only with resuscitated cells from 6 months. In contrast, when the inducing copper concentration was the highest, only the cells recovered from 0- to 2-day-old VBNC cells produced symptoms, since culturable cells were recovered only within the first 2 days. Therefore, whenever VBNC cells regained their culturability, they also recovered their pathogenicity.
DISCUSSION
This work has shown, for the first time, that copper induces the VBNC state in E. amylovora, as described for other plant-pathogenic bacteria (1, 16, 17). The aim of this work has been to study the survival strategy of this bacterium against copper as a stress factor and not as a growth inhibitor, which involved monitoring the long-term survival of this pathogen in a mineral medium with very low copper-complexing ability at different concentrations of copper below its MIC.
Total and viable cell counts of E. amylovora remained at high levels in all the experiments, irrespective of the copper concentration or the strain assayed. Thus, copper did not produce cellular lysis and did not kill all the E. amylovora cells under the assayed conditions, since there was a difference of only around 1 to 2 logarithmic orders between total and viable cells. However, there was a decrease in the culturable cell counts (down to 104 CFU/ml) in the copper-free AB medium, which was probably due to nutrient starvation, as recently reported for E. amylovora in water by Biosca et al. (6). At the three the copper concentrations tested, the culturability of E. amylovora fell below 1 CFU/ml despite the high numbers of viable cells (108 to 106), indicating the presence of a high VBNC cell fraction in the bacterial population. This is in agreement with reports on other phytopathogenic bacteria, such as R. solanacearum (17) and A. tumefaciens (1). However, at least at the highest copper concentration assayed, E. amylovora entered into the VBNC state earlier than other plant pathogens (1, 16, 17). The number of VBNC cells at the end of the survival experiment (270 days) was inversely proportional to copper concentration, as described for X. campestris pv. campestris (16). As metal concentration increased, E. amylovora entered into the VBNC state more quickly, and perhaps this sudden shift caused more cells to die, reducing the fraction of the bacterial population in that state.
To determine the viability of nonculturable cells (23, 28), the LIVE/DEAD kit (8) and the CTC dye (11) were chosen, and a difference of 1 logarithmic order between the results was observed. This has been already reported for other bacteria regardless of the use of epifluorescence microscopy or flow cytometry (8, 9, 28), probably due to the fact that respiration is a more stringent criterion of viability than membrane integrity (8).
The copper concentrations assayed in the present study and in previous works (1, 16, 17) are lower than those used in agriculture. However, the fact that Cu2+ ions are significantly complexed in the leaves of treated plants should be taken into account (24, 25). Moreover, inoculum levels, time and methods of application, plant species or cultivar, weather conditions, and the physiological cell state of the host plant greatly affect the soluble copper available for the bacteria in plant organs (29). All these events can decrease the available toxic ions on treated plants. Therefore, our results provide a good basis for understanding how E. amylovora faces copper in nature.
Once the induction of the VBNC state in E. amylovora by copper was demonstrated, the possibility of resuscitation by different agents which complex Cu2+ ions was studied. KB broth and pear juice were the most powerful copper-complexing compounds, removing 100% of free ions from the AB medium, followed by EDTA, asparagine, and citric acid. When these different compounds were added to copper-induced VBNC cells, KB broth and fresh immature pear juice also enabled the highest levels of resuscitation even from 9-month-old VBNC cells. This is probably also due to their nutrient contribution to bacterial cell multiplication. Asparagine permitted the restoration of 75-day-old VBNC cells to culturability, probably due to its complexing power (15) and its contribution as a nitrogen source for E. amylovora (33). However, EDTA and citric acid resuscitated copper-induced VBNC cells for only 18 days after entry into the nonculturable state. Although EDTA was well able to complex Cu2+ ions in the absence of bacterial cells, its ability to chelate divalent cations destabilizes the gram-negative bacterial outer membrane (21). The high resuscitation ability of KB broth versus the nonculturability on solid medium could be due to the uniform availability of nutrients and chelators in the liquid medium, in addition to the lack of growth inhibitors that can be present in agar, together with a more active metabolism under shaking conditions (5).
Regarding the possible emergence of copper resistance as a survival mechanism in itself during the period under study at the copper concentrations assayed, resuscitated cells did not show any increase in the copper MIC, which is a prerequisite for evidence of resistance.
In contrast to most previous studies, where resuscitation was achieved from cells that were in the VBNC state for a few days (19, 23), this work shows the recovery of long-term copper-induced VBNC cells (up to 9 months for the two lowest concentrations). These results seem to support the hypothesis that the VBNC state is part of the life cycle of E. amylovora. However, the resuscitation of VBNC cells induced at the highest copper concentration was achieved for only 2 days. Since the VBNC state can be considered a process in which bacterial cells progressively adapt to adverse environmental conditions (27), perhaps the rapid entry of E. amylovora into the VBNC state at 0.05 mM Cu2+ could hinder successful reversion of the nonculturable state.
Resuscitation is the keystone of the VBNC state hypothesis (7), so the demonstration of a true reversion was indispensable in our study. In order to differentiate resuscitation from regrowth, it was imperative to determine the probability that a given sample contains any culturable cell prior to resuscitation assays, according to the work of Kell et al. (19). Since in our dilution experiments culturable restored cells were recovered even when P was <0.0000001, this must be due to resuscitation and not to regrowth of undetectable culturable cells, according to the work of Whitesides and Oliver (37).
Epidemiological studies of plant-pathogenic bacteria are usually based only on the results of plate counts (38), but VBNC cells could play significant roles in the life cycle of bacteria (36). Furthermore, the maintenance of the cellular integrity of nonculturable cells permits genetic material of the pathogen to persist in the environment (36). Although VBNC E. amylovora cells were pathogenic for only 5 days, they were able to regain their culturability and pathogenicity. The long-term resuscitation with immature pear juice could be related to the contact between the bacterium and some substances from its natural host and could be involved in the recurrent infections of fire blight in copper-treated crops.
Regarding bacterial morphology, the visualization by SEM of E. amylovora cells in AB medium without copper showed the typical rod shape. However, cells maintained in the presence of copper had an altered morphology with a partial increase in the thickness of the external layer. It has been reported that copper ions increase the level of the exopolysaccharide (EPS) amylovoran (4) and that these ions are accumulated on the surface of E. amylovora cells (41). Moreover, it is known that bacterial EPSs have a cation-binding capacity (18). In accordance with all these previous studies, the bacterial morphology observed by SEM could be due to EPSs with bound copper ions. Studies of the role of EPSs in the survival of E. amylovora in copper environments are now under way.
Overall, our results demonstrate, for the first time, the induction of the VBNC state in E. amylovora by copper and the possibility that this state could represent a survival strategy under certain adverse environmental conditions. These VBNC cells remain undetected on KB medium plates, and under favorable conditions, they can multiply and regain their pathogenicity, representing a hazard for the host plants. Further studies on the interaction of copper with E. amylovora and the VBNC state are needed to improve our understanding of the life cycle of this pathogen and to optimize the fire blight control strategies.
Acknowledgments
This work was supported through projects AGL-2001-2349-C03-02 and AGL-2004-07799-C03-02 from the Spanish Ministry of Science and Technology (MCYT) and GV-04B313 and GV05/214 from Generalitat Valenciana. M. Ordax wants to thank the MCYT for being awarded a predoctoral fellowship. E. Marco-Noales has a contract from the Ministry of Education and Science of Spain (Programa INIA/CC.AA).
We thank the Servicio de Microscopía Electrónica (Universidad de Valencia), J. M. Gasol (Centro Mediterráneo de Investigaciones Marinas y Ambientales, CMIMA, CSIC), and E. Carbonell and J. Pérez (Departamento de Biometría, IVIA) for expert technical assistance in scanning electron microscopy, flow cytometry, and statistical analysis, respectively. We also are grateful to M. A. Cambra (Centro de Protección Vegetal, Gobierno de Aragón), and E. Montesinos (Universidad de Gerona) for providing the immature pear fruits and, finally, to “Speakenglish” Valencia and F. Barraclough for revising the English text.
REFERENCES
- 1.Alexander, E., D. Pham, and T. R. Steck. 1999. The viable-but-nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum. Appl. Environ. Microbiol. 65:3754-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Amounts of antimicrobial used, p. 44-45. In Opinion of the scientific steering committee on antimicrobial resistance. Unit B3—Management of scientific committees II. Directorate General XXIV Consumer Policy and Consumer Health Protection. European Commission, Brussels, Belgium.
- 3.Anonymous. 2004. Diagnostic protocols for regulated pests. Erwinia amylovora. Bull. OEPP/EPPO Bull. 34:159-171. [Google Scholar]
- 4.Bereswill, S., S. Jock, P. Bellemann, and K. Geider. 1998. Identification of Erwinia amylovora by growth in the presence of copper sulfate and by capsule staining with lectin. Plant Dis. 82:158-164. [DOI] [PubMed] [Google Scholar]
- 5.Biosca, E. G., P. Caruso, E. Bertolini, B. Álvarez, J. L. Palomo, M. T. Gorris, and M. M. López. 2005. Improved detection of Ralstonia solanacearum in culturable and VBNC state from water samples at low temperatures, p. 501-506. In C. Allen, P. Prior, and A. C. Hayward (ed.), Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, Minn.
- 6.Biosca, E. G., E. Marco-Noales, M. Ordax, and M. M. López. Long-term starvation-survival of Erwinia amylovora in sterile irrigation water. Acta Hort., in press.
- 7.Bogosian, G., and E. V. Bourneuf. 2001. A matter of bacterial life and death. EMBO Rep. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulos, L., M. Prévost, B. Barbeau, J. Coallier, and R. Desjardins. 1999. LIVE/DEAD BacLight: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 37:77-86. [DOI] [PubMed] [Google Scholar]
- 9.Créach, V., A. C. Baudoux, G. Bertru, and B. L. Rouzic. 2003. Direct estimate of active bacteria: CTC use and limitations. J. Microbiol. Methods 52:19-28. [DOI] [PubMed] [Google Scholar]
- 10.Donat, V., E. G. Biosca, A. Rico, J. Peñalver, M. Borruel, D. Berra, T. Basterretxea, J. Murillo, and M. M. López. 2005. Erwinia amylovora strains from outbreaks of fire blight in Spain: phenotypic characteristics. Ann. Appl. Biol. 146:105-114. [Google Scholar]
- 11.Dufour, P., and M. Colon. 1992. The tetrazolium reduction method for assessing the viability of individual bacterial cells in aquatic environments: improvements, performance and application. Hydrobiologia 232:211-218. [Google Scholar]
- 12.European Norm EN 1040. 1997. Chemical disinfectants and antiseptics. Basic bactericidal activity. Test method and requirements (phase 1). European Commission, Brussels, Belgium.
- 13.European Norm EN 1276. 1997. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic, and institutional areas. Test methods and requirements (phase 2, step 1). European Commission, Brussels, Belgium.
- 14.Gasol, J. M., U. L. Zweifel, F. Peters, J. A. Fuhrman, and A. Hagström. 1999. Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl. Environ. Microbiol. 65:4475-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geider, K. 1999. Interference of copper sulphate in growth of Erwinia amylovora. J. Phytopathol. 147:521-526. [Google Scholar]
- 16.Ghezzi, J. I., and T. R. Steck. 1999. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol. Ecol. 30:203-208. [DOI] [PubMed] [Google Scholar]
- 17.Grey, B. E., and T. R. Steck. 2001. The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection. Appl. Environ. Microbiol. 67:3866-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutnick, D. L., and H. Bach. 2000. Engineering bacterial biopolymers for the biosorption of heavy metals; new products and novel formulations. Appl. Microbiol. Biotechnol. 54:451-460. [DOI] [PubMed] [Google Scholar]
- 19.Kell, D. B., A. S. Kaprelyants, D. H. Weichart, C. R. Harwood, and M. R. Barer. 1998. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek 73:169-187. [DOI] [PubMed] [Google Scholar]
- 20.King, E. O., M. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:401-407. [PubMed] [Google Scholar]
- 21.Leive, L. 1965. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem. Biophys. Res. Commun. 21:290-296. [DOI] [PubMed] [Google Scholar]
- 22.Marco-Noales, E., E. G. Biosca, and C. Amaro. 1999. Effects of salinity and temperature on long-term survival of the eel pathogen Vibrio vulnificus biotype 2 (serovar E). Appl. Environ. Microbiol. 65:1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDougald, D., S. A. Rice, D. Weichart, and S. Kjelleberg. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25:1-9. [Google Scholar]
- 24.Menkissoglu, O., and S. E. Lindow. 1991. Relationship of free ionic copper and toxicity to bacteria in solutions of organic compounds. Phytopathology 81:1258-1263. [Google Scholar]
- 25.Menkissoglu, O., and S. E. Lindow. 1991. Chemical forms of copper on leaves in relation to the bactericidal activity of cupric hydroxide deposits on plants. Phytopathology 81:1263-1270. [Google Scholar]
- 26.Norelli, J. L., A. L. Jones, and H. S. Aldwinckle. 2003. Fire blight management in the twenty-first century. Plant Dis. 87:756-765. [DOI] [PubMed] [Google Scholar]
- 27.Nyström, T. 2001. Not quite dead enough: on bacterial life, culturability, senescence, and death. Arch. Microbiol. 176:159-164. [DOI] [PubMed] [Google Scholar]
- 28.Oliver, J. D. 1993. Formation of viable but nonculturable cells, p. 239-272. In S. Kjelleberg (ed.), Starvation in bacteria. Plenum Press, New York, N.Y.
- 29.Psallidas, P. G., and J. Tsiantos. 2000. Chemical control of fire blight, p. 199-234. In J. L. Vanneste (ed.), Fire blight: the disease and its causative agent, Erwinia amylovora. CABI Publishing, Wallingford, United Kingdom.
- 30.Roszak, B. D., and R. R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena, D., N. Joshi, and S. Srivastava. 2002. Mechanism of copper resistance in a copper mine isolate Pseudomonas putida strain S4. Curr. Microbiol. 45:410-414. [DOI] [PubMed] [Google Scholar]
- 32.Thomson, S. V. 2000. Epidemiology of fire blight, p. 9-36. In J. L. Vanneste (ed.), Fire blight: the disease and its causative agent, Erwinia amylovora. CABI Publishing, Wallingford, United Kingdom.
- 33.Tolbert, N. E. 1964. Nitrogen requirement and metabolism of Erwinia amylovora. Physiol. Plant 17:44-48. [Google Scholar]
- 34.Van der Zwet, T., and S. V. Beer. 1995. Fire blight—its nature, prevention, and control. A practical guide to integrated disease management. USDA agriculture information bulletin no. 631. U.S. Department of Agriculture, Washington, D.C.
- 35.Van der Zwet, T., and H. L. Keil. 1979. Fire blight—a bacterial disease of rosaceous plants. USDA agriculture handbook 510. U.S. Department of Agriculture, Washington, D.C.
- 36.Weichart, D. H. 1999. Stability and survival of VBNC cells—conceptual and practical implications. In C. R. Bell, M. Brylinsky, and P. Johnson-Green (ed.), Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology. Atlantic Canada Society for Microbial Ecology, Halifax, Canada.
- 37.Whitesides, M. D., and J. D. Oliver. 1997. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl. Environ. Microbiol. 63:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, M., and S. E. Lindow. 2000. Viable but nonculturable cells in plant-associated bacterial populations, p. 731-736. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 39.Winslow, C.-E. A., J. Broadhurst, R. E. Buchanan, C. Krumwiede, L. A. Rogers, and G. H. Smith. 1920. The families and genera of the bacteria. Final report of the Committee of the Society of American Bacteriologists on characterization and classification of bacterial types. J. Bacteriol. 5:191-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zevenhuizen, L. P. T. M., J. Dolfing, E. J. Eshuis, and I. J. Scholten-Koerselman. 1979. Inhibitory effects of copper on bacteria related to the free ion concentration. Microb. Ecol. 5:139-146. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Y., S. Jock, and K. Geider. 2000. Genes of Erwinia amylovora involved in yellow color formation and release of a low-molecular-weight compound during growth in the presence of copper ions. Mol. Gen. Genet. 264:233-240. [DOI] [PubMed] [Google Scholar]