Abstract
Two quaternary ammonium silanes (QAS) were used to coat silicone rubber tracheoesophageal shunt prostheses, yielding a positively charged surface. One QAS coating [(trimethoxysilyl)-propyldimethyloctadecylammonium chloride] was applied through chemical bonding, while the other coating, Biocidal ZF, was sprayed onto the silicone rubber surface. The sprayed coating lost its stability within an hour, while the chemically bonded coating appeared stable. Upon incubation in an artificial throat model, allowing simultaneous adhesion and growth of yeast and bacteria, all coated prostheses showed significant reductions in the numbers of viable yeast (to 12% to 16%) and bacteria (to 27% to 36%) compared with those for silicone rubber controls, as confirmed using confocal laser scanning microscopy after live/dead staining of the biofilms. In situ hybridization with fluorescently labeled oligonucleotide probes showed that yeasts expressed hyphae on the untreated and Biocidal ZF-coated prostheses but not on the QAS-coated prostheses. Whether this is a result of the positive QAS coating or is due to the reduced number of bacteria is currently unknown. In summary, this is the first report on the inhibitory effects of positively charged coatings on the viability of yeasts and bacteria in mixed biofilms. Although the study initially aimed at reducing voice prosthetic biofilms, its relevance extends to all biomedical and environmental surfaces where mixed biofilms develop and present a problem.
Biofilm formation is the leading cause for the failure of biomedical prostheses (9, 13), including tracheoesophageal shunt prostheses, used for speech rehabilitation in patients after total laryngectomy because of a malignant laryngeal tumor. Tracheoesophageal shunt prostheses are made of a silicone rubber (SR) tube capped on one end with a one-way valve and are placed between the esophagus and the trachea. The valve of the prosthesis constitutes its esophageal side, and the one-way mechanism allows air to pass from the tracheal side, but fluids passing the esophagus are blocked from entering the trachea. Microorganisms readily form a biofilm on the esophageal side of a prosthesis, which leads to dysfunction of the valve and induces leakage of fluids in the trachea or increased airflow resistance during speech (3, 15). Consequently, the useful lifetime of a voice prosthesis ranges from 3 to 6 months (10, 15, 16).
Tracheoesophageal shunt prosthetic biofilms contain a mixture of yeasts and bacteria, including Candida species, Staphylococcus and Streptococcus species, and Rothia dentocariosa. Especially Candida albicans, Candida tropicalis, and R. dentocariosa are known to reduce the lifetimes of tracheoesophageal shunt prostheses in vivo (5). Antimicrobials or antifungal agents, or both, have been administered to patients in order to eradicate these biofilms (18), but the biofilm offers effective protection against antimicrobials, to which planktonic organisms are usually susceptible (2, 7). Therefore, preventive measures seem a better way to deal with these biofilms. Gottenbos et al. (8) described the antibacterial properties of a positively charged organosilane quaternary ammonium compound [3-(trimethoxysilyl)-propyldimethyloctadecylammonium chloride (referred to below as QAS, for quaternary ammonium silane)] coating on silicone rubber against a variety of different bacterial strains in vitro and moreover demonstrated the effectiveness of QAS coatings against a Staphylococcus aureus biofilm in vivo. Similarly, Biocidal ZF, a commercially available disinfectant containing quaternary ammonium compounds as the only specified active ingredient, is used for coating incubators and sterile cabinets to protect cell cultures from microbial contamination. Neither the QAS coating nor Biocidal ZF has ever been investigated for its efficacy against mixed fungal and bacterial biofilms.
The aim of this study was to evaluate the inhibitory effects of QAS and Biocidal ZF coatings against the development of a mixed fungal and bacterial biofilm on silicone rubber tracheoesophageal shunt prostheses in vitro, in order to develop new strategies for the prevention of microbial colonization of silicone rubber voice prostheses.
MATERIALS AND METHODS
Tracheoesophageal shunt prostheses.
“Ultra Low Resistance” silicone rubber Groningen button tracheoesophageal shunt prostheses were supplied by Médin Instruments and Supplies (Groningen, The Netherlands). The “Ultra Low Resistance” Groningen button tracheoesophageal shunt prosthesis consists of a shaft with two flanges with a semicircular slit of 200° in the hat of the esophageal flange, functioning as a one-way valve. The prosthesis is made of implant-grade silicone rubber.
Silanization and surface characterization.
The tracheoesophageal shunt prostheses were cleaned in a 2% RBS 35 detergent solution (Omniclean, Breda, The Netherlands) under simultaneous sonication and thoroughly rinsed in demineralized water, disinfected in 70% ethanol, washed with sterile Millipore-Q water, and dried overnight at 80°C under aseptic conditions. For coating with Biocidal ZF, tracheoesophageal shunt prostheses were sprayed twice with Biocidal ZF (WAK-Chemie Medical GmbH, Steinbach, Germany), fully covering the valve with the Biocidal, followed by exposure to ambient air and drying for 20 h under aseptic conditions. For QAS coating (8), prostheses were oxidized in a glow-discharge reactor (a DC modified Edwards sputter coater S150B) by argon plasma treatment, carried out under 5 × 102 Pa argon pressure at a power of 7 W for 5 min, followed by exposure to ambient air. Subsequently, each oxidized voice prosthesis was immediately immersed in 0.5% QAS (Dow Corning Corporation, Carrollton, Ky.) in Millipore water. Coated tracheoesophageal shunt prostheses were allowed to react and dry at 80°C for 20 h (8) under aseptic conditions. Sheets of SR (25 by 76 mm) were similarly treated for surface characterization.
For surface characterization, QAS- and Biocidal-coated SR was washed for 30 min in phosphate-buffered saline (PBS), followed by rinsing with demineralized water. The chemical compositions of uncoated, QAS-coated, and Biocidal-coated SR surfaces were determined by X-ray photoelectron spectroscopy (XPS) using an S-Probe spectrometer (Surface Science Instruments, Mountain View, CA) at a spot size of 250 by 1,000 μm, and X-rays were produced using an aluminum anode. A scan of the overall spectrum in the binding energy range of 1 to 1,200 eV at low resolution (pass energy, 150 V) was recorded, followed by scans over a 20-eV binding energy range at high resolution (pass energy, 50 eV) for C1s (carbon), O1s (oxygen), N1s (nitrogen), Si2p (silicon), and Cl2p (chloride). The area under the peak, after linear background subtraction, was used to calculate the peak intensities after correction with sensitivity factors provided by the manufacturer. The elemental surface compositions were expressed as atoms percent, by setting %C + %O + %N + %Si + %Cl to 100%.
Zeta potentials of the surfaces were derived from the pressure dependence of the streaming potentials by employing a parallel plate flow chamber of which the top and bottom plate were constituted by uncoated, QAS-coated, or Biocidal-coated SR sheets fixed on Perspex plates (25 by 76 mm), separated by a 0.2-mm Teflon gasket. Two rectangular platinum electrodes (5.0 by 25.0 mm) were located at both ends of a parallel plate flow chamber (17). Streaming potentials were measured over 1 h in PBS (10 mM potassium phosphate and 150 mM NaCl, pH 7.0) at 10 different pressures ranging from 5.103 to 20.103 Pa, and each pressure was applied for 10 s in both directions.
Advancing type water contact angles were measured at room temperature with a homemade contour monitor using the sessile drop technique.
Determination of in vitro cytotoxicity.
In order to ensure that potential future applications of these coatings would not be impeded because of cytotoxicity, silicone rubber sheets with a QAS coating were sent to a reference laboratory (Toxicon Europe NV, Leuven, Belgium). Briefly, extracts of QAS-coated silicone rubber were prepared at 37°C for 24 h by using 12.1 ml minimum essential medium supplemented with serum (MEM complete) for 72.5 cm2. Extracts of positive (natural rubber) and negative (bare silicone rubber) controls were also prepared to verify the proper functioning of the test system. The extracts were then tested for cytotoxicity using L929 mouse fibroblast cell culture (USP 28-NF 23). QAS-coated silicone rubber may be considered noncytotoxic if none of the cultures exposed show more than mild reactivity. The toxicity measurements were performed at an authorized test institute (Toxicon, Europe NV, Leuven, Belgium) following tests for in vitro cytotoxicity (EN/ISO 10993-5).
Biofilm formation.
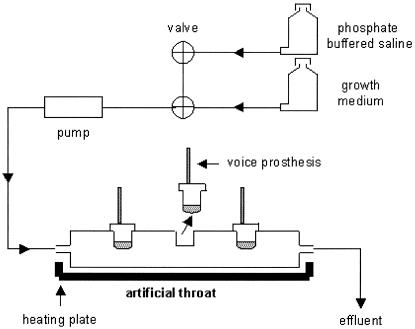
A modified Robbins device made of stainless steel was used as an artificial throat (Fig. 1) to grow biofilms (14). Each artificial throat was equipped with three Groningen Ultra Low Resistance tracheoesophageal shunt prostheses: an uncoated, a QAS-coated, and a Biocidal-coated prosthesis. During the experiment, the artificial throat was maintained at a temperature between 36°C and 37°C, as in a laryngectomized patient.
FIG. 1.
Schematic presentation of the modified Robbins device, used as an artificial throat and equipped with three Groningen button tracheoesophageal shunt prostheses.
To grow tracheoesophageal shunt prosthetic biofilms as found in laryngectomized patients, artificial throats were inoculated for 5 h with a combination of bacteria and yeasts previously isolated from explanted Groningen tracheoesophageal shunt prostheses. This combination comprised Candida tropicalis GB 9/9, Candida albicans GBJ 13/4A, Staphylococcus aureus GB 2/1, Staphylococcus epidermidis GB 9/6, Streptococcus salivarius GB 24/9, and Rothia dentocariosa GBJ 52/2B and was cultured in a mixture of 30% brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) and 70% defined yeast medium [per liter, 7.5 g glucose, 3.5 g (NH4)2SO4, 1.5 g l-asparagine, 10 mg l-histidine, 20 mg dl-methionine, 20 mg dl-tryptophan, 1 g KH2PO4, 500 mg MgSO4 · 7H2O, 500 mg NaCl, 500 mg CaCl2 · 2H2O, 100 mg yeast extract, 500 μg H3BO3, 400 μg ZnSO4 · 7H2O, 120 μg Fe(III)Cl3, 200 μg Na2MoO4 · 2H2O, 100 μg KI, and 40 μg CuSO4 · 5H2O]. After inoculation, a biofilm was allowed to grow on the tracheoesophageal shunt prostheses for 3 days, by filling the devices with growth medium. From day 4 to day 7, the artificial throats were perfused three times a day with 250 ml PBS. After each perfusion, the prostheses were blown through with compressed air at three different pressures (10, 15, and 20 cm H2O) to mimic shunt esophageal speech and to mobilize the valve system.
Subsequently, the prostheses were left in the moist environment of the artificial throats. At the end of each day, the devices were filled with growth medium for 30 min and left overnight in the moist environment of the drained artificial throats. The tracheal sides of the prostheses were left in ambient air, a situation similar to that with a stoma (14).
Evaluation of biofilms.
On day 8 of an experiment, tracheoesophageal shunt prostheses were removed from the artificial throats. Biofilm formation on the valve side of the prosthesis was assessed by determining the number of colony-forming yeasts and bacteria (CFU). To this end, biofilms were removed by scraping and sonication and were subsequently serially diluted. After the serial dilutions were plated on MRS (de Man, Rogosa, and Sharpe) agar plates for yeasts and blood agar plates for bacteria, plates were incubated at 37°C in an aerobic incubator for 3 days prior to enumeration. In each experimental run, an untreated silicone rubber prosthesis was inserted as a control, and the number of bacterial and yeast CFU on the esophageal surface of each prosthesis was determined separately and expressed as a percentage of that for the control to ensure consistency of biofilm formation in each run.
Two artificial throats were used for imaging biofilm formation on the valve side of the prostheses by confocal laser scanning microscopy (CLSM). Voice prostheses of one artificial throat were visualized after fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. FISH was performed using a modification of previously described protocols (1, 6, 12). After removal from the artificial throat, the tracheoesophageal shunt prostheses underwent the following preparation steps: conservation for 24 h in sterile PBS, fixation for 24 h in a 4%-paraformaldehyde solution at 4°C, and conservation for at least 24 h in an ethanol-PBS (1:1) solution. After those preparation steps, the valves of the prostheses were cut into small cross-sections and attached to glass slides. The fixed samples were hybridized (in a closed moist chamber at 50°C) in a volume of 200 μl of prewarmed (50°C) hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl, pH 7.2, and 0.01% sodium dodecyl sulfate) mixed with 10 μl of 100-ng/μl rhodamine-labeled EUB-338 probe (5′-GCTGCCTCCCGTAGGAGT-3′) for detection of bacteria and 10 μl of 100-ng/μl fluorescein isothyocyanate-labeled EUK-516 probe (5′-ACCAGACTTGCCCTCC-3′) for visualization of the yeasts (1) (Eurogentec, Seraing, Belgium). After 17 to 19 h, the slides were washed in a prewarmed (50°C) washing buffer for 15 min to remove unbound probes, rinsed with sterile Millipore water, carefully dried with tissues, mounted in Vectashield medium for fluorescence (Vector Laboratories, Inc., Burlingame, CA), and covered with a coverslip. Confocal images were obtained using a 20× oil immersion objective of a model LEICA TCS SP2 CLSM (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany).
Samples from the second artificial throat intended for imaging were subjected to live/dead baclight (Molecular Probes, Leiden, The Netherlands) staining kits for yeasts and bacteria. Directly after removal of the prostheses from the artificial throat, prostheses were stained for 15 min in the dark at room temperature with both the live/dead viability stain, containing SYTO 9 dye (3.34 mM) and propidium iodide (20 mM), and the live/dead yeast viability kit containing FUN-1 cell stain (10 μM) and calcofluor white M2R staining (25 μM), but in our experience the two-color fluorescent probe, FUN-1, sufficed for determining yeast viability. Series of about 20 images were made of each biofilm on the prostheses by using a 20× water immersion objective and were stacked into overlay projections.
Statistical analysis.
All experiments with the artificial throats were done in quadruplicate, and the quantitative data were statistically compared to those for the control. A Wilcoxon signed-rank test was used for the statistical analysis; a P value of <0.05 was considered statistically significant.
RESULTS
The surface characteristics of the silicone rubber prior to and after QAS and Biocidal coating are summarized in Table 1. The presence of a QAS coating increases %N and %Cl relative to those in uncoated SR, whereas the presence of the Biocidal coating is not evident from the XPS data, likely because its layer is too thin for detection by XPS. Water contact angles are similar on QAS-coated (100°) and uncoated (108°) SR, but the Biocidal coating creates a more hydrophilic SR surface (40°). Most importantly, the zeta potential of SR, authentically negative, becomes positive after QAS and Biocidal coating (16 and 29 mV, respectively). The Biocidal coating, however, becomes negatively charged within 1 h, whereas the QAS coating stays positively charged. The cytotoxicity of the QAS-coated silicone rubber was tested, and mild biological reactivity (grade 2) was observed in the L929 mammalian cells at 48 h postexposure. The observed cellular response obtained from the positive-control extract (grade 3) and the negative-control extract (grade zero) confirmed the suitability of the test system. The QAS-coated silicone rubber can therefore be considered noncytotoxic.
TABLE 1.
Chemical surface composition, equilibrium water contact angles, and zeta potentials in PBS of untreated, QAS-coated, and Biocidal-coated SR
| Surface property | Value in:
|
||
|---|---|---|---|
| Untreated SR | QAS-coated SR | Biocidal-coated SR | |
| %C | 49 | 63 | 49 |
| %O | 26 | 19 | 25 |
| %Si | 25 | 14 | 26 |
| %N | 0 | 2.6 | 0 |
| %Cl | 0 | 2.3 | 0 |
| Equilibrium water contact angle (°) | 108 | 100 | 40 |
| Zeta potential (mV) | −15 | +16 | +29 |
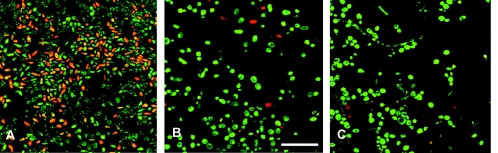
The percentages of viable yeasts and bacteria harvested from QAS- and Biocidal-coated SR, as well as the total number of microorganism cultures per cm2 of prosthesis surface, are shown in Table 2. Significantly (P < 0.05) fewer viable bacteria and yeasts are harvested from the QAS coating than from authentic silicone rubber prostheses, while the Biocidal coating also shows a reduction, which is, however, not significant. These numbers are confirmed qualitatively in Fig. 2, showing CLSM images of the prosthesis surfaces after live/dead staining. Note that some hyphae are observed on the Biocidal coating but are absent on the QAS-coated prosthesis.
TABLE 2.
Decreases in percentages of viable bacteria and yeasts isolated from the tracheoesophageal shunt prostheses coated with QAS or Biocidal with respect to untreated prosthesesa
| Coating | % of:
|
Total microorganisms (CFU/cm2) | |
|---|---|---|---|
| Total bacteriab | Total yeastb | ||
| None | 100 | 100 | 2.5 × 106 ± 1.5 × 106 |
| QAS | 36* ± 16 | 12* ± 9 | 0.8 × 106* ± 0.3 × 106 |
| Biocidal | 27 ± 32 | 16 ± 15 | 0.6 × 106* ± 0.7 × 106 |
Results were obtained in four independent experiments and are means ± standard deviations. For both bacteria and yeasts, the number of organisms of the untreated prostheses was set at 100%. Asterisks indicate results significantly different from those for untreated prostheses (P < 0.05 by the Wilcoxon signed-rank test).
The numbers of viable bacterial and yeast CFU on untreated silicone rubber prostheses amounted, respectively, to 2.1 × 106 and 3.8 × 105 per cm2 on the esophageal side of the Low Resistance Groningen Button tracheoesophageal shunt prostheses.
FIG. 2.
CLSM images of surfaces of Groningen button tracheoesophageal shunt prostheses after live/dead staining for yeasts and bacteria. Red and green indicate dead and live cells, respectively, of both yeasts and bacteria. Bar, 40 μm. (A) Untreated prosthesis; (B) QAS coating; (C) Biocidal coating.
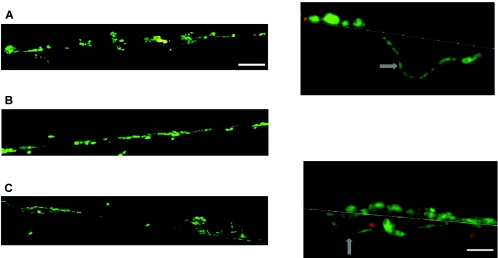
Figure 3 shows CLSM images of cross-sections of biofilms on the valves of the silicone rubber tracheoesophageal shunt prostheses prior to and after coating. As can be seen, the biofilm on the untreated prosthesis is thicker than those on the coated prostheses. The high magnifications for the control and the Biocidal coating show ingrowth of hyphae into the silicone rubber. No hyphae of yeasts have been observed in the biofilm on the QAS-coated surface.
FIG. 3.
CLSM images of cross-sections of tracheoesophageal shunt prosthetic biofilms after in situ hybridization with fluorescence-labeled oligonucleotide probes, making bacterial cells appear red and yeasts appear green. Arrows in the magnifications point to hyphae. Bar for overviews, 40 μm; bar for magnifications, 10 μm. (A) Untreated prosthesis; (B) QAS coating; (C) Biocidal coating.
DISCUSSION
In this study, silicone rubber tracheoesophageal shunt prostheses were coated with QAS and Biocidal ZF coatings to evaluate their inhibitory effects against the development of a mixed fungal and bacterial biofilm on these prostheses. QAS coatings turned out to be stable coatings that were not cytotoxic in a first evaluation due to the stable bound state of the QAS molecules (note that the Biocidal ZF coating is not stable and was therefore not tested for its cytotoxicity). Thus, QAS coatings constitute a new strategy for prevention of microbial colonization of silicone rubber surfaces in voice prostheses, but also for prevention of microbial colonization of medical devices in general, and can be helpful in prevention of resistance of microorganisms to antibiotics or antimycotics.
The surface characteristics of the coated tracheoesophageal shunt prostheses showed that the Biocidal coating was not evident from the XPS data, probably because the Biocidal coating is thinner than the depth of information of XPS (3 to 5 nm). In contrast, water contact angles and the zeta potential, both measured on the outer surface layer with an information depth of several angstroms, clearly demonstrated the presence of the coating. The zeta potential of the Biocidal coating quickly becomes negative, indicating its instability. In this respect it should be noted that the commercially available antimicrobial fluid, Biocidal ZF, is normally used as a coating for incubators, which have to be cleaned and recoated every 14 days. This is in contrast to the chemical bonding established for the QAS coating.
Gottenbos et al. (8) reported that the positively charged QAS coating affects the viability of gram-negative bacteria as well as of gram-positive bacteria in single-strain bacterial biofilms. Here it is demonstrated that such a coating also reduces the number of viable bacteria and yeasts in mixed biofilms, as demonstrated by plate counting and CLSM after live/dead staining. Immobilized QAS molecules are known to interact with cell membranes of adhering bacteria, presumably causing membrane leakage and cell death (8, 11). The mechanisms of action of QAS causing death in yeast is not known, but it seems to impede the formation of hyphae (see Fig. 3). Little is known also about the influence of the bacterial presence on the expression of hyphae in yeast. Consequently, the absence of hyphae could be either a direct effect of the coating or an indirect effect caused by the absence of bacteria on QAS-coated surfaces.
This study demonstrates for the first time that the viability of both yeasts and bacteria in mixed biofilms is affected by positively charged QAS coatings on silicone rubber. Because QAS coatings are nontoxic, clinical application could increase the useful lifetime of tracheoesophageal shunt prostheses by decreasing biofilm formation in vivo, since ingrowth of yeasts is held mainly responsible for deterioration of the silicone rubber in vivo (4). The relevance of the current findings extends, however, to all biomedical and environmental applications where mixed biofilms develop and present a problem.
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 3.De Carpentier, J. P., W. D. J. Ryder, I. S. Grad, S. R Saeed, and T. J. Woolford. 1996. Survival times of Provox valves. J. Laryngol. Otol. 110:37-42. [DOI] [PubMed] [Google Scholar]
- 4.Ell, S. R. 1996. Candida— ‘the cancer of silastic.’ J. Laryngol. Otol. 110:240-242. [PubMed] [Google Scholar]
- 5.Elving, G. J., H. C. van der Mei, H. J. Busscher, R. van Weissenbruch, and F. W. J. Albers. 2002. A comparison of the microbial composition of voice prosthetic biofilms in patients requiring frequent versus infrequent replacements. Ann. Otol. Rhinol. Laryngol. 111:200-203. [DOI] [PubMed] [Google Scholar]
- 6.Franks, A. H., H. J. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, P., J. Das, and I. Foley. 1997. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 11:160-167. [DOI] [PubMed] [Google Scholar]
- 8.Gottenbos, B., H. C. van der Mei, F. Klatter, P. Nieuwenhuis, and H. J. Busscher. 2002. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 23:1417-1423. [DOI] [PubMed] [Google Scholar]
- 9.Gristina, A. G. 1987. Biomaterial-centred infection: microbial adhesion versus tissue integration. Science 237:1586-1595. [DOI] [PubMed] [Google Scholar]
- 10.Hilgers, F. J. M., and A. J. M. Balm. 1993. Long-term results of vocal rehabilitation after total laryngectomy with the low resistance, indwelling Provox voice prosthesis system. Clin. Otolaryngol. 18:517-523. [DOI] [PubMed] [Google Scholar]
- 11.Isquith, A. J., A. Abbott, and P. A. Walters. 1972. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl. Microbiol. 24:859-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen, G. J., M. Mooibroek, J. Idema, H. J. Harmsen, G. W. Welling, and J. E. Degener. 2000. Rapid identification of bacteria in blood cultures by using fluorescently labeled oligonucleotide probes. J. Clin. Microbiol. 38:814-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khardori, N., and M. Yassien. 1995. Biofilms in device-related infections. J. Ind. Microbiol. 15:141-147. [DOI] [PubMed] [Google Scholar]
- 14.Leunisse, C., R. van Weissenbruch, H. J. Busscher, H. C. van der Mei, and F. W. J. Albers. 1999. The artificial throat: a new method for standardization of in vitro experiments with tracheo-oesophageal voice prostheses. Acta Otolaryngol. (Stockholm) 119:604-608. [PubMed] [Google Scholar]
- 15.Mahieu, H. F., H. K. F. Van Saene, H. J. Rosing, and H. K. Schutte. 1986. Candida vegetations on silicone voice prostheses. Arch. Otolaryngol. Head Neck Surg. 112:321-325. [DOI] [PubMed] [Google Scholar]
- 16.Van de Hoogen, F. J. A., M. J. Oudes, G. Hombergen, H. F. Nijdam, and J. J. Manni. 1996. The Groningen, Nijdam and Provox voice prostheses: a prospective clinical comparison based on 845 replacements. Acta Otolaryngol. (Stockholm) 116:119-124. [DOI] [PubMed] [Google Scholar]
- 17.Van Wagenen, R. A., and J. D. Andrade. 1980. Flat plate streaming potential investigations: hydrodynamics and electrokinetic equivalency. J. Coll. Interf. Sci. 76:305-314. [Google Scholar]
- 18.Van Weissenbruch, R., S. Bouckaert, J. P. Remon, H. J. Nelis, R. Aerts, and F. W. J. Albers. 1997. Chemoprophylaxis of fungal deterioration of the Provox tracheoesophageal voice prosthesis in post-laryngectomy patients. Ann. Otol. Rhinol. Laryngol. 106:329-337. [DOI] [PubMed] [Google Scholar]