Abstract
In the arsenic resistance gene cluster from the large linear plasmid pHZ227, two novel genes, arsO (for a putative flavin-binding monooxygenase) and arsT (for a putative thioredoxin reductase), were coactivated and cotranscribed with arsR1-arsB and arsC, respectively. Deletion of the ars gene cluster on pHZ227 in Streptomyces sp. strain FR-008 resulted in sensitivity to arsenic, and heterologous expression of the ars gene cluster in the arsenic-sensitive Streptomyces strains conferred resistance on the new hosts. The pHZ227 ArsB protein showed homology to the yeast arsenite transporter Acr3p. The pHZ227 ArsC appears to be a bacterial thioredoxin-dependent ArsC-type arsenate reductase with four conserved cysteine thioredoxin-requiring motifs.
Arsenic is a toxic metalloid and a cause of cancer that exists in natural and polluted industrial environments. Arsenic resistance genes have been identified in circular plasmids and chromosomes in bacteria (10). Arsenic resistance gene clusters in the eukaryotic model organisms Saccharomyces cerevisiae and Saccharomyces douglasii consist of three genes, ACR1, ACR2, and ACR3 (2, 5, 21), whose functions are similar to those of arsR, arsC, and arsB, respectively, of bacteria (10). However, none of the three gene products shows significant sequence similarity to the bacterial ars gene products.
New arsenic resistance gene clusters other than the initial arsRDABC type continue to emerge, and varied mechanisms seem to occur in diverse biological systems. The ars operon in the skin (for sigK-intervening) element of Bacillus subtilis contains four genes in the order arsR, ORF2, arsB, and arsC. Although ORF2 homologs have not been found (17), it is known to be essential for arsenic resistance. The large extrachromosomal replicon pNRC100 in Halobacterium sp. strain NRC-1 harbors an arsADRC-R2M-type arsenic resistance gene cluster involving a putative arsenite(III)-methyltransferase (20). The circular plasmid pWCFS103 in Lactobacillus plantarum carries a uniquely organized arsRDDB-type arsenic resistance gene cluster (13) that lacks the arsenate reductase gene arsC and contains two copies of the arsD regulatory gene.
Linear plasmids have been reported to encode diverse biological functions, yet arsenic resistance systems in linear plasmids have not been studied in detail. Here, we report the analysis of an arsenic resistance (ars) gene cluster from a large linear plasmid, pHZ227, in Streptomyces sp. strain FR-008. This determinant is compared with the putative ars gene clusters from Streptomyces coelicolor A3(2) (1) and Streptomyces sp. strain F2 (GenBank accession number AY943951, submitted by another group).
General methods and techniques.
The general methods used for handling Escherichia coli and Streptomyces species have been described previously (11, 16). Sequencing was performed at the Chinese National Human Genome Center, Shanghai, China. The sequence data were analyzed with FramePlot 3.0 beta. DNA and deduced protein sequence homology searches were performed by using the BLAST and FASTA programs (http://www.ncbi.nlm.nih.gov).
Cosmids covering pHZ227 and pHZ228 from the Streptomyces sp. strain FR-008 genomic library.
The linear plasmid pHZ227 band was cut and isolated from a pulsed-field gel electrophoresis gel and labeled with α-32P as a probe to hybridize against a Streptomyces sp. strain FR-008 genomic library in Escherichia coli comprising 1,920 colonies. Hybridizing cosmids (46) were obtained, and eight overlapping cosmids (pHZ1239 to pHZ1246) were aligned and mapped to form a contig. Three positive clones were also obtained for the smaller linear plasmid pHZ228 of Streptomyces sp. strain FR-008, which has not been mapped in detail.
Generation of a deletion in the ars gene cluster by targeted gene replacement.
The ars gene cluster was removed from a 7,568-bp PstI fragment (GenBank accession number DQ231520) by digestion with NruI and PmaCI and replaced with the apramycin resistance gene [acc(3)IV] as a blunt-ended SmaI-EcoRV fragment from pHGF9827 (L. Bai, personal communication). This construct was cloned into pHZ1358 (19) to yield pJTU1908 for gene replacement. pJTU1908 was transferred by conjugation from E. coli ET12567(pUZ8002) into Streptomyces sp. strain FR-008. Thiostrepton-sensitive and apramycin-resistant colonies were selected from the initial thiostrepton-resistant exconjugants after two rounds of apramycin-selective growth. The selected thiostrepton-sensitive and apramycin-resistant strain (WL1) was shown to have the expected gene replacement by PCR amplification using oligonucleotide primers WL1-1 (5′-GGTCCTTCGCCTCCAACA-3′) and WL1-2 (5′-ATCACCGCCAGGTCCAAG-3′).
RT-PCR analysis.
Fresh spores of Streptomyces sp. strain FR-008 were separately inoculated into 10 ml TSBY growth medium (3% Difco trytic soy broth powder, 34% sucrose, 1% Difco yeast extract, pH 7.2) in three batches with 250 μM As(III), with 500 μM As(V), and without arsenic as a control, respectively. The cultures were incubated with agitation at 30°C for 30 h. Total RNA was isolated using the RNeasy minikit (QIAGEN) and treated with DNase I for 7 h. Reverse transcription (RT)-PCR was performed in a Thermo Hybaid PCR system (0.2 S) using the OneStep RT-PCR kit and Q-Solution (QIAGEN).
Genomic and phenotypic comparisons of the two Streptomyces strains producing identical candicidin complexes.
Streptomyces sp. strain FR-008 and Streptomyces griseus IMRU3570 (a gift from José Gil, Universidad de León, Spain) are independent isolates, one from China and the other from Spain, known to synthesize the antibiotic complex of the candicidin compounds (4). A comparison of the strains by pulsed-field gel electrophoresis analysis revealed two additional bands (ca. 130 and 30 kb) that were present in Streptomyces sp. strain FR-008 but absent in S. griseus IMRU3570 (Fig. 1), apart from chromosomal bands. Two-dimensional pulsed-field gel electrophoresis revealed no further separation of these bands after UV irradiation. These bands were also found to be sensitive to ExoIII exonuclease, but not to λ exonuclease, consistent with the properties of other reported linear plasmids and chromosomes in Streptomyces (reference 12 and data not shown). The two linear plasmids of Streptomyces sp. strain FR-008 were named pHZ227 and pHZ228 (Fig. 1).
FIG. 1.
Identification of linear plasmids in Streptomyces sp. strain FR-008 by PFGE. Undigested chromosomes and plasmids (pHZ227 and pHZ228) of Streptomyces sp. strain FR-008 (lane 1) and S. griseus IMRU3570 (lane 2) and intact samples (lanes 1 and 2) digested with endonuclease VspI (lanes 3 and 4) were run in parallel with size standards (S). Xme, undigested chromosomes.
When Streptomyces sp. strain FR-008 and S. griseus IMRU3570 were compared for sensitivity to heavy metals, including mercuric chloride, sodium arsenite, and sodium arsenate, neither grew on SFM (2% soy flour, 2% mannitol, 2% agar) plates containing 50 μM mercuric chloride. However, Streptomyces sp. strain FR-008 grew on the plates supplemented with arsenite and arsenate at concentrations of 5 mM and 100 mM (data not shown).
Linear plasmid pHZ227 is involved in arsenic resistance.
Four representative overlapping cosmids (pHZ1239, pHZ1240, pHZ1244, and pHZ1246) covering almost the entire region of pHZ227 and three cosmid derivatives carrying fragments from pHZ228 were introduced into S. griseus IMRU3570 (6) by conjugation. Interestingly, cosmids pHZ1239 and pHZ1240 enabled S. griseus IMRU3570 to grow on SFM plates supplemented with 5 mM arsenite and 100 mM arsenate (data not shown).
Identification and sequence analysis of the ars gene cluster.
The fact that arsenic resistance genes were likely located on the overlapping region between pHZ1239 and pHZ1240 prompted us to sequence cosmid pHZ1239. Six putative genes likely to be involved in Streptomyces arsenic resistance (designated arsR2, arsO, arsB, arsR1, arsC, and arsT) were located on an internal 7,568-bp PstI fragment (Fig. 2). The DNA and deduced protein sequences had been deposited in GenBank under the accession number DQ231520. The overall G+C content of the six ars genes is 69.9%. They are arranged in an unusual manner, with arsR1, arsB, and arsO constituting one operon and arsC and arsT as another divergently transcribed operon. The 3′ end of arsB and the 5′ end of arsO overlapped by 4 bp (GTGA). The same 4-bp overlap (GTGA) was also observed at the 3′ end of arsC and the 5′ end of arsT. An extensive NCBI Conserved Domain search was performed for these putative proteins. ArsB belongs to the yeast arsenite efflux pump ACR3 family. It showed remarkable homology to Acr3p from Saccharomyces cerevisiae (33% amino acid identity) and Saccharomyces douglasii (31% identity), but not to bacterial arsB gene products, such as those encoded by E. coli plasmid R773 and Staphylococcus aureus plasmid pI258.
FIG. 2.
(Top) Schematic presentation of the linear plasmid pHZ227 with putative terminal proteins. (A) The arsenic resistance gene cluster (thick shaded bar) is located in the overlapping region as a PstI fragment between pHZ1239 and pHZ1240. (B) Genetic organization of the ars gene cluster, with the region indicated by open arrows replaced by the 1.4-kb acc(3)IV gene to obtain mutant WL1. (C) A series of deletion clones for the characterization of arsenite [As(III)] and arsenate [As(V)] resistance in S. griseus IMRU3570 and in S. lividans TK24 (in parentheses). +, able to confer arsenic resistance; −, unable to confer arsenic resistance; w, grew weakly. (D) RT-PCR analysis of cotranscriptions of arsR1BO and arsCT, using primers schematically located above or below the putative ars gene cluster in panel B. PCR products were obtained using template RNA extracted from Streptomyces sp. strain FR-008 under uninduced (lanes 2, 5, and 8), arsenite-induced (lanes 3, 6, and 9) or arsenate-induced (lanes 4, 7, and 10) conditions. Primers RB-1 (5′-GATCGAGTCGTCCCCACCCC-3′) and RB-2 (5′-CGGAGCCAAGCAG CCAACC-3′) (lanes 2 to 4), BO-1 (5′-TCGGCCGGGGAGAACAGGTG-3′) and BO-2 (5′-CGGTCCTGATCGGCCTGGTC-3′) (lanes 5 to 7), and CT-1 (5′-CGTCTTCCCCGGCAAGCGCTA-3′) and CT-2 (5′-CGAACAGCACGGGCCTCAGC-3′) (lanes 8 to 10) were used as primer pairs. Results obtained using different primer pairs are separated by vertical lines.
In contrast, ArsC was a homolog of bacterial arsenate reductases, such as ArsC from S. aureus plasmid pI258 (9) (36% amino acid identity) and Staphylococcus xylosus plasmid pSX267 (15) (35% amino acid identity), but shared no homology with the Acr2p yeast arsenate reductases from S. cerevisiae and S. douglasii. ArsO was homologous to flavin-binding monooxygenases, as it was 57% identical to a putative monooxygenase (ZP_00420634) from Burkholderia vietnamiensis G4 and 48% identical to a putative monooxygenase (NP_744078) from Pseudomonas putida KT2440. ArsT showed extensive homology to numerous thioredoxin reductases, such as thioredoxin reductases from Streptomyces coelicolor A3(2) (71% amino acid identity to CAA63076) and Streptomyces clavuligerus (73% amino acid identity to CAA79940). The other two putative proteins (ArsR1 and ArsR2) showed significant homology to ArsR proteins from many arsenic systems, suggesting their roles as repressors for the arsenic resistance genes. Several genes flanking both sides of the six ars genes could not be assigned to a role in arsenic resistance, including the complete orf2 and incomplete orf1 (whose putative products showed homology to monooxygenases involved in K+ transport) and the complete orf3 and orf4 (whose putative products showed no significant homology to known proteins in the protein database) (Fig. 2).
Localization and functional analyses of the ars gene cluster.
The 5,091-bp NdeI-MluI fragment, containing only six putative ars genes, was cloned into a phage ΦC31-derived integrative vector, pSET152 (11), for the construction of pJTU1910. When pJTU1910 was introduced into S. griseus strain IMRU3570, the derivative strain gained resistance to both 5 mM arsenite and 100 mM arsenate, implying that this NdeI-MluI fragment contained the complete arsenic resistance gene cluster.
Evidence that the above-mentioned six ars genes were directly responsible for arsenic resistance in their native host, Streptomyces sp. strain FR-008, came from deletion of the putative ars gene cluster from pHZ227 by targeted gene replacement. The resulting mutant, WL1, displayed sensitivity to 5 mM arsenite and 100 mM arsenate comparable to that of S. griseus IMRU3570. Arsenic resistance was restored when pHZ1239 and pHZ1240 were introduced into WL1, while the control cosmids (pHZ1244 and pHZ1246) had no such effect. Thus, the arsRBOCT gene cluster lying on linear plasmid pHZ227 was demonstrated to be responsible for arsenic resistance in Streptomyces sp. strain FR-008.
To determine the functions of individual genes in the pHZ227 arsenic resistance gene cluster, a series of pSET152 derivative clones carrying different sets of the six putative ars genes were assayed for arsenic resistance in S. griseus IMRU3570 (Fig. 2). Vector pSET152 was used as a negative control throughout. Plasmids pJTU91 (lacking arsT), pJTU94 (lacking arsR2 and arsO), and pJTU92 (lacking arsR2, arsO, and arsT) conferred resistance to both arsenite and arsenate, suggesting that a PvuII fragment carrying an arsB-arsR1-arsC gene cassette (pJTU92) was sufficient for conferring resistance to As(III), as well as As(V), on S. griseus IMRU3570. Additionally, further partial removal of arsC (pJTU98 and pJTU99) abolished resistance to As(V) but not to As(III) (data not shown), while a clone (pJTU93) removing arsB but maintaining arsC and arsR1 lost resistance to As(III) and to As(V) completely in S. griseus IMRU3570 (data not shown). The most striking difference in the heterologous expression of these clones in Streptomyces lividans TK24 was that the absence of arsT (as in pJTU91 and pJTU92 [Fig. 2C]) abolished resistance to As(V).
ArsB appears to be an arsenite efflux pump protein required for resistance to both arsenite and arsenate, while ArsC is only necessary for resistance to arsenate. This is in accord with the known function of ArsC as an arsenate reductase for the reduction of As(V) to As(III), which is then effluxed by ArsB. Thioredoxin is required for arsenate reduction by ArsC of S. aureus plasmid pI258 (8), and an independent functional trxA (thioredoxin) gene in the E. coli chromosome is required for T. ferrooxidans ArsC-mediated arsenate resistance in E. coli (3). An equivalent function is likely to be mediated by arsT, a putative thioredoxin reductase gene in the pHZ227 ars gene cluster. This agrees well with the proposal that the thioredoxin reductase gene participates in the reduction of arsenate to arsenite with the reducing power from NADPH → thioredoxin reductase → thioredoxin → ArsC → arsenate (8).
arsR1BO and arsCT constitute two cotranscribed operons.
Total RNA was isolated from arsenite-induced, arsenate-induced, and uninduced mycelia of Streptomyces sp. strain FR-008. RT-PCR results using different sets of primers are shown in Fig. 2D. As expected, arsR1, arsB, and arsO were cotranscribed, as RT-PCR with two sets of primers (RB-1 and RB-2 and BO-1 and BO-2) resulted in two expected 249-bp fragments. Likewise, the expected 250-bp fragment was obtained using CT-1 and CT-2, specific for the putative arsC-arsT. No transcription was observed for these genes under uninduced conditions. We therefore concluded that arsR1BO and arsCT constitute two independently cotranscribed operons.
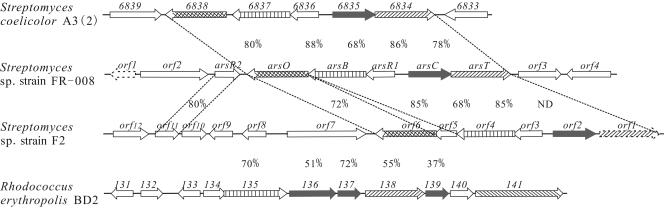
With the rapidly increasing information from genome-sequencing projects, a number of potential ars gene clusters have been annotated in chromosomes. An extensive BLAST search using the pHZ227 ars gene cluster against NCBI databases identified related putative ars gene clusters (Fig. 3). Among these, pHZ227 ars genes showed the highest similarities to putative ars genes from the S. coelicolor A3(2) genome open reading frames (ORFs). One of the two sets of putative arsenic resistance genes in S. coelicolor A3(2) (SCO6834 to SCO6838 of GenBank accession no. AL939129) (Fig. 3) is located in a tRNA region, suggesting acquisition from an exogenous genomic island (1). The other ars gene cluster (SCO3696 to SCO3701 of GenBank accession no. AL939117) contains an arsR2 homolog (ORF SCO3696) and a partial arsT (ORF SCO3701). The ars genes from pHZ227 also showed significant homology with the genes of the putative arsenic resistance gene cluster (G+C content 69.4%) from Streptomyces sp. strain F2, recently deposited in GenBank by a different Shanghai research group (Fig. 3), although the starting orf1 encoding a putative thioredoxin reductase is an incomplete gene sequence. An extra gene (orf5), whose role is unknown, was found between arsB and arsO homologs. Though the arsenic resistance genes in S. coelicolor A3(2) and Streptomyces sp. strain F2 have not been functionally analyzed, wild-type S. coelicolor A3(2) has been shown to be resistant to arsenic (7).
FIG. 3.
Genetic organizations of genes involved in arsenic resistance in Streptomyces sp. strain FR-008 (GenBank accession number DQ231520) with S. coelicolor A3(2) (GenBank accession number AL939129), Streptomyces sp. strain F2 (GenBank accession number AY943951), and Rhodococcus erythropilis BD2 (GenBank accession number AY223810). The gene numbers correspond to the numbers in the genome database. Only one of the two arsenic resistance gene clusters (SCO6834 to SCO6838, harboring full-length arsT but lacking arsR2 homology) from S. coelicolor A3(2) is shown. Homologous genes are aligned with crosshatching (arsO), vertical lines (arsB), hatching (arsT), and gray (arsC) for comparison. The numbers between the dotted lines and above R. erythropilis BD2 are the percentages of amino acid sequence identity between the related ars proteins of Streptomyces sp. strain FR-008.
Additionally, pBD2, a linear plasmid from Rhodococcus erythropolis BD2, which displayed an arsenic resistance phenotype, carries several arsenic resistance gene homologs (18) whose genetic organizations differ dramatically from that of pHZ227 (Fig. 3). First, linear plasmid pBD2 carries an arsA homolog (ORF 141 in Fig. 3) encoding an ATPase typical of arsenic resistance gene clusters in gram-negative bacteria that is absent in pHZ227. In contrast to three independent ORFs (ORFs 136, 137, and 139 in Fig. 3), encoding putative ArsC in pBD2, there is only one arsC gene in pHZ227. In addition, pBD2 contains a putative thioredoxin reductase gene (an arsT homolog) but apparently does not have a homolog for a flavin-binding monooxygenase gene (arsO).
The genetic arrangement of arsO and arsT in pHZ227 implies that they are coupled with arsR1-arsB and arsC, respectively. Nonetheless, plasmids pJTU91 lacking arsT and pJTU94 lacking arsO both conferred resistance to arsenic on S. griseus IMRU3570, although the arsT function was absolutely required for arsenate resistance in S. lividans TK24 (Fig. 2C). Since the putative thioredoxin reductase activity of ArsT is known to be widely distributed in bacteria [e.g., ArsT is highly homologous to at least five putative thioredoxin reductases, ORFs CAB71847, CAB42713, CAA63076, CAA07451, and CAA63075 in GenBank accession number AL645882, in S. coelicolor A3(2)], it is possible that such activity is recruited from endogenous chromosomal ArsT homologs. This may explain why most known arsenic resistance gene clusters harboring a thioredoxin-dependent arsC do not contain an arsT (thioredoxin reductase) gene or an arsO (flavin-binding monooxygenase) homolog.
A flavin-binding monooxygenase has not previously been characterized in known arsenic resistance systems. Although no obvious similarity was found, the role of ArsO in Acr3p-type arsenite efflux systems may be similar to that of ArsH, a protein showing weak homology to oxidoreductases (3, 14), in the arsenic resistance systems of some gram-negative bacteria.
All of the ars gene clusters in Streptomyces have high G+C contents (ca. 70%), a general feature of Streptomyces genomes which nevertheless contrasts with the generally lower (< 50%) G+C contents of arsenic gene clusters found in other organisms. Moreover, the arsT gene (encoding a possible thioredoxin reductase) was found only in a relatively high-G+C-content arsenic resistance gene cluster (65%) from the linear plasmid pBD2 of gram-positive Rhodococcus erythropolis BD2. Also unusual for pHZ227 ars clusters are the hypothetical flavin-binding monooxygenase ArsO, the similarity of the arsenite transporter (ArsB) to that of the yeast Acr3p family, and the similarity of the arsenate reductase (ArsC) to bacterial ArsC. Taken together, these features may constitute an unusual entity specific to Streptomyces and other gram-positive organisms with high G+C contents.
Acknowledgments
We thank Justin Maresh for critical reading of the manuscript.
This work received support from the Ministry of Science and Technology (2003CB114205), the National Science Foundation of China, the Ph.D. Training Fund of the Ministry of Education, and the Shanghai Municipal Council of Science and Technology.
REFERENCES
- 1.Bentley, S. D., K. F. Chater, and A. M. Cerdeno-Tarraga. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 2.Bobrowicz, P., R. Wysocki, G. Owsianik, A. Goffeau, and S. Ulaszewski. 1997. Isolation of three contiguous genes, ACR1, ACR2 and ACR3, involved in resistance to arsenic compounds in the yeast Saccharomyces cerevisiae. Yeast 13:819-828. [DOI] [PubMed] [Google Scholar]
- 3.Butcher, B. G., S. M. Deane, and D. E. Rawlings. 2000. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, S., X. Huang, X. Zhou, L. Bai, J. He, K. J. Jeong, S. Y. Lee, and Z. Deng. 2003. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem. Biol. 10:1065-1076. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh, M., J. Shen, and B. P. Rosen. 1999. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96:5001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil, J. A., P. Liras, G. Naharro, J. R. Villanueva, and J. F. Martin. 1980. Regulation by aromatic amino acids of the biosynthesis of candicidin by Streptomyces griseus. J. Gen. Microbiol. 118:189-195. [DOI] [PubMed] [Google Scholar]
- 7.Hanel, F., H. Krugel, and G. Fiedler. 1989. Arsenical resistance of growth and phosphate control of antibiotic biosynthesis in Streptomyces. J. Gen. Microbiol. 135:583-591. [DOI] [PubMed] [Google Scholar]
- 8.Ji, G., and S. Silver. 1992. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc. Natl. Acad. Sci. USA 89:9474-9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji, G., and S. Silver. 1992. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J. Bacteriol. 174:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur, P., and B. P. Rosen. 1992. Plasmid-encoded resistance to arsenic and antimony. Plasmid 27:29-40. [DOI] [PubMed] [Google Scholar]
- 11.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics: a laboratory manual. John Innes Foundation, Norwich, United Kingdom.
- 12.Kinashi, H., and M. Shimaji-Murayama. 1991. Physical characterization of SCP1, a giant linear plasmid from Streptomyces coelicolor. J. Bacteriol. 173:1523-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Maury, L., F. J. Florencio, and J. C. Reyes. 2003. Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 185:5363-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenstein, R., A. Peschel, B. Wieland, and F. Götz. 1992. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J. Bacteriol. 174:3676-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Sato, T., and Y. Kobayashi. 1998. The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J. Bacteriol. 180:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stecker, C., A. Johann, C. Herzberg, B. Averhoff, and G. Gottschalk. 2003. Complete nucleotide sequence and genetic organization of the 210-kilobase linear plasmid of Rhodococcus erythropolis BD2. J. Bacteriol. 185:5269-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, Y., X. Zhou, J. Liu, K. Bao, G. Zhang, G. Tu, T. Kieser, and Z. Deng. 2002. ‘Streptomyces nanchangensis’, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 148:361-371. [DOI] [PubMed] [Google Scholar]
- 20.Wang, G., S. P. Kennedy, S. Fasiludeen, C. Rensing, and S. DasSarma. 2004. Arsenic resistance in Halobacterium sp. strain NRC-1 examined by using an improved gene knockout system. J. Bacteriol. 186:3187-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wysocki, R., P. Bobrowicz, and S. Ulaszewski. 1997. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 272:30061-30066. [DOI] [PubMed] [Google Scholar]