Abstract
Bacteria belonging to the Roseobacter clade of the α-Proteobacteria occupy a wide range of environmental niches and are numerically abundant in coastal waters. Here we reveal that Roseobacter-like bacteria may play a previously unrecognized role in the oxidation and cycling of manganese (Mn) in coastal waters. A diverse array of Mn(II)-oxidizing Roseobacter-like species were isolated from Elkhorn Slough, a coastal estuary adjacent to Monterey Bay in California. One isolate (designated AzwK-3b), in particular, rapidly oxidizes Mn(II) to insoluble Mn(III, IV) oxides. Interestingly, AzwK-3b is 100% identical (at the 16S rRNA gene level) to a previously described Pfiesteria-associated Roseobacter-like bacterium, which is not able to oxidize Mn(II). The rates of manganese(II) oxidation by live cultures and cell-free filtrates are substantially higher when the preparations are incubated in the presence of light. The rates of oxidation by washed cell extracts, however, are light independent. Thus, AzwK-3b invokes two Mn(II) oxidation mechanisms when it is incubated in the presence of light, in contrast to the predominantly direct enzymatic oxidation in the dark. In the presence of light, production of photochemically active metabolites is coupled with initial direct enzymatic Mn(II) oxidation, resulting in higher Mn(II) oxidation rates. Thus, Roseobacter-like bacteria may not only play a previously unrecognized role in Mn(II) oxidation and cycling in coastal surface waters but also induce a novel photooxidation pathway that provides an alternative means of Mn(II) oxidation in the photic zone.
In surface environments, Mn cycles between the following three dominant oxidation states: Mn(II), which exists as the soluble ion Mn(H2O)62+ (when it is undersaturated with respect to MnCO3), and Mn(III) and Mn(IV), which typically precipitate as single- or mixed-valence, sparingly soluble (oxy)(hydr)oxides (referred to collectively as oxides below). The oxidation of Mn(II) has sweeping environmental ramifications in that Mn(III, IV) oxides are effective scavengers of nutrients and metals, potent oxidants of both organic constituents (e.g., humic acids) and inorganic constituents (e.g., HS−, CrVI), and terminal electron acceptors for anaerobic respiration (see reference 52 and references therein). While the oxidation of aqueous Mn(II) by O2 is thermodynamically favorable in most surface environments, the reaction is kinetically limited except in the presence of catalysts [e.g., Mn(III, IV) oxide surfaces] (14, 16, 29). A number of bacteria have been found to mediate the oxidation of Mn(II), increasing the rate of oxidation by up to 5 orders of magnitude compared to the rates of surface-catalyzed abiotic reactions (36, 51). Thus, the dominant pathway of Mn(II) oxidation in the environment is likely microbially mediated.
Manganese(II)-oxidizing bacteria have been found in a wide range of environments (54), including marine waters (35), freshwaters (22, 26), sediments, nodules (17, 18, 42, 44), ferromanganese cave deposits (39), hydrothermal vents (28), and submarine basalt surfaces (55). The ability to oxidize Mn(II) has been observed in a diverse group of bacteria, including the low-G+C-content gram-positive bacteria (Firmicutes), Actinobacteria, and α-, β-, and γ-Proteobacteria (53, 54). Genetic and/or biochemical characterization of six well-studied bacteria, Bacillus sp. strain SG-1 (19, 56), Leptothrix discophora strain SS-1 (10, 12), α-proteobacterium SD-21 (21), Pedomicrobium sp. strain ACM 3067 (31), and Pseudomonas putida strains MnB1 and GB-1 (9, 15, 21, 40), has revealed that genes or enzymes related to multicopper oxidases (MCOs) appear to be involved in Mn(II) oxidation. Multicopper oxidases are a structurally and functionally diverse family of enzymes that are able to oxidize a number of (in)organic substrates [e.g., Fe(II), diphenolics] (10, 43). While MCOs appear to be universally involved in the bacterial oxidation of Mn(II), the underlying biochemical mechanisms and physiological and ecological advantages of Mn(II) oxidation remain unresolved.
Recent studies have revealed that bacteria belonging to the Roseobacter clade of the α-Proteobacteria are ubiquitous in marine environments (25) and often account for a substantial fraction of 16S rRNA gene clone libraries and metagenomic libraries (5, 34, 57). Furthermore, Roseobacter-like species inhabit a diverse array of marine habitats, including coastal and open-ocean waters, hypersaline lakes, sediments, and Arctic and Antarctic sea ice, and are found in association with submarine basalts, seaweed, algal blooms, and dinoflagellates (34, 55). In terms of biogeochemical cycling, many members of the Roseobacter clade appear to possess the widespread ability to degrade dimethylsulfoniopropionate (DMSP) (23, 24, 33, 34), an abundant organic sulfur compound produced by marine algae. However, the role of Roseobacter-like bacteria in the transformation and cycling of metals, such as Mn, is largely unknown.
Here we report that Roseobacter-like bacteria may play a previously unrecognized role in the oxidation of Mn(II) in coastal environments. Coastal waters and sediments adjacent to Monterey Bay in California harbor a diverse array of Mn(II)-oxidizing bacteria belonging to the Roseobacter clade. Below, one planktonic isolate (designated AzwK-3b) is characterized in detail to provide insight into the mechanisms and conditions conducive to Mn(II) oxidation in the Roseobacter clade.
MATERIALS AND METHODS
Site description.
Lower Azevedo Pond and Upper Azevedo Pond are agriculturally impacted, shallow salt marshes adjacent to Elkhorn Slough in California (11). Tidal restrictions and high nutrient loads result in complete O2 consumption in the top few millimeters of sediment, which produces a steep redox gradient in the surface sediments (4, 11). In Upper Azevedo Pond, light-regulated (e.g., photosynthetic) O2 fluctuations are coupled with extreme diel redox Mn cycling, with dissolved Mn removal rates on the order of 1 μM h−1 during oxygenated events (4). Lower Azevedo Pond consists of sulfide-rich sediments, often with a striking Mn oxide veneer reminiscent of desert varnish. Sediment samples (5-cm cores) and surface water samples were obtained in May and July 2004 from Lower Azevedo Pond and Upper Azevedo Pond and were stored on ice for transport back to the lab and immediate initiation of Mn(II) oxidizer enrichments. At the time of collection (8 July 2004), Upper Azevedo Pond had a temperature, dissolved oxygen content, conductivity, salinity, and pH of 19°C, 53%, 53 mS, 36 ppt, and 8.3, respectively. On 5 May 2004, Lower Azevedo Pond had a temperature, dissolved oxygen content, conductivity, salinity, and pH of 23°C, 49%, 45 mS, 28 ppt, and 8.2, respectively.
Mn(II)-oxidizing bacteria enrichments.
A dilution series (10−2 to 10−9) of sediment and water samples (100 μl) was spread on plates containing a variety of seawater-based (75%) enrichment media, including K, M, and Leptothrix (Lept) media, as described previously (55), J medium, and J medium amended with 10 mM acetate (JAC medium), which contained 1.5 mM NH4Cl, 2 mM HCO3, 73 μM KH2PO4, and 10 ml of Wolfe's vitamin supplement (ATCC). Each medium was amended with 100 μM MnCl2 (pH 7.6). Mn(II)-oxidizing bacteria were identified by testing brown colonies with the Mn(III, IV) oxide colorimetric dye leucoberbelin blue (LBB) (30).
Physiological experiments.
The growth of Mn(II)-oxidizing isolate AzwK-3b as a function of temperature (4, 25, 32, 37, and 42°C), salinity (0 to 15% Na), pH (pH 6 to 9), and Mn(II) concentration (0 to 2 mM) was determined by incubating AzwK-3b in K medium prepared with artificial seawater (K-ASW) for 1 week in the dark and measuring the optical density at 600 nm. For pigment analysis, bacterial cell pellets (0.2 g, wet weight) of dark-grown cultures of AzwK-3b were extracted with 2 ml of acetone-methanol (7:2, vol/vol), and absorption spectra were collected at 200 to 1,100 nm.
Oxidation experiments.
Duplicate cultures of isolate AzwK-3b were grown at 25°C in 1-liter flasks containing 250 ml of K-ASW on an orbital shaker (150 rpm) in the presence and absence of MnCl2 (100 μM), fluorescent light, and Fe(III) citrate (5 to 50 μM). The impact of pH on Mn(II) oxidation was investigated at pH values ranging from 6.5 to 8.5 using 10 mM HEPES buffer. Cultures were sampled every 12 h to determine growth (based on the optical density at 600 nm with 200 μM ascorbate added to dissolve Mn oxides) and the Mn(III, IV) oxide concentration (with LBB). Cell culture filtrates were obtained by growing AzwK-3b to the mid-exponential phase and filtering the cell culture (100 ml) twice through a 0.22-μm filter. Washed cells were obtained by growing cells to the mid-exponential phase in K-ASW (100 ml), harvesting the cells by centrifugation at 7,000 × g for 15 min at 5°C, and resuspending the cells in 20 ml (5× concentrated) of 10 mM HEPES buffer (pH 7.5). The concentration of Mn(III, IV) oxides was determined by colorimetric quantification using LBB (30) relative to a standard curve for KMnO4 and was confirmed by measuring the concentration of Mn removed by filtration (0.22-μm filter) using inductively coupled plasma optical emission spectroscopy. Manganese oxidation was confirmed by X-ray absorption near-edge structure (XANES) spectroscopy of filtered (pore size, 0.22 μm) cultures, conducted at beamline 11-2 at the Stanford Synchrotron Radiation Laboratory. Background-subtracted, normalized spectra (SIXPACK/IFEFIT) (37, 59) were fitted using a Gaussian function (PeakFit, Systat, Inc.) of three fixed energy peaks corresponding to Mn(II) (6,553 eV), Mn(III) (∼6,558 eV), and Mn(IV) (6,562 eV).
DNA extraction, PCR amplification, and restriction fragment length polymorphism analysis.
DNA was extracted from axenic Mn(II)-oxidizing isolates using a DNeasy DNA extraction kit (QIAGEN). Bacterial 16S rRNA gene fragments were amplified from DNA extracts using primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) in a standard 30-cycle PCR performed with Hot Star Taq polymerase (QIAGEN). PCR products were cloned into the vector pCR2.1 by using a TOPO-TA cloning kit (Invitrogen). Restriction fragment length polymorphism analysis was performed by digestion of 27F/1492R PCR products with HhaI for 2.5 h, followed by electrophoresis in 2% agarose gels to identify redundant phylotypes.
Sequencing and phylogenetic analysis.
Sequencing of both strands of 27F/1492R PCR products was performed with the T7 and M13 vector primers and internal 16S rRNA primers 338F (5′-ACTCCTACGGGAGGCAGC-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′), using 3100 and 3700 capillary sequencers (PE Applied Biosystems). Nucleotide sequences were assembled and edited using Sequencher v.4.2 (GeneCodes Corp.). The initial alignment of amplified bacterial 16S rRNA sequences and closely related sequences identified by BLAST (1, 6) was performed by using the automated 16S rRNA sequence aligner of the ARB software package (32) and the SSU Prokaryote database from the Ribosomal Database Project. Ambiguously and incorrectly aligned positions were aligned manually on the basis of the conserved primary sequence and the secondary structure. Bacterial phylogenetic associations were determined from 1,182 masked positions using a neighbor-joining algorithm. Phylogenetic trees were created based on distance analysis using the neighbor-joining algorithm of the software program PAUP (version 4.0b10) with a Jukes-Cantor correction (50). The robustness of inferred topologies was tested by bootstrap resampling using the same distance model (1,000 replicates).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of the Mn(II)-oxidizing isolates reported in this study have been deposited in the GenBank database under accession numbers DQ223017 to DQ223021.
RESULTS AND DISCUSSION
Mn(II) oxidation in the Roseobacter clade.
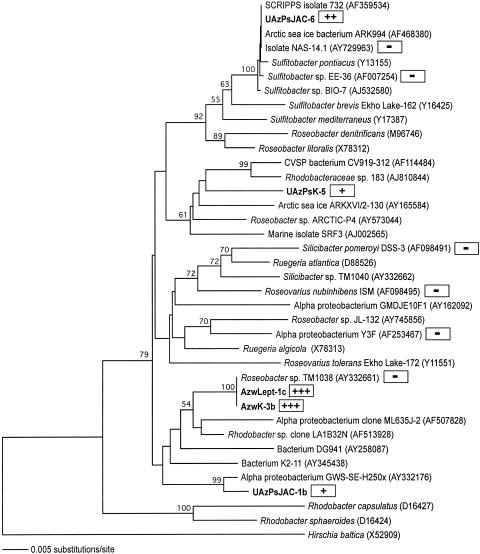
The Mn-rich surface sediments and overlying water column in Lower Azevedo Pond and Upper Azevedo Pond harbor a number of unique Mn(II) oxidizers, including organisms spanning several distinct lineages within the Roseobacter clade of the α-Proteobacteria (Fig. 1). Three isolates were obtained from the upper 2 cm of submerged sediment in Upper Azevedo Pond and were identified by formation of light brown Mn oxide-encrusted, LBB-positive colonies on K medium (UazPsK-5) and J and JAC media (UAzPsJAC-1b and UAzPsJAC-6). The 16S rRNA database sequences most closely related to the sequence of UAzPsJAC-1b are those of isolate GWS-SE-H250x (98% similarity for 1,267 bases), which was obtained from the oxic sediment layer of the German Wadden Sea, and isolate K2-11 (95% similarity for 1,326 bases), which was isolated from the water column of Lake Kauhako in Hawaii. The 16S rRNA gene sequence of isolate UAzPsJAC-6 falls in a cluster of Sulfitobacter-like bacteria and is most closely related (99% similar for 1,324 bases) to isolate NAS-14.1, which was cultivated using DMSP-amended media from the North Atlantic Ocean. Isolate UazPsK-5 defines a distinct lineage, with the closest phylogenetic relative (96% similar for 1,320 bases) being Rhodobacteraceae strain 183, a marine methyl halide-oxidizing bacterium (41). In the shallow (depth, <0.5 m) water column of Lower Azevedo Pond, a Mn(II)-oxidizing bacterium (LBB positive) exhibiting 100% 16S rRNA gene identity to a dinoflagellate (Pfiesteria piscicida)-associated DMSP-degrading Roseobacter-like isolate (33) was obtained on both K (AzwK-3b) and Lept (AzwLept-1c) media (Fig. 1). The Mn(II) oxidation capacities of these isolates varied, and AzwK-3b (which was similar if not identical to AzwLept-1c) was the most proficient Mn(II)-oxidizing isolate, which prompted further characterization (Fig. 1).
FIG. 1.
Neighbor-joining phylogenetic tree of the Roseobacter clade in the α-Proteobacteria. Mn(II)-oxidizing isolates obtained in this study are indicated by boldface type. The relative levels of Mn(II) oxidation by the Elkhorn Slough isolates and reference Roseobacter-like species are indicated in the shaded boxes [−, weak to no Mn(II) oxidation; +++, strong Mn(II) oxidation on K medium plates containing 100 μM Mn(II), pH 7.5]. The tree is based on 1,182 nucleotide positions, and Hirschia baltica is the outgroup. Bootstrap values greater than 50% are indicated at nodes.
Recently, Roseobacter-like bacteria capable of oxidizing Mn(II) were isolated from submarine basalt surfaces at and near Loihi Seamount (55). The majority of those bacteria were most similar to strains of Sulfitobacter, particularly Sulfitobacter dubius, a thiosulfate-oxidizing heterotroph. Mn(II)-oxidizing organisms in this group may be ecologically, as well as phylogenetically, diverse, influencing Mn(II) oxidation from coastal surface waters to the deep sea. However, other well-studied, cultured members of the Roseobacter clade have limited or no Mn(II)-oxidizing ability (Fig. 1). While the Mn(II)-oxidizing UAzPsJAC-6 isolate exhibits 99% 16S rRNA sequence similarity to NAS-14.1 and Sulfitobacter sp. strain EE-36, neither NAS-14.1 nor EE-36 appears to oxidize Mn(II) when grown on various media. Moreover, Roseobacter-like isolate TM1038 does not appear to oxidize Mn(II), despite its 100% 16S rRNA gene sequence identity to our Mn(II)-oxidizing isolate AzwK-3b (and AzPwLept-1c), exemplifying the incongruity between 16S rRNA-based phylogeny and biogeochemical function. The biochemical and genetic variation between AzwK-3b and TM1038 may provide insight into the mechanisms of Mn(II) oxidation and is currently being investigated.
Rates and pathways of Mn(II) oxidation.
To obtain a better understanding of the mechanisms and conditions conducive to Mn(II) oxidation by Roseobacter-like bacteria, we further characterized Lower Azevedo Pond isolate AzwK-3b and the factors controlling the rates and extent of Mn(II) oxidation. AzwK-3b is a gram-negative rod with an average size of 0.5 by 1.5 μm. AzwK-3b grew at wide ranges of temperature (15 to 47°C), pH (pH 6.5 to 9), salinity (1.5 to 15% NaCl), and Mn(II) concentration (0 to 2 mM). Optimal growth occurred at 30°C, pH 7.5, and in the presence of 2.5% NaCl. No growth was observed in the absence of NaCl. Peak Mn(II) oxidation occurred with 200 μM Mn(II) and at pH 7.5. On solid media, AzwK-3b produced distinct dark brown precipitates in the presence of Fe(III) but a diffuse light brown halo under low-Fe conditions. However, while addition of Fe(III) citrate (5 and 50 μM) increased the growth yield of AzwK-3b [ca. 25% increase with 50 μM Fe(III)], the presence of Fe(III) did not influence the extent or rate of Mn(II) oxidation (data not shown).
Although AzwK-3b did not produce pigmented colonies on solid media, when it was grown to high cell densities in liquid media, the cell pellet had pale pink pigmentation. Some pigmented members of the Roseobacter clade are aerobic anoxygenic phototrophs that contain bacteriochlorophyll a and carotenoids (34). Peaks corresponding to carotenoids and bacteriochlorophyll a, however, were not detected in absorption spectra of methanol-acetone extracts from cells of AzwK-3b (data not shown).
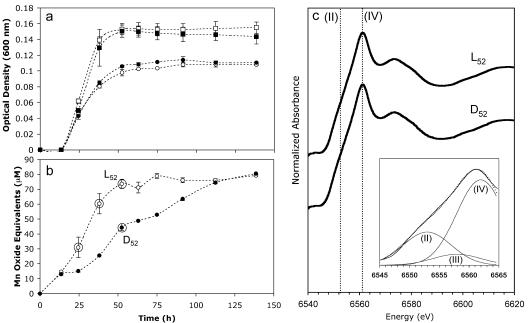
The presence of 100 μM Mn(II) decreased the overall growth rate and yield of AzwK-3b by ∼25% in liquid K medium at pH 7.5 (Fig. 2a). This result is in contrast to previous findings which showed that there was an increase in the growth yield of the Mn(II)-oxidizing α-proteobacterium strain SD-21 in the presence of Mn(II) (20). However, while growth was inhibited, AzwK-3b rapidly oxidized Mn(II) at a rate [ca. 6.5 μmol Mn(II)/day] that was consistent with the oxidation rates of other Mn(II)-oxidizing strains (Fig. 2b). After ∼140 h of incubation, 80% of the Mn(II) was oxidized, while no oxidation occurred in sterile liquid media incubated under equivalent conditions. AzwK-3b began oxidizing Mn(II) during early exponential phase (Fig. 2), whereas most Mn(II)-oxidizing bacteria, with the exception of Leptothrix discophora (8) and the budding α-proteobacterium Pedomicrobium sp. strain ACM 3067 (31), oxidize Mn(II) during stationary phase (8, 20, 40, 54). Within 1 day, Mn(II) oxidation was visually apparent due to a golden discoloration of the liquid K medium, and this was followed by the formation of discrete brown oxide particles (<2 mm) after 2 to 3 days of incubation. Regardless of the reaction time and the presence of light, the Mn particulates were composed of approximately 66% Mn(IV), 9% Mn(III), and 25% Mn(II) (r2 = 0.997) (Fig. 2c), present as the layered Mn oxide birnessite, which is consistent with the Mn oxides formed by Bacillus sp. strain SG-1 (3, 60).
FIG. 2.
Growth of (a) and Mn(II) oxidation by (b) AzwK-3b over time. Duplicate cultures were incubated in the light (open symbols) or in the dark (solid symbols) in the presence (circles) or in the absence (squares) of 100 μM MnCl2. Cell density (a) was determined by adding 200 μM ascorbic acid to remove Mn oxides prior to measurement of the optical density at 600 nm. The first standard deviations for duplicate cultures are indicated by error bars. The circled data points in panel b indicate times when XANES analysis was performed. (c) Manganese K-edge XANES spectra of filtered particulates (pore size, >0.2 μm) produced in the light (L52) and in the dark (D52) following 52 h of incubation. The dotted lines indicate maximum edge positions for Mn(II) and Mn(IV). (Inset) Three-component Gaussian fit (dotted line) to the absorption edge of D52 (solid line) using binding energy positions indicative of Mn(II) (6,553 eV), Mn(III) (6,558 eV, approximate), and Mn(IV) (6,562 eV).
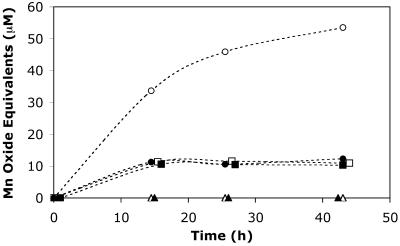
Although light did not appear to influence the speciation of the particulate Mn oxides produced, the rates of Mn(II) oxidation differed in response to light. The initial (ca. 12-h) Mn(II) oxidation rates in the light and dark were equivalent, suggesting that similar oxidative mechanisms were operating (Fig. 2b). After 12 h, however, the rate of oxidation in the light was substantially higher than the rate of oxidation in the dark. The manganese oxide concentration plateaued (∼80 μM) following ca. 75 h in the light but took nearly twice as long to reach an equivalent level in the dark. This finding is again in contrast to previous results, which showed that strain SD-21 exhibited somewhat lower Mn(II) oxidation rates in the light (20). In addition, decreased Mn(II) oxidation rates during the day in coastal waters of the Bahama Islands have been attributed to photoinhibition of Mn(II)-oxidizing bacteria (46). Considering that the cell growth of AzwK-3b was the same in the presence and absence of light (Fig. 2a), the higher rates of Mn(II) oxidation in the light appeared to be a function of photochemical oxidation. Correspondingly, cell-free filtrates (pore size, <0.22 μm) of cultures grown in the absence of Mn(II) oxidized Mn(II) to a much greater extent when they were incubated in the light than in the dark (Fig. 3). In fact, oxidation was nearly twice as fast with filtrates as with live cultures. Furthermore, filtrates that were obtained from dark-grown cultures exhibited oxidation potentials that were equivalent to those of light-grown cultures, indicating that enhanced oxidation was not a direct metabolic response to light.
FIG. 3.
Mn(II) (100 μM) oxidation over time by cell-free filtrates (circles), heat-treated (95°C, 10 min) cell-free filtrates (triangles), and washed cells (squares) of AzwK-3b in the presence (open symbols) and absence (solid symbols) of light.
Manganese(II) oxidation in the dark by both live cultures and cell-free filtrates suggested that photooxidation was not the only operative mechanism. Minor oxidation occurred in the cell-free filtrates incubated in the dark, although the levels were ca. sixfold less than those in the light (Fig. 3). During filtration, cell lysis could release enzymes (e.g., MCOs) into the filtrate, causing Mn(II) oxidation in the dark. Manganese(II) oxidation was not observed with filtrates that were initially heated to 95°C for 10 min, a treatment sufficient to denature most active enzymes (Fig. 3). Furthermore, washed cell suspensions in 10 mM HEPES buffer oxidized minor amounts of Mn(II), regardless of the presence of light (i.e., photooxidation was not operative) (Fig. 3). Considering that the washed cells consisted of “clean” cells with photoactive components from the spent media removed, oxidation by the washed cells is likely a consequence of cell surface oxidases. Interestingly, the amount of Mn(II) oxidized by washed cells (both in the dark and in the light) was equivalent to that oxidized by cell-free filtrates incubated in the dark (Fig. 3). It appeared that oxidation in the filtrates incubated in the dark occurred via an enzymatic pathway, most likely via oxidases that were released by cell lysis during filtration. Considering the widespread involvement of MCOs in Mn(II) oxidation in physiologically and phylogenetically diverse bacteria (54), the operative Mn(II) oxidase in this system may also be an MCO. In fact, one or more putative MCO genes have been identified within genomes of numerous members of the Roseobacter clade, including Roseobacter, Silicibacter, Sulfitobacter, and Roseovarius species. Addition of the MCO inhibitor azide (43) completely inhibited oxidation of Mn(II) in both the light and the dark; however, we also observed abiotic Mn oxide reduction by azide, which complicated interpretation of the results (data not shown). Furthermore, addition of Cu (an MCO cofactor) resulted in a slight increase in Mn(II) oxidation when preparations were incubated in the dark, but no enhancement of oxidation was observed in the light (data not shown). The role of MCOs in Mn(II) oxidation by AzwK-3b is, therefore, unclear and is currently being investigated further.
For direct Mn(II) oxidation by MCOs to be the only operative pathway in the light, we would need to invoke light enhancement of MCO function, which has not been reported previously. Thus, we propose that AzwK-3b uses two Mn(II) oxidation mechanisms. The initial Mn(II) oxidation both in the presence and in the absence of light appears to be directly enzymatically mediated, as suggested by the equivalent initial (12-h) oxidation rates regardless of light (Fig. 2b), partial oxidation by the filtrates incubated in the dark (Fig. 3), and equivalent Mn(II) oxidation in the light and dark by washed cells (Fig. 3). Following the initial oxidation, however, there is a secondary photochemical mechanism in the light, which is coupled with continued enzymatic and autocatalytic activity that also occurs in the dark.
Photochemical reactions in Mn cycling.
Light-induced reduction and subsequent dissolution of Mn oxides is considered an important reaction pathway in the cycling of Mn in surface environments (46, 47). In fact, it has been proposed that one potential reason for the evolutionary selection of bacterial Mn(II) oxidation is to produce low-molecular-weight utilizable organic compounds from high-molecular-weight (e.g., humic) substances via photoreduction of bacterially produced MnO2 (49). A range of mechanisms have been proposed for photoreduction of MnO2, including reaction with O2•− and H2O2 produced by humic acid illumination (48), electron transfer by unknown chromophores and reductants present in microbially produced Mn oxides (48), and direct electron transfer from excited states of sorbed organic matter to the oxide surface (7, 45, 58). In contrast to photoreduction, Mn(II) photooxidation has been largely unexplored. Nico et al. (38) found that reactive oxygen species (primarily superoxide, O2•−) formed through illumination of humic substances may induce photochemical oxidation of Mn(II) at rates comparable to those reported for biological oxidation. Photooxidation has also been observed in an anaerobic sodium chloride solution irradiated with UV light (λ, <240 nm), presumably via UV-stimulated electron transfer from aqueous Mn(II) to H+ (2). We suspect that an intermediate photoactive metabolite was involved in Mn(II) oxidation in our cultures due to the lack of photooxidation during the initial growth by live cultures and by washed cells (Fig. 3) in the light, even in the presence of the initial enzymatically produced Mn oxides. For instance, photoactive constituents (e.g., organic metabolites) in the aqueous milieu may be illuminated to form reactive oxygen species (e.g., H2O2, O2•−, 1O2, and OH·) that ultimately oxidize Mn(II). In fact, Mn(II) incorporated either intracellularly or as a cofactor for the Mn-dependent enzyme superoxide dismutase serves as a protectant for bacteria, such as Lactobacillus and Deinococcus sp., by reacting with reactive oxygen species produced by ionizing radiation or bacterial metabolism (13, 27). Photoactive bacterial metabolites (e.g., organic compounds) may therefore represent a novel oxidant of Mn(II). Interestingly, we screened a number of phylogenetically diverse Mn(II)-oxidizing isolates also obtained from Elkhorn Slough and observed similar light-enhanced Mn(II) oxidation rates to various degrees (data not shown). Thus, bacterially induced photooxidation may provide an alternative and widespread means of Mn(II) oxidation in surface environments and potentially contribute to the cycling of Mn in the photic zone.
Acknowledgments
We thank Mary Ann Moran (University of Georgia), Alison Buchan (University of Tennessee), and Robert Belas (University of Maryland Biotechnology Institute) for providing Roseobacter strains. We also thank Sam Webb at the Stanford Synchrotron Radiation Laboratory (SSRL) for helpful discussions and for providing beamtime. XANES spectra were collected on beamline 11-2 at SSRL, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy Office of Basic Sciences.
The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health National Center for Research Resources Biomedical Technology Program.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anbar, A. D., and H. D. Holland. 1992. The photochemistry of manganese and the origin of banded iron formations. Geochim. Cosmochim. Acta 56:2595-2603. [DOI] [PubMed] [Google Scholar]
- 3.Bargar, J. R., B. M. Tebo, and J. E. Villinski. 2000. In situ characterization of Mn(II) oxidation by spores of the marine Bacillus sp. strain SG-1. Geochim. Cosmochim. Acta 64:2775-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck, N. G., and K. W. Bruland. 2000. Diel biogeochemical cycling in a hyperventilating shallow estuarine environment. Estuaries 23:177-187. [Google Scholar]
- 5.Beja, O., M. T. Suzuki, J. F. Heidelberg, W. C. Nelson, C. M. Preston, T. Hamada, J. A. Eisen, C. M. Fraser, and E. F. DeLong. 2002. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630-633. [DOI] [PubMed] [Google Scholar]
- 6.Benson, D. A., I. Karsh-Mizrachi, D. J. Lipman, J. Ostell, B. A. Rapp, and D. L. Wheeler. 2000. GenBank. Nucleic Acids Res. 28:15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertino, D. J., and R. G. Zepp. 1991. Effects of solar-radiation on manganese oxide reactions with selected organic-compounds. Environ. Sci. Technol. 25:1267-1273. [Google Scholar]
- 8.Boogerd, F. C., and J. P. de Vrind. 1987. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 169:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwers, G. J., J. P. de Vrind, P. L. Corstjens, P. Cornelis, C. Baysse, and E. W. de Vrind-de Jong. 1999. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 65:1762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwers, G. J., E. Vijgenboom, P. L. A. M. Corstjens, J. P. M. de Vrind, and E. W. de Vrind-De Jong. 2000. Bacterial Mn2+ oxidizing systems and multicopper oxidases: an overview of mechanisms and functions. Geomicrobiol. J. 17:1-24. [Google Scholar]
- 11.Caffrey, J. M., N. Harrington, and B. B. Ward. 2002. Biogeochemical processes in a small California estuary. 1. Benthic fluxes and pore water constituents reflect high nutrient freshwater inputs. Mar. Ecol. Prog. Ser. 233:39-53. [Google Scholar]
- 12.Corstjens, P. L. A. M., J. P. M. de Vrind, T. Goosen, and E. W. de Vrind-De Jong. 1997. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol. J. 14:91-108. [Google Scholar]
- 13.Daly, M. J., E. K. Gaidamakova, V. Y. Matrosova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M. V. Omelchencko, H. M. Kostandarithes, K. S. Makarova, L. P. Wackett, J. K. Fredrickson, and D. Ghosal. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025-1028. [DOI] [PubMed] [Google Scholar]
- 14.Davies, S. H. R., and J. J. Morgan. 1989. Manganese(II) oxidation kinetics on metal oxide surfaces. J. Colloid Interface Sci. 129:63-77. [Google Scholar]
- 15.de Vrind, J. P., G. J. Brouwers, P. L. Corstjens, J. den Dulk, and E. W. de Vrind-de Jong. 1998. The cytochrome c maturation operon is involved in manganese oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 64:3556-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diem, D., and W. Stumm. 1984. Is dissolved Mn2+ being oxidized by O−2 in the absence of Mn-bacteria or surface catalysts. Geochim. Cosmochim. Acta 48:1571-1573. [Google Scholar]
- 17.Douka, C. 1977. Study of the bacteria from manganese concretions. Soil Biol. Biochem. 9:89-97. [Google Scholar]
- 18.Ehrlich, H. L. 1968. Bacteriology of manganese nodules. II. Manganese oxidation by cell-free extract from a manganese nodule bacterium. Appl. Microbiol. 16:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis, C. A., K. L. Casciotti, and B. M. Tebo. 2002. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp. strain SG-1. Arch. Microbiol. 178:450-456. [DOI] [PubMed] [Google Scholar]
- 20.Francis, C. A., E. M. Co, and B. M. Tebo. 2001. Enzymatic manganese(II) oxidation by a marine α-proteobacterium. Appl. Environ. Microbiol. 67:4024-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis, C. A., and B. M. Tebo. 2001. cumA multicopper oxidase genes from diverse Mn(II)-oxidizing and non-Mn(II)-oxidizing Pseudomonas strains. Appl. Environ. Microbiol. 67:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiorse, W. C. 1984. Biology of iron- and manganese-depositing bacteria. Annu. Rev. Microbiol. 38:515-550. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez, J. M., J. S. Covert, W. B. Whitman, J. R. Henriksen, F. Mayer, B. Scharf, R. Schmitt, A. Buchan, J. A. Fuhrman, R. P. Kiene, and M. A. Moran. 2003. Silicibacter pomeroyi sp. nov. and Roseovarius nubinhibens sp. nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. Int. J. Syst. Evol. Microbiol. 53:1261-1269. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez, J. M., R. P. Kiene, and M. A. Moran. 1999. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the α-subclass of the class Proteobacteria. Appl. Environ. Microbiol. 65:3810-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez, J. M., and M. A. Moran. 1997. Numerical dominance of a group of marine bacteria in the α-subclass of the class Proteobacteria in coastal seawater. Appl. Environ. Microbiol. 63:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory, E., and J. T. Staley. 1982. Widespread distribution of ability to oxidize manganese among freshwater bacteria. Appl. Environ. Microbiol. 44:509-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jukubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 28.Juniper, S. K., and B. M. Tebo. 1995. Microbe-metal interactions and mineral deposition at hydrothermal vents, p. 219-254. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Boca Raton, Fla.
- 29.Junta, J. L., and M. F. Hochella, Jr. 1994. Manganese(II) oxidation at mineral surfaces: a microscopic and spectroscopic study. Geochim. Cosmochim. Acta 58:4985-4999. [Google Scholar]
- 30.Krumbein, W. E., and H. J. Altman. 1973. A new method for detection and enumeration of manganese-oxidizing and -reducing microorganisms. Helgol. Wiss. Meeresunters. 25:347-356. [Google Scholar]
- 31.Larsen, E. I., L. I. Sly, and A. G. McEwan. 1999. Manganese(II) adsorption and oxidation by whole cells and a membrane fraction of Pedomicrobium sp. ACM 3067. Arch. Microbiol. 171:257-264. [Google Scholar]
- 32.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, T. R., and R. Belas. 2004. Dimethylsulfoniopropionate metabolism by Pfiesteria-associated Roseobacter spp. Appl. Environ. Microbiol. 70:3383-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran, M. A., J. M. Gonzalez, and R. P. Kiene. 2003. Linking a bacterial taxon to sulfur cycling in the sea: studies of the marine Roseobacter group. Geomicrobiol. J. 20:375-388. [Google Scholar]
- 35.Nealson, K. H. 1978. The isolation and characterization of marine bacteria which catalyze manganese oxidation, p. 847-858. In W. E. Krumbein (ed.), Environmental biogeochemistry and geomicrobiology: methods, metals, and assessment. Ann Arbor Science, Ann Arbor, Mich.
- 36.Nealson, K. H., B. M. Tebo, and R. A. Rosson. 1988. Occurrence and mechanisms of microbial oxidation of manganese. Adv. Appl. Microbiol. 33:279-318. [Google Scholar]
- 37.Newville, M. 2001. EXAFS analysis using FEFF and FEFFIT. J. Synchotron Radiat. 8:96-100. [DOI] [PubMed] [Google Scholar]
- 38.Nico, P. S., C. Anastasio, and R. J. Zasoski. 2002. Rapid photo-oxidation of Mn(II) mediated by humic substances. Geochim. Cosmochim. Acta 66:4047-4056. [Google Scholar]
- 39.Northup, D. E., S. M. Barns, L. E. Yu, M. N. Spilde, R. T. Schelble, K. E. Dano, L. J. Crossey, C. A. Connolly, P. J. Boston, D. O. Natvig, and C. N. Dahm. 2003. Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves. Environ. Microbiol. 5:1071-1086. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki, M., T. Sugita, M. Shimizu, Y. Ohode, K. Iwamoto, E. W. de Vrind-de Jong, J. P. de Vrind, and P. L. Corstjens. 1997. Partial purification and characterization of manganese-oxidizing factors of Pseudomonas fluorescens GB-1. Appl. Environ. Microbiol. 63:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schafer, H., I. R. McDonald, P. D. Nightingale, and J. C. Murrell. 2005. Evidence for the presence of a CmuA methyltransferase pathway in novel marine methyl halide-oxidizing bacteria. Environ. Microbiol. 7:839-852. [DOI] [PubMed] [Google Scholar]
- 42.Schutt, C. 1978. Distribution and identification of manganese-precipitating bacteria from noncontaminated ferromanganese nodules, p. 868-878. In W. E. Krumbein (ed.), Environmental biogeochemistry and geomicrobiology: methods, metals, and assessment. Ann Arbor Science, Ann Arbor, Mich.
- 43.Solomon, E. I., U. M. Sundaram, and T. E. Machonkin. 1996. Multicopper oxidases and oxygenases. Chem. Rev. 96:2563-2605. [DOI] [PubMed] [Google Scholar]
- 44.Stein, L. Y., M. T. La Duc, T. J. Grundl, and K. H. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]
- 45.Stone, A. T., and J. J. Morgan. 1984. Reduction and dissolution of manganese(III) and manganese(IV) oxides by organics. 2. Survey of the reactivity of organics. Environ. Sci. Technol. 18:617-624. [DOI] [PubMed] [Google Scholar]
- 46.Sunda, W. G., and S. A. Huntsman. 1990. Diel cycles in microbial manganese oxidation and manganese redox speciation in coastal waters of the Bahama Islands. Limnol. Oceanogr. 35:325-338. [Google Scholar]
- 47.Sunda, W. G., and S. A. Huntsman. 1987. Microbial oxidation of manganese in a North Carolina estuary. Limnol. Oceanogr. 32:552-564. [Google Scholar]
- 48.Sunda, W. G., and S. A. Huntsman. 1994. Photoreduction of manganese oxides in seawater. Mar. Chem. 46:133-152. [Google Scholar]
- 49.Sunda, W. G., and D. J. Kieber. 1994. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature 367:62-64. [Google Scholar]
- 50.Swofford, D. L. 1999. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, Mass.
- 51.Tebo, B. M. 1991. Manganese-II oxidation in the suboxic zone of the Black Sea. Deep-Sea Res. 38(Suppl. 2A):S883-S906. [Google Scholar]
- 52.Tebo, B. M., J. R. Bargar, B. G. Clement, G. J. Dick, K. J. Murray, D. L. Parker, R. Verity, and S. M. Webb. 2004. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 32:287-328. [Google Scholar]
- 53.Tebo, B. M., W. C. Ghiorse, L. G. van Waasbergen, P. L. Siering, and R. Caspi. 1997. Bacterially mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies, p. 225-266. In J. F. Banfield and K. H. Nealson (ed.), Geomicrobiology: interactions between microbes and minerals, vol. 35. The Mineralogical Society of America, Washington, D.C. [Google Scholar]
- 54.Tebo, B. M., H. A. Johnson, J. K. McCarthy, and A. S. Templeton. 2005. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 13:421-428. [DOI] [PubMed] [Google Scholar]
- 55.Templeton, A. S., H. Staudigel, and B. M. Tebo. 2005. Diverse Mn(II)-oxidizing bacteria isolated from submarine basalts at Loihi Seamount. Geomicrobiol. J. 22:127-139. [Google Scholar]
- 56.van Waasbergen, L. G., M. Hildebrand, and B. M. Tebo. 1996. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J. Bacteriol. 178:3517-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Y. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y. H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
- 58.Waite, T. D., I. C. Wrigley, and R. Szymczak. 1988. Photoassisted dissolution of colloidal manganese oxide in the presence of fulvic acid. Environ. Sci. Technol. 22:778-785. [DOI] [PubMed] [Google Scholar]
- 59.Webb, S. M. 2005. SIXPack, a graphical user interface for XAS analysis using IFEFFIT. Phys. Scripta T115:1011-1012. [Google Scholar]
- 60.Webb, S. M., B. M. Tebo, and J. R. Bargar. 2005. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1. Am. Mineral. 90:1342-1357. [Google Scholar]