Abstract
Microbial oxidation and precipitation of manganese at deep-sea hydrothermal vents are important oceanic biogeochemical processes, yet nothing is known about the types of microorganisms or mechanisms involved. Here we report isolation of a number of diverse spore-forming Mn(II)-oxidizing Bacillus species from Guaymas Basin, a deep-sea hydrothermal vent environment in the Gulf of California, where rapid microbially mediated Mn(II) oxidation was previously observed. mnxG multicopper oxidase genes involved in Mn(II) oxidation were amplified from all Mn(II)-oxidizing Bacillus spores isolated, suggesting that a copper-mediated mechanism of Mn(II) oxidation could be important at deep-sea hydrothermal vents. Phylogenetic analysis of 16S rRNA and mnxG genes revealed that while many of the deep-sea Mn(II)-oxidizing Bacillus species are very closely related to previously recognized isolates from coastal sediments, other organisms represent novel strains and clusters. The growth and Mn(II) oxidation properties of these Bacillus species suggest that in hydrothermal sediments they are likely present as spores that are active in oxidizing Mn(II) as it emerges from the seafloor.
Deep-sea hydrothermal vents are a major source of dissolved Mn(II) for the world's oceans (14), and Mn has long been used as a valuable tracer of hydrothermal vent emissions (2, 8, 15, 21, 22, 29). Hydrothermal fluids mix with oxygenated seawater in hydrothermal plumes, where dissolved Mn(II) is oxidized and precipitated by microorganisms to form particulate Mn(III/IV) oxides. These biogenic minerals are biogeochemically important because they can scavenge and/or oxidize a number of elements and compounds (38) and because they contribute to metalliferous sediments and deposits that are widespread in areas surrounding midocean ridges and back arc basins (14, 22). Although bacteria are known to catalyze Mn(II) oxidation in many environments (38), including deep-sea hydrothermal vents (7, 9-12, 27), very little is known about the organisms or mechanisms involved in this oxidation/precipitation process.
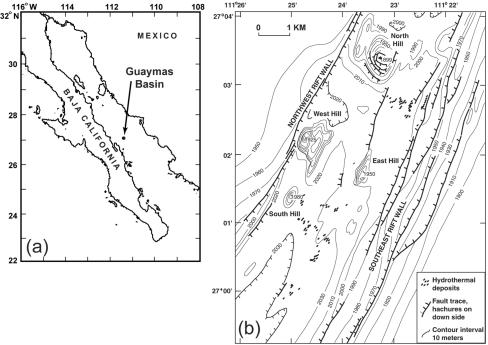
Guaymas Basin (GB) in the Gulf of California (Fig. 1) is a hydrothermally impacted, semienclosed basin where Mn(II) oxidation and precipitation of Mn oxides are particularly intense (7). It is an unusual hydrothermal system due to its close proximity to the coast, where high sedimentation rates maintain a thick blanket of organic compound-rich sediment over the ridge axis. Hydrothermal solutions ascend through and react with this overlying sediment, resulting in fluids that emerge from the seafloor and are enriched in Mn relative to Fe (42), a condition which may favor bacterially mediated Mn(II) oxidation (27). Indeed, a previous geochemical modeling study showed that Mn(II) oxidation rates are very high in GB hydrothermal plumes, and scanning electron microscopy of the resulting Mn oxide particles revealed Mn oxide-encrusted bacteria, providing evidence that Mn(II) oxidation is bacterially mediated (7).
FIG. 1.
(a) Location of the study site, Guaymas Basin in the Gulf of California. (b) Detailed sketch map of the seafloor features at the study site, adapted from reference 23.
The goal of the work described here was to identify bacteria involved in the rapid Mn(II) oxidation and precipitation observed in GB hydrothermal sediments and plumes. Because Mn(II)-oxidizing bacteria are polyphyletic and difficult to identify based on 16S rRNA gene sequences alone, we employed culture-based methods. Many phylogenetically diverse Mn(II)-oxidizing bacteria were identified; here we describe one of the most abundant cultured groups, spore-forming Bacillus species. It has been known for some time that there are marine Bacillus spores that catalyze Mn(II) oxidation (31, 33), and many diverse Bacillus species produce spores that oxidize Mn(II) (19). Bacillus sp. strain SG-1 is one of the most-studied model Mn(II)-oxidizing bacteria (1, 18, 31, 33, 40, 41) and is thought to oxidize Mn(II) via the multicopper oxidase MnxG (40, 43), which is deposited on the outermost layer of the spore coat (exosporium) during sporulation (18). The predicted amino acid sequence of MnxG has several short but highly conserved copper binding motifs that are characteristic of multicopper oxidases, a diverse family of enzymes that use multiple types of copper ions as cofactors in the oxidation of a wide variety of organic and inorganic compounds (36). The research presented here expands the previously recognized diversity of Mn(II)-oxidizing Bacillus spores and is the first to identify functional genes involved in Mn(II) oxidation from a deep-sea hydrothermal vent environment.
MATERIALS AND METHODS
Sample collection, strain isolation, and growth experiments.
Samples were collected aboard the R/V Atlantis on the HOLA-1 cruise to Guaymas Basin in April and May 2002 (Chief Scientist, Jim Cowen). Hydrothermal plume samples were obtained within a square area defined by 27°0′N, 111°23′W at its southeastern corner and 27°2.2′N, 111°26′W at its northwestern corner (Fig. 1). Plumes were detected based on turbidity anomalies, as determined by a transmissometer. Plume water samples were collected in 10-liter Niskin bottles on a conductivity-temperature-depth rosette from depths ranging from 1,500 m to 2,000 m. Seawater to be used for culturing was transferred directly from Niskin bottles to sterile 50-ml centrifuge tubes. The presence of particulate Mn oxides in hydrothermal plumes was confirmed by filtering ∼10 liters of water onto a 0.22-μm membrane and then adding 10 μl leukoberbelin blue (LBB) spot test reagent (24) directly to the filter and observing the reagent form a dark blue color. Background nonplume samples were obtained from deep water with no turbidity anomaly. Sediment samples were collected by push cores with the DSV Alvin, and the temperature of the sediments was measured in situ with a titanium thermocouple on the robotic arm of Alvin. Onboard ship, the surface sediments from the cores were transferred to sterile Eppendorf tubes and diluted with sterile seawater. To select for spores, some samples were incubated (pasteurized) at 80°C for 20 min. Seawater and diluted sediment samples were spread on Lept, J, J-acetate, J-succinate, M, or K medium plates. Lept medium (4) contained 10 mM HEPES (pH 7.5) and 100 μM MnCl2. J, M, and K media (30) were modified by adding 20 mM HEPES (pH 7.8) and 100 μM MnCl2 after autoclaving. J-acetate and J-succinate media were J medium supplemented with 10 mM acetate and succinate, respectively. Plates were incubated at either room temperature or 4°C. Mn(II)-oxidizing strains were isolated based on the formation of brown Mn oxides on colonies, which were confirmed to be Mn oxides with LBB (24). Cell morphology was determined by examining isolates by light microscopy; spore-forming organisms were apparent due to the presence of phase-bright spores. Growth experiments were conducted in 10 ml K medium incubated with shaking at 150 rpm at room temperature, 37°C, 50°C, 55°C, or 60°C. The optical density at 600 nm was determined every 6 to 12 h using a Spectronic 20 spectrophotometer (Milton Roy Company).
DNA extraction, PCR, cloning, and sequencing.
DNA extraction, PCR, and TOPO cloning of 16S rRNA (primers 27f and 1492r) and mnxG (primers MnxGIf and MnxGIr) genes were performed as described previously (19). Initially, no mnxG PCR products were obtained from strains GB02-25, -27, and -31, but mnxG was successfully amplified by using degenerate primers MnxGUf (CAGRTGRATRTGCTGGCCGAT) and MnxGBr1 (RAAIARRTGTRCRTGRAARAA). Both strands of 16S rRNA gene PCR products were sequenced with the 27f, 338f, 536r, 1074r, 1055f, and 1492r primers. mnxG genes were TOPO cloned into pCR2.1 and sequenced with the M13f and M13r primers. Sequencing was done with either an Amersham Pharmacia Megabace 500 (Scripps Institution of Oceanography Marine Biology Research Division) or ABI 3100 (Sequegene, Inc., and Center for AIDS Research at University of California, San Diego) automated sequencer.
Phylogenetic analysis.
16S rRNA genes were aligned with the program Sequencher, and alignments were checked and edited manually. MnxG amino acid sequences were aligned with the program Clustal X (http://www-igbmc.u-strasbg.fr/BioInfo/ClustalX/Top.html) using default settings, with the following exceptions: for pairwise alignment parameters, the gap opening was set to 1.00 and the gap extension was set to 0.01; and for multiple-alignment parameters, the gap opening was set to 1.00 and the gap extension was set to 0.10. PAUP (version 4.0b10) was used to generate phylogenetic trees for both 16S rRNA and MnxG sequences. Trees were constructed by using neighbor joining (see Fig. 2) and maximum parsimony; these two methods gave similar results.
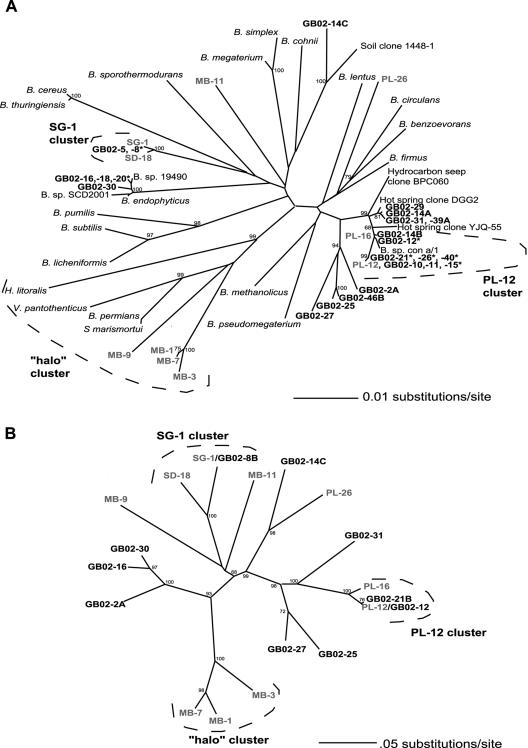
FIG. 2.
Unrooted neighbor-joining phylogenetic trees based on 16S rRNA sequences (A) and MnxG amino acid sequences (B). Mn(II)-oxidizing isolates from Guaymas Basin are indicated by boldface type and are designated “GB02-.” An asterisk indicates that multiple isolates were found to have identical 16S rRNA gene sequences (Table 1). Mn(II)-oxidizing isolates from coastal San Diego sediments are indicated by gray type. Additional Bacillus species, isolates, and clones are not thought to be capable of Mn(II) oxidation and are included for reference. Bootstrap values based on 1,000 replicates (>65%) are indicated at the branch points.
Spore purification and Mn(II) oxidation assays.
Spores were purified as described previously (33), except that the 0.15 M NaCl wash was supplemented with 0.3 to 1 mM ascorbate to remove Mn oxides formed during culturing. Mn(II) oxidation rate assays were performed in 500-μl reaction mixtures containing 3 μM CuCl2, 800 μM MnCl2, and 20 mM HEPES (pH 7.8) in MilliQ-filtered water. Reaction mixtures with no spores were incubated at 4°C, room temperature, 37°C, 50°C, or 70°C for equilibration, 100 μl of purified spores was added to each mixture, and then the mixtures were incubated for 10 h at the same temperatures. The relative quantity of Mn oxides formed was determined by adding 50 μl of the assay mixture to 250 μl of LBB reagent (24) and measuring the absorption at 620 nm with a Perkin-Elmer Lambda Bio UV/VIS spectrophotometer. All reactions were performed in triplicate.
Nucleotide sequence accession numbers.
All 16S rRNA gene sequences have been deposited in the GenBank database under the nucleotide sequence accession numbers shown in Table 1. The accession numbers for the mnxG nucleotide sequences are as follows: DQ079011 for GB02-2A, DQ079012 for GB02-8B, DQ079013 for GB02-12, DQ079014 for GB02-14C, DQ079015 for GB02-16, DQ079016 for GB02-21B, DQ079017 for GB02-25, DQ079018 for GB02-27, DQ079019 for GB02-30, and DQ079020 for GB02-31.
TABLE 1.
Mn(II)-oxidizing Bacillus strains used in this study
| Accession no. | Isolate(s) | Sourceb | Medium | Temp (°C)e |
|---|---|---|---|---|
| DQ079008 | GB02-2A | Mat-covered sediment | K | 4-31 |
| DQ078996 | GB02-5 | Mat-covered sediment (HT)c | K | 4-31 |
| DQ078996 | GB02-8A, -8B, -8C, -8D (4)a | Mat-covered sediment | K-vpwd | 75-82 |
| DQ078999 | GB02-10, -11 (2)a | Deep nonplume water (depth, 1,486 m) | M | 3 |
| DQ079005 | GB02-12, -12A, -12B (3)a | Black hydrothermal sediment (HT)c | K-vpwd | 24-66 |
| DQ079001 | GB02-14A | Sediment (HT)c | M | 3 |
| DQ078995 | GB02-14B | Sediment (HT)c | M | 3 |
| DQ079004 | GB02-14C | Sediment (HT)c | M | 3 |
| DQ078999 | GB02-15 | Plume (depth, 1,812 m) | M | 3 |
| DQ079006 | GB02-16, -18, -20 (3)a | Sediment (HT)c | Lept | 3 |
| DQ078998 | GB02-21B, -21C (2)a | Plume (depth, 1,812 m) | M | 3 |
| DQ079010 | GB02-25 | Mat-covered sediment | K | 4-31 |
| DQ078998 | GB02-26A, -26B, -26C (3)a | Mat-covered sediment | M-vpwd | 75-82 |
| DQ078997 | GB02-27 | Sediment (HT)c | K | 3 |
| DQ079002 | GB02-29A, -29B, -29C, -29D (4)a | Sediment (HT)c | K, J-acetate | 3 |
| DQ079007 | GB02-30 | Sediment (HT)c | K | 3 |
| DQ079000 | GB02-31 | Sediment (HT)c | K | 3 |
| DQ079003 | GB02-39A | Sediment (HT)c | M | 3 |
| DQ078998 | GB02-40A, -40B, -40C (3)a | Mat-covered sediment (HT)c | M | 75-82 |
| DQ079009 | GB02-46B | Sediment (HT)c | K | 3 |
The numbers in parentheses indicate the numbers of isolates that were sequenced and found to have identical 16S rRNA gene sequences.
All sediment samples were collected at a depth of ∼2,000 m.
HT indicates that samples were treated at 80°C for 20 min prior to plating.
vpw indicates that the medium was made with GB vent plume water.
Temperature of the sample upon collection.
RESULTS AND DISCUSSION
GB in the Gulf of California is a semienclosed basin with a sediment-covered hydrothermal system, and previous studies have suggested that in this area bacteria play an important role in the rapid oxidation and precipitation of hydrothermally derived dissolved Mn(II) (7, 27). Despite the important role of bacteria in the oxidation and precipitation of Mn at GB and other deep-sea hydrothermal vents (7, 9-12, 27), little is known about the organisms or mechanisms involved in this biomineralization. The objective of this study was to identify Mn(II)-oxidizing bacteria in GB hydrothermal sediments and plumes. Hydrothermal plumes were detected based on turbidity anomalies, and the presence of a large standing stock of particulate Mn oxide was confirmed by filtering plume water onto 0.22-μm filters. The brown particulate matter that collected on filters was determined to be Mn oxide based on the LBB test (24) and rapid dissolution and disappearance of the brown color upon addition of ascorbic acid. All detectable particulate Mn oxide passed through a 5-μm filter but was trapped on a 0.22-μm filter, which is consistent with previous observations that Mn oxides at GB are dominated by bacterium-size particles.
Isolation and diversity of Mn(II)-oxidizing Bacillus spores from sediments and plumes.
Mn(II)-oxidizing bacteria are known to be polyphyletic, which makes it difficult to identify them based on 16S rRNA sequences alone, so we focused on culture-based approaches. A large number of heterotrophic Mn(II)-oxidizing bacteria were isolated, including α- and γ-proteobacteria (especially Pseudoalteromonas and Microbulbifer spp.). Here we focus on one of the most abundant groups of bacteria that we isolated, endospore-forming Bacillus species, which were cultured from high- and low-temperature sediments, as well as from hydrothermal plumes and nonhydrothermally affected deep waters (Table 1). In general, the number of Mn(II)-oxidizing colonies obtained from hydrothermal plumes was greater than the number of Mn(II)-oxidizing colonies obtained from nonplumes and the number of Mn(II)-oxidizing colonies obtained from hydrothermal sediments was greater than the number of Mn(II)-oxidizing colonies obtained from cold sediments (Table 2). To specifically select for spore-forming bacteria, some sediment samples were incubated at 80°C for 20 min (Table 1). Based on microscopic and 16S rRNA sequence analyses, all Mn(II)-oxidizing colonies examined after this treatment were spore-forming Bacillus species. More than 25% of the colonies recovered from hydrothermal sediments after heat treatment oxidized Mn(II), whereas only 6.7% of the colonies recovered from nonhydrothermal sediments subjected to the same heat treatment oxidized Mn(II) (Table 2).
TABLE 2.
Plate counts of Mn(II)-oxidizing bacteria
| Source | No. of Mn oxide-encrusted colonies | Total no. of colonies | % of colonies that oxidized Mn |
|---|---|---|---|
| Plumes | 146 | 2,250 | 6.5 |
| Nonplumes | 3 | 359 | 1.9 |
| Hydrothermal sediments | 144 | 1,248 | 11.5 |
| Nonhydrothermal sediments | 91 | 2,188 | 4.1 |
| Pasteurized hydrothermal sedimentsa | 132 | 523 | 25.2 |
| Pasteurized nonhydrothermal sedimentsa | 41 | 608 | 6.7 |
Pasteurization consisted of heating at 80°C for 20 min.
Analysis of 16S rRNA genes of the Mn(II)-oxidizing spores from GB revealed that while many of them fall into clusters defined in a previous study of Mn(II)-oxidizing Bacillus spores from coastal sediments (19), many others represent distinct lineages (GB02-14C) and two new clusters (GB02-2A, -25, -27, and -46B; and GB02-16, -18, -20, and -30) (Fig. 2A). Several of the isolates were found to have identical 16S rRNA genes (Table 1). A number of isolates (GB02-10, -11, -12, -12A, -12B, -14B, -15, -21B, -21C, -26A, -26B, -26C, -40A, -40B, and -40C) from GB fell into the PL-12 cluster, a phylogenetically tight group that includes organisms and clones isolated from various environments, such as the Korean traditional fermented seafood jeotgal (Bacillus jeotgali), a uranium mine tailings pile, a hydrocarbon seep, and rice paddy-associated anoxic bulk soil (see reference 19 and references therein). Several other 16S rRNA gene sequences that are present in the GenBank database but have not been published also fall into the PL-12 cluster, including an isolate from concretions of siderite from an arsenic-polluted aquifer in West Bengal, India (Bacillus sp. strain Cona/1) and a clone retrieved from a pink microbial mat in the Spectacles Hot Spring in Rehai, Tengchong, China (Bacillus sp. clone YJQ-55). Another group of GB isolates (GB-14A, -29, -31, and -39A) forms a group just outside the PL-12 cluster along with another clone from the hot springs in Rehai, Tengchong, China (Bacillus sp. clone DGG2). Isolates GB02-2A, -25, -27, and -46B form a loose group that is only distantly related (∼97% identical) to the PL-12 cluster or any other sequences in the GenBank database.
GB02-14C is very different than any other previously recognized Mn(II) oxidizer and is most closely related to uncultured soil bacterium clone 1448-1 (39). Four isolates (GB02-16, -18, -20, and -30) form a distinct cluster along with three strains not known to oxidize Mn(II): Bacillus endophyticus, an organism isolated from the inner tissues of cotton plants (32); Bacillus sp. strain 19490, an isolate from a biodeteriorated mural painting (20); and Bacillus sp. strain SCD-2001, a fluorescent soil isolate (26). Three of these Mn(II)-oxidizing isolates (GB02-16, -18, and -20) have identical 16S rRNA gene sequences and were isolated on Lept freshwater medium, but they also grow on the seawater-based K medium. Five GB isolates (GB02-5, -8A, -8B, -8C, and -8D) have identical 16S rRNA gene sequences that fall into the SG-1 cluster.
mnxG, a gene believed to encode a multicopper oxidase that catalyzes Mn(II) oxidation in Bacillus sp. strain SG-1 (40, 43) and other Mn(II)-oxidizing Bacillus strains (19), was amplified from all GB Mn(II)-oxidizing isolates, indicating that these deep-sea hydrothermal vent isolates likely share a copper-dependent mechanism of Mn(II) oxidation with previously characterized Mn(II)-oxidizing Bacillus spores. mnxG could not be amplified from non-Mn(II)-oxidizing Bacillus spores (19), and mnxG is not present in the genomes of any non-Mn(II)-oxidizing Bacillus species sequenced to date, suggesting that mnxG is a good genetic marker for the ability of Bacillus spp. to oxidize Mn(II). We were unable to amplify mnxG with primers mnxGIf and mnxGIr from several of our Mn(II)-oxidizing isolates, so we designed new degenerate primers targeting conserved copper-binding regions. The degenerate primers yielded good amplification of mnxG from diverse Mn-oxidizing sporeformers, suggesting that these primers may be suitable for amplifying mnxG directly from environmental DNA in order to obtain a culture-independent view of the diversity of this gene in the environment.
Phylogenetic trees constructed based on predicted MnxG amino acid sequences (Fig. 2B) are very similar to trees based on 16S rRNA genes (Fig. 2A), which is consistent with the hypothesis that there has been no extensive horizontal transfer of mnxG genes in these organisms (19). However, there are a few exceptions among the GB isolates. Based on 16S rRNA, GB02-2A falls near the PL-12 cluster (Fig. 2A), whereas the MnxG phylogeny places it with the GB02-16 cluster (Fig. 2B). Also, the MB-9 MnxG sequence does not group with the “halo” cluster as it does when 16S rRNA is used. The “halo” cluster is a group of organisms so named because of the ability of its members to grow under high-salt conditions (19). In view of MB-9's lower tolerance for salt (19) and its only loose phylogenetic affiliation, it seems that this isolate should not be considered a member of the “halo” cluster. More phylogenetic analysis with additional genes is required to resolve the exact phylogenetic relationships among the deeply branching Mn(II) oxidizers (MB-9) and the organisms for which 16S rRNA and MnxG sequences give different results (GB02-2A).
Growth and Mn(II) oxidation properties of Bacillus isolates.
Due to the ability of Bacillus spores to persist under harsh conditions over long periods, it is not clear whether the GB isolates are metabolically active as vegetative cells in the sediments and plumes where we isolated them or if they are deposited in these areas with sinking organic matter from surface waters or river runoff. To address this question, we determined the growth characteristics of a selection of isolates at a range of temperatures. Several strains (including GB02-8B and -27) had an optimum growth rate at 50°C, while other isolates grew best at room temperature or 37°C (e.g., GB02-12) (data not shown). These results indicate that the organisms are mesophiles or perhaps slight thermophiles, suggesting that while the GB Mn(II)-oxidizing Bacillus isolates may be able to grow in some of the moderately hot sediments sampled, they were most likely present only as spores in sediment samples at temperatures above 60°C (Table 1). Spore-forming Bacillus species capable of Mn(II) oxidation seem to be able to grow at wide ranges of temperatures and salt concentrations (19), which makes it difficult to infer whether these organisms are specifically adapted to marine and/or hydrothermal environments.
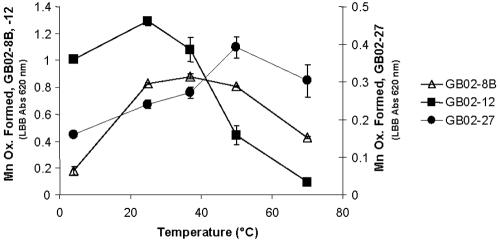
Regardless of whether the Bacillus isolates are metabolically active in GB hydrothermal sediments and plumes, Mn(II)-oxidizing activity is associated with the spores and is therefore likely to occur in a wide range of conditions. To verify that Mn(II)-oxidizing activity could occur at high temperatures, we assayed Mn(II) oxidation by purified spores of several isolates at 4, 25, 37, 50, and 70°C. The activity-versus-temperature profile varied for different spores, but there was always significant activity at 50°C and there was usually significant activity at 70°C (Fig. 3). However, there was no clear correlation between the optimal growth temperature and the optimal temperature for Mn(II) oxidation. Nevertheless, together with the growth characteristics (mesophilic, not thermophilic), these data suggest that the Bacillus species isolated here are present as active Mn(II)-oxidizing spores at the sediment-water interface of hydrothermal sediments. Therefore, Mn(II)-oxidizing Bacillus spores in surface sediments might play an important role in oxidizing Mn(II), perhaps contributing to the short residence time of dissolved Mn in the Guaymas Basin.
FIG. 3.
Relative amounts of Mn oxidized at different temperatures by Bacillus spores isolated from Guaymas Basin. Mn Ox., Mn oxide; Abs 620 nm, absorbance at 620 nm.
Mn-oxidizing spores from other deep-sea hydrothermal vents.
Because GB represents a unique deep-sea hydrothermal vent environment, we sequenced the 16S rRNA genes from several Mn(II)-oxidizing bacteria from our culture collection isolated on previous expeditions to hydrothermal vents at the Juan de Fuca Ridge (B. Tebo, unpublished results). Two of these isolates were spore-forming bacilli; one fell into the PL-12 cluster, and one fell into the SG-1 cluster. The third isolate was also a gram-positive bacterium and was most closely related to Microbacterium oxydans (34). Results from our laboratory indicate that Mn(II)-oxidizing spores of Bacillus species can also be isolated from Vailulu'u (A. Templeton and B. Tebo, unpublished results), an underwater volcano in the South Pacific (37). Thus, Mn(II)-oxidizing Bacillus spores seem to be widespread in deep-sea hydrothermal vent environments.
Our results show that diverse Mn(II)-oxidizing Bacillus spores with mnxG multicopper oxidase genes can be isolated from GB hydrothermal sediments and plumes, extending the previously recognized diversity of Mn(II)-oxidizing Bacillus spores and mnxG genes. Although there have been several brief descriptions of isolation of Mn(II)-oxidizing bacteria from deep-sea hydrothermal vents (13, 16, 17), this study provides the first 16S rRNA gene-based identification of such bacteria. The mnxG multicopper oxidase genes reported here are the first functional genes involved in Mn(II) oxidation to be recovered from deep-sea hydrothermal vents, where bacteria are thought to play a crucial role in mediating rapid Mn(II) oxidation and precipitation of Mn oxides on massive, globally distributed scales. Mn(II)-oxidizing Bacillus spores exhibit extremely rapid and stable Mn(II)-oxidizing activity, and their presence in GB hydrothermal sediments and plumes could significantly contribute to the rapid Mn(II) oxidation that takes place at GB.
Bacillus species are generally recognized as organisms that are ubiquitous in many environments and have been isolated from marine sediments (3, 35) and several deep-sea hydrothermal vents, including Guaymas Basin (5, 28). However, few studies that we are aware of have addressed the numerical or biogeochemical significance of Bacillus spores in the marine environment, especially in the water column. One possible explanation for the presence of Bacillus spp. in the water column at GB is that they are attached to suspended particulate matter (6), which could include fine sediment particles entrained by ascending hydrothermal fluids. Previous work in our laboratory demonstrated that a significant fraction of Mn(II)-oxidizing activity in coastal San Diego sediments is heat resistant, which is consistent with catalysis by a spore coat enzyme (25). Our results suggest that Bacillus species (and gram-positive organisms in general) could play important but previously unrecognized roles in biogeochemical cycles in the oceanic water column. More cultivation-independent studies using molecular and biogeochemical approaches are needed to determine whether Bacillus spores such as those identified here significantly affect Mn biogeochemistry in Guaymas Basin and other deep-sea hydrothermal vent environments.
Acknowledgments
We thank the captain and crew of R/V Atlantis and the pilots and crew of DSV Alvin, who made this work possible. We are grateful to Chief Scientist Jim Cowen for allowing us to participate in the cruise and to Jasper Konter for assistance in the preparation of the maps.
This work was supported by the Superfund Basic Research Program (NIEHS grant ES10337 to UCSD) and the NSF (grant OCE-0352081). G.J.D. was supported in part by an NSF graduate research fellowship.
REFERENCES
- 1.Bargar, J. R., B. M. Tebo, U. Bergmann, S. M. Webb, P. Glatzel, V. Q. Chiu, and M. Villalobos. 2005. Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am. Mineral 90:143-154. [Google Scholar]
- 2.Bolger, G. W., P. R. Betzer, and V. V. Gordeev. 1978. Hydrothermally-derived manganese suspended over the Galapagos Spreading Center. Deep-Sea Res. 25:721-733. [Google Scholar]
- 3.Bonde, G. J. 1981. Bacillus from marine habitats: allocation to phena established by numerical techniques, p. 181-215. In R. C. W. Berkeley and M. Goodfellow (ed.), The aerobic endospore-forming bacteria: classification and identification. Academic Press, New York, N.Y.
- 4.Boogerd, F. C., and J. P. M. de Vrind. 1987. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 169:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccamo, D., T. Maugeri, and C. Gugliandolo. 2001. Identification of thermophilic and marine bacilli from shallow thermal vents by restriction analysis of their amplified 16S rDNA. J. Appl. Microbiol. 91:520-524. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, A. C., and J. M. Gieskes. 1984. Water column anomalies associated with hydrothermal activity in the Guaymas Basin, Gulf of California. Earth Planet. Sci. Lett. 68:57-72. [Google Scholar]
- 7.Campbell, A. C., J. M. Gieskes, J. E. Lupton, and P. F. Lonsdale. 1988. Manganese geochemistry in the Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 52:345-357. [Google Scholar]
- 8.Coale, K. H., C. S. Chin, G. J. Massoth, K. S. Johnson, and E. T. Baker. 1991. In situ chemical mapping of dissolved iron and manganese in hydrothermal plumes. Nature 352:325-328. [Google Scholar]
- 9.Cowen, J. P., M. A. Bertram, E. T. Baker, G. J. Massoth, R. A. Feely, and M. Summit. 1998. Geomicrobial transformation of manganese in Gorda Ridge event plumes. Deep-Sea Res. II 45:2713-2738. [Google Scholar]
- 10.Cowen, J. P., and Y. H. Li. 1991. The influence of a changing bacterial community on trace metal scavenging in a deep-sea particle plume. J. Mar. Res. 49:517-542. [Google Scholar]
- 11.Cowen, J. P., G. J. Massoth, and E. T. Baker. 1986. Bacterial scavenging of Mn and Fe in a mid- to far-field hydrothermal particle plume. Nature 322:169-171. [Google Scholar]
- 12.Cowen, J. P., G. J. Massoth, and R. A. Feely. 1990. Scavenging rates of dissolved manganese in a hydrothermal vent plume. Deep-Sea Res. 37:1619-1637. [Google Scholar]
- 13.Durand, P., D. Prieur, C. Jeanthon, and E. Jacq. 1990. Occurrence and activity of heterotrophic manganese oxidizing bacteria associated with alvinellids (polychaetous annelids) from a deep hydrothermal vent site on the East Pacific Rise. C. R. Acad. Sci. Ser. III 310:273-278. [Google Scholar]
- 14.Edmond, J. M., K. L. Von Damm, R. E. McDuff, and C. I. Measure. 1982. Chemistry of hot springs on the East Pacific Rise and their effluent dispersal. Nature 297:187-191. [Google Scholar]
- 15.Edmonds, H. N., P. J. Michael, E. T. Baker, D. P. Connelly, J. E. Snow, C. H. Langmuir, H. J. B. Dick, R. Muhe, C. R. German, and D. W. Graham. 2003. Discovery of abundant hydrothermal venting on the ultraslow-spreading Gakkel ridge in the Artic Ocean. Nature 421:252-256. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich, H. L. 1983. Manganese oxidizing bacteria from a hydrothermally active area on the Galapagos Rift. Ecol. Bull 35:357-366. [Google Scholar]
- 17.Ehrlich, H. L. 1985. Mesophilic manganese-oxidizing bacteria from hydrothermal discharge areas at 21° north on the East Pacific Rise, p. 186-194. In D. E. Caldwell, J. A. Brierley, and C. L. Brierley (ed.), Planetary ecology. Van Nostrand Reinhold Co., New York, N.Y.
- 18.Francis, C. A., K. L. Casciotti, and B. M. Tebo. 2002. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Arch. Microbiol. 178:450-456. [DOI] [PubMed] [Google Scholar]
- 19.Francis, C. A., and B. M. Tebo. 2002. Enzymatic manganese(II) oxidation by metabolically dormant spores of diverse Bacillus species. Appl. Environ. Microbiol. 68:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyrman, J., and J. Swings. 2001. 16S rDNA sequence analysis of bacterial isolates from biodeteriorated mural paintings in the Servilia Tomb (Necropolis of Carmona, Seville, Spain). Syst. Appl. Microbiol. 24:417-422. [DOI] [PubMed] [Google Scholar]
- 21.Klinkhammer, G., P. Rona, M. Greaves, and H. Elderfield. 1985. Hydrothermal manganese plumes in the Mid-Atlantic Ridge rift valley. Nature 314:727-731. [Google Scholar]
- 22.Klinkhammer, G. P., and A. Hudson. 1986. Dispersal patterns for hydrothermal plumes in the southern Pacific using manganese as a tracer. Earth Planet Sci. Lett. 79:241-249. [Google Scholar]
- 23.Koski, R. A., P. F. Lonsdale, W. C. Shanks, M. E. Berndt, and S. S. Howe. 1985. Mineralogy and geochemistry of a sediment-hosted hydrothermal sulfide deposit from the Southern Trough of Guaymas Basin, Gulf of California. J. Geophys. Res. 90:6695-6707. [Google Scholar]
- 24.Krumbein, W. E., and H. J. Altmann. 1973. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol. Wiss. Meeresunters. 25:347-356. [Google Scholar]
- 25.Lee, Y. 1994. Microbial oxidation of cobalt: characterization and its significance in marine environments. Ph.D. thesis. University of California, San Diego.
- 26.Magyarosy, A., J. Z. Ho, H. Rapoport, S. Dawson, J. Hancock, and J. D. Keasling. 2002. Chlorxanthomycin, a fluorescent, chlorinated, pentcyclic pyrene from a Bacillus sp. Appl. Environ. Microbiol. 68:4095-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandernack, K. W., and B. M. Tebo. 1993. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim. Cosmochim. Acta 57:3907-3923. [Google Scholar]
- 28.Marteinsson, V. T., J. L. Birrien, C. Jeanthon, and D. Prieur. 1996. Numerical taxonomic study of thermophilic Bacillus isolated from three geographically separated deep-sea hydrothermal vents. FEMS Microbiol. Ecol. 21:255-266. [Google Scholar]
- 29.Massoth, G. J., E. T. Baker, R. A. Feely, J. E. Lupton, R. W. Collier, J. F. Gendron, K. K. Roe, S. M. Maenner, and J. A. Resing. 1998. Manganese and iron in hydrothermal plumes resulting from the 1996 Gorda Ridge Event. Deep-Sea Res. II 45:2683-2712. [Google Scholar]
- 30.Nealson, K. H. 1978. The isolation and characterization of marine bacteria which catalyze manganese oxidation, p. 847-858. In W. E. Krumbein (ed.), Environmental biogeochemistry and geomicrobiology, vol. 3. Methods, metals and assessment. Ann Arbor Science Publishers Inc., Ann Arbor, MI. [Google Scholar]
- 31.Nealson, K. H., and J. Ford. 1980. Surface enhancement of bacterial manganese oxidation: implications for aquatic environments. Geomicrobiol. J. 2:21-37. [Google Scholar]
- 32.Reva, O. N., V. V. Smirnov, B. Pettersson, and F. G. Priest. 2002. Bacillus endophyticus sp. nov., isolated from the inner tissues of cotton plants (Gossypium sp.). Int. J. Syst. Evol. Microbiol. 52:101-107. [DOI] [PubMed] [Google Scholar]
- 33.Rosson, R. A., and K. H. Nealson. 1982. Manganese binding and oxidation by spores of a marine bacillus. J. Bacteriol. 151:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumann, P., F. A. Rainey, J. Burghardt, E. Stackebrandt, and N. Weiss. 1999. Reclassification of Brevibacterium oxydans (Chatelain and Second 1966) as Microbacterium oxydans comb. nov. Int. J. Syst. Bacteriol. 49:175-177. [DOI] [PubMed] [Google Scholar]
- 35.Siefert, J. L., M. Larios-Sanz, L. K. Nakamura, R. A. Slepecky, J. H. Paul, E. R. B. Moore, G. E. Fox, and J. P. Jurtshuk. 2000. Phylogeny of marine Bacillus isolates from the Gulf of Mexico. Curr. Microbiol. 41:84-88. [DOI] [PubMed] [Google Scholar]
- 36.Solomon, E. I., U. M. Sundaram, and T. E. Machonkin. 1996. Multicopper oxidases and oxygenases. Chem. Rev. 96:2563-2605. [DOI] [PubMed] [Google Scholar]
- 37.Staudigel, H., S. R. Hart, A. A. P. Koppers, C. Constable, R. Workman, M. Kurz, and E. T. Baker. 2004. Hydrothermal venting at Vailulu'u seamount: the smoking end of the Samoan chain. Geochem. Geophys. Geosyst. 5:1-25. [Google Scholar]
- 38.Tebo, B. M., J. R. Bargar, B. G. Clement, G. J. Dick, K. J. Murray, D. Parker, R. Verity, and S. M. Webb. 2004. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet Sci. 32:287-328. [Google Scholar]
- 39.Valinsky, L., G. D. Vedova, A. J. Scupham, S. Alvey, A. Figueroa, B. Yin, R. J. Hartin, M. Chrobak, D. E. Crowley, T. Jiang, and J. Borneman. 2002. Analysis of bacterial community composition by oligonucleotide fingerprinting of rRNA genes. Appl. Environ. Microbiol. 68:3243-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Waasbergen, L. G., M. Hildebrand, and B. M. Tebo. 1996. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J. Bacteriol. 178:3517-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Waasbergen, L. G., J. A. Hoch, and B. M. Tebo. 1993. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J. Bacteriol. 175:7594-7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Von Damm, K. L., J. M. Edmond, C. I. Measures, and B. Grant. 1985. Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 49:2221-2237. [Google Scholar]
- 43.Webb, S. M., G. J. Dick, J. R. Bargar, and B. M. Tebo. 2005. Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). Proc. Natl. Acad. Sci. USA 102:5558-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]