Abstract
Four sausage batters (17.59% beef, 60.67% pork, and 17.59% pork fat) were inoculated with two commercial starter culture organisms (>7 log10 CFU/g Pediococcus pentosaceus and 6 log10 CFU/g Staphylococcus carnosus) and a five-strain cocktail of nonpathogenic variants of Escherichia coli O157:H7 to yield 6 to 7 log10 CFU/g. Microencapsulated allyl isothiocyanate (AIT) was added to three batters at 500, 750, or 1,000 ppm to determine its antimicrobial effects. For sensory analysis, separate batches with starter cultures and 0, 500, or 750 ppm microencapsulated AIT were produced. Sausages were fermented at ≤26°C and 88% relative humidity (RH) for 72 h. Subsequently sausages were dried at 75% RH and 13°C for at least 25 days. The water activity (aw), pH, and levels of starter cultures, E. coli O157:H7, and total bacteria were monitored during fermentation and drying. All sausages showed changes in the initial pH from 5.57 to 4.89 and in aw from 0.96 to 0.89 by the end of fermentation and drying, respectively. Starter culture numbers were reduced during sausage maturation, but there was no effect of AIT on meat pH reduction. E. coli O157:H7 was reduced by 6.5 log10 CFU/g in sausages containing 750 and 1,000 ppm AIT after 21 and 16 days of processing, respectively. E. coli O157:H7 numbers were reduced by 4.75 log10 CFU/g after 28 days of processing in treatments with 500 ppm AIT, and the organism was not recovered from this treatment beyond 40 days. During sensory evaluation, sausages containing 500 ppm AIT were considered acceptable although slightly spicy by panelists.
In 1982 Escherichia coli O157:H7 was first recognized as a human pathogen which caused hemorrhagic colitis (29). Since then, human infections attributed to E. coli O157:H7 have been reported in over 30 countries, reaching alarming annual incidence rates of 8 per 100,000 inhabitants in some areas of Canada, the United States, and Scotland (23). Foods of animal origin are considered the main source of E. coli O157:H7 illnesses in humans (34). Most outbreaks of E. coli O157:H7 have been linked with the consumption of undercooked meats, especially ground beef or hamburger patties (4, 31). Fermented meat products had long been thought to be relatively safe due to their low pH, their low water activity (aw), and the presence of curing salts (nitrite and nitrate). However, an important wake-up call occurred in 1994, when 23 individuals in the states of Washington and California were infected with E. coli O157:H7 as a consequence of consumption of dry-cured salami (5). After this outbreak, the U.S. Department of Agriculture Food Safety and Inspection Service required meat processors to validate that processing dry and semidry fermented sausages resulted in at least a 5-log10-unit reduction in numbers of E. coli O157:H7 cells (28). The ability of E. coli O157:H7 to adapt to acid environments (11) has caused this microorganism to be regarded as one of the most dangerous pathogens in fermented meat products. Several studies have shown that E. coli O157:H7 is able to survive the processes of fermentation, drying, and storage when this microorganism is present at high numbers (14, 16, 27). The use of different starter cultures during fermentation has been unsuccessful in achieving the required 5-log10-unit reduction of E. coli O157:H7 (12, 22). Heat treatments seem to be the best way to reduce the numbers of this pathogen in semidry sausages; however, physical characteristics of dry sausage change during heating, and the products no longer resemble uncooked fermented sausages (16).
Alternative means for reducing the viability of E. coli O157:H7 in food systems are being studied, and one of these is the addition of the natural antimicrobial allyl isothiocyanate (AIT) to meat. AIT is one of several naturally occurring compounds formed when glucosinolates present in cruciferous vegetables are hydrolyzed by the enzyme myrosinase (8). AIT is a potent inhibitor of a large number of pathogenic microorganisms (9, 19), and it has been successfully used for elimination of E. coli O157:H7 from ground beef, roast beef, and hamburger patties (24, 26, 32). In general the application of AIT in the food industry has been limited due to its volatility; however, this obstacle was overcome by microencapsulation (6).
Microencapsulation of liquid volatile compounds can be successfully accomplished in two steps. First, an emulsion of the volatile compound is made in an aqueous dispersion of a suitable “wall” material, which also functions as the emulsifier. Second, the microencapsulated emulsion must be dried under controlled conditions so as to diminish the loss of the encapsulated material by volatilization (20).
The objectives of this study were to determine whether microencapsulated AIT was able to reduce the levels of E. coli O157:H7 in dry fermented sausages by at least 5 log10 units and to evaluate the acceptability of uncontaminated AIT-treated products to consumers.
MATERIALS AND METHODS
AIT microcapsule preparation and analysis. (i) Gum acacia emulsion.
The procedure of Chacon et al. (6) was used to prepare a gum acacia emulsion. A 30% (wt/wt) solution of a commercial spray-dried gum acacia powder (SD prehydrated; Nealanders International Inc., Mississauga, ON, Canada) in deionized water was homogenized using a high-speed mixer (Omni-mixer; Ivan Sorvall Inc., Norwalk, CT) set at 4,800 rpm for 25 min. The solution was covered and held overnight at 5°C to allow complete hydration of the gum acacia for use as the wall material. The emulsion was prepared by adding a solution of 94% pure AIT (Sigma, St. Louis, MO) as the core material to the gum acacia solution at a core/wall ratio of 1:4. The mixture was emulsified using the Omni-mixer at 8,000 rpm for 2 min.
(ii) Freeze drying.
Five hundred milliliters of the gum acacia-AIT emulsion was spread over individual aluminum trays (19.4 cm by 19.4 cm by 4.4 cm) covered with wax paper to form a layer approximately 1.6 cm thick. Emulsions were frozen by holding them at −40°C for about 8 h. Frozen emulsions were placed in a freeze-dryer (model 10-146 MP; Virtis Corp., Gardiner, NY) and held initially at −70°C and 0.5 mm Hg atmospheric pressure for about 70 h. Dried emulsions were chopped using a food processor (model FP 1000-04; Black and Decker, Brockville, ON, Canada) for 30 s to provide the microencapsulated powder.
(iii) AIT calibration.
Six solutions containing 20, 50, 100, 300, 500, and 1,000 ppm of 94% AIT in hexane (high-pressure liquid chromatography grade; Fisher Scientific, Ottawa, ON, Canada) were used as standards for preparing the AIT calibration curve. A 10-μl aliquot of each solution was taken with a gas-tight 10-μl syringe (Hamilton Co., Reno, NV) and injected into a gas-liquid chromatograph (Varian Star 3400cx; Varian Chromatography Systems, Walnut Creek, CA) operated using the following: a BD5MS column (30 m by 0.25 mm [inside diameter]; 25-μm wall thickness; J&W Scientific Inc., Folsom, CA), helium carrier gas, and a column temperature that increased from 60 to 95°C at a rate of 12.5°C/min and was held at 95°C for 45 s. The inlet and flame ionization detector temperatures were set at 250°C. The area under the curve obtained from the chromatograph software (Varian Star chromatography, Walnut Creek, CA) was plotted against the AIT concentration to prepare the calibration curve.
(iv) Determination of AIT in microcapsules.
Lyophilized powder (0.5 g) from the chopped emulsion was placed in a screw-cap test tube (16 by 100 mm), which was closed and stored at −40°C for analysis of AIT by gas-liquid chromatography. Distilled water (2 ml) was added to each powder sample and mixed at 2,500 rpm (Vortex mixer; Fisher Scientific) for 1 min. Samples were left at 5°C for 6 h with mixing at 2-h intervals in order to ensure the complete dissolution of the wall material, and then 6 ml of hexane (high-pressure liquid chromatography grade; Fisher Scientific) was added to the sample and mixed for 1 min. The precipitated wall material was allowed to stand for 30 min, mixed, and held again for 30 min. A 10-μl aliquot of the hexane phase was taken with a gas-tight 10-μl syringe (Hamilton Co., Reno, NV) and injected into the gas-liquid chromatograph, which was operated using the same conditions as for preparation of the AIT calibration curve. The amount of AIT in the powder was obtained by calculating the area under the AIT response curve by comparison with the calibration curve for AIT.
Bacterial strain preparation.
Dry-cured sausages were fermented using the strains Pediococcus pentosaceus UM 116P and Staphylococcus carnosus UM 110M, which were isolated from lyophilized commercial meat starter cultures (Trumark LTII M and LTII, respectively; Rector Foods Ltd., Mississauga, ON, Canada). P. pentosaceus was maintained in deMan Rogosa Sharpe (MRS) broth (BBL, Becton Dickinson, Sparks, MD) containing 20% glycerol at −70°C. S. carnosus was maintained in Trypticase soy broth (TSB) (BBL, Becton Dickinson) containing 20% glycerol at −70°C. P. pentosaceus was subcultured twice in MRS broth (Trypticase soy agar was used for S. carnosus) incubated at 37°C before use in the experiments. Overnight cultures (16 h, at the late logarithmic phase of growth) were centrifuged at 8,000 × g for 10 min, washed in 0.1% (wt/vol) peptone water, and suspended in 0.1% peptone water to achieve 9 log CFU/ml by standardization at an optical density of 600 nm using a spectrophotometer (Ultrospec 2000; Pharmacia Biotech, Baie d'Urfe, QC, Canada).
Five human isolates of E. coli O157:H7 which had mutated and become nonpathogenic (verotoxigenic negative) during storage (strains 3581, 0304, 0627, and 0628 and a nonmotile strain, 1840) were provided by Rafig Ahmed, National Microbiology Laboratory, Public Health Agency, Canadian Science Centre for Human and Animal Health, Winnipeg, MB, Canada. These organisms were comparable in their resistance toward AIT to pathogenic human clinical and hamburger isolates of E. coli O157:H7 (6, 24, 26). These strains were maintained in TSB (BBL) and subcultured twice at 37°C for 18 h before use in experiments. After the last incubation, 40 ml of each E. coli O157:H7 culture was centrifuged at 8,000 × g for 10 min at 10°C (Sorvall RC-5; Du Pont, Newtown, CT). E. coli O157:H7 cells were washed in 40 ml of 0.1% peptone water and collected by centrifugation. Cell pellets were standardized at an optical density of 0.250 at 600 nm by dilution in peptone water and were monitored with the spectrophotometer. E. coli O157:H7 suspensions were mixed to obtain an equal number of cells of each strain in the inoculation cocktail.
Dry fermented sausage manufacture for E. coli O157:H7 challenge.
Four salami batches of approximately 10 kg each were processed in the Department of Food Science pilot plant. The batch composition is shown in Table 1. Sausage batters were prepared by chopping 1°C pork fat, pork, and beef (purchased from a local wholesaler) in a precooled (1°C) Titane 40 rotating bowl meat cutter (Dadaux, Bersaillin, France). P. pentosaceus and S. carnosus were added to reach a final inoculation level of 7 log10 CFU/g. All batters were also inoculated with the five-strain cocktail of nonvirulent E. coli O157:H7 to yield approximately 7 log10 CFU/g. To these batters, cervelat spice mix (Hermann Laue Spice Co. Inc., Uxbridge, ON, Canada), Rapidur (a proprietary mixture of corn syrup solids and dextrose), pickle cure concentrate containing 6.25% NaNO2 (which gave a final concentration of 193 ppm of nitrite) plus 1% NaHCO3, and 2.9% (wt/wt) salt (Canada Compound Western Ltd., Winnipeg, MB) were added and chopped (≤5 min) to achieve a granular (3-mm) consistency of meat and fat particles. AIT microcapsules (containing 44.14 mg of AIT/g of microcapsules) were added to the first three sausage batters at 1.13%, 1.69%, and 2.26% (wt/wt) to yield 500, 750, and 1,000 ppm of AIT, respectively. These concentrations were used because preliminary testing indicated that <500 ppm AIT would not cause the extent of E. coli O157:H7 lethality desired. No AIT was added to the control salami batter. Fibrous casings (55-mm diameter; Kalle GmbH & Co., Wiesbaden, Germany) were presoaked in lukewarm water (40°C for 30 min) before use. Sausage batters at 4°C were stuffed with a piston stuffer (Mainca model EM30; Equipamientos Carnicos, S. L. Barcelona, Spain) into the casings to achieve a final weight of about 500 g. Sausages were transferred to a single-cage smokehouse (Allroundsystem Rondette with a Titan programmable controller; Maurer AG, Reichenau, Germany) and processed by fermentation at 26°C and 88% relative humidity (RH) for 24 h to reach a pH of <5.3. Thereafter the fermentation temperature and RH were decreased stepwise (six times at 12-h intervals, where temperature and RH were reduced by 2°C and 2.2%, respectively) from 26 to 14°C and 88% to 75% RH over 72 h, with intermittent smoking (from electrically fired hardwood chips) for 30 min at 48, 60, and 72 h. The degree-hour guidelines of Agriculture and Agri-Food Canada (1) were followed when calculating the permitted time and temperature combinations during fermentation. Fermentation was followed by drying at 13°C and 75% RH for 25 days.
TABLE 1.
Formulation used for manufacture of dry fermented sausage
| Ingredient(s) | Composition (%, wt/wt) |
|---|---|
| Beef fronts (85% lean) | 17.59 |
| Pork (90% lean) | 60.67 |
| Pork fat | 17.59 |
| Spice mix | 0.44 |
| Salt | 2.90 |
| Dextrose + corn syrup solids (Rapidur)a | 0.69 |
| Pickle cure concentratea | 0.31 |
From Canada Compound Western Ltd., Winnipeg, MB, Canada.
Microbial analysis of dry fermented sausages.
Each dry fermented sausage treatment was analyzed microbiologically after stuffing (0 days) and after 3, 6, 9, 16, 21, and 28 days of fermentation and ripening. For E. coli O157:H7, additional analyses were made after 35, 40, and 45 days of processing. For microbial analysis, 11 g sausage meat was taken in triplicate from each of two cross-sectioned sausages, placed in a sterile stomacher bag (Filtra-bag; VWR International, Edmonton, AB, Canada), and homogenized in a stomacher (model 400; A. J. Seward, London, United Kingdom) with 99 ml of 0.1% peptone water. The total number of bacteria and numbers of P. pentosaceus, and S. carnosus cells were determined by serial dilution in 0.1% peptone water and plating in duplicate on Trypticase soy agar, MRS agar, and mannitol salt agar (BBL), respectively, using a spiral plater (Autoplate 4000; Spiral Biotech, Norwood, MA). E. coli O157:H7 numbers were obtained by spiral plating of the diluted samples on sorbitol MacConkey agar (BBL) modified with a cefixime tellurite supplement (Oxoid, Hampshire, England) to yield the medium CT-SMAC (35). All plates were incubated at 37°C for 24 to 48 h aerobically, except for P. pentosaceus plates, which were incubated at 45°C anaerobically using the BBL GasPak system. In order to determine if E. coli O157:H7 cells were killed or injured in the dry fermented sausage samples as a result of AIT exposure, an additional 25 g of meat from samples showing no growth after AIT treatment was examined for viable E. coli O157:H7 cells after 24 h of resuscitation at 37°C in 225 ml TSB, using an immunomagnetic separation (IMS) technique (Dynabeads anti-E. coli O157:H7; Dynal, Oslo, Norway) performed according to the manufacturer's instructions. Fifty microliters of the IMS complex was spread onto CT-SMAC plates and incubated at 37°C for 24 h. Presumptive E. coli O157:H7 colonies from CT-SMAC plates were confirmed to be E. coli by using API 20E biochemical test strips (bioMérieux Vitek, Inc., Hazelwood, MO).
Chemical analysis. (i) Water activity and pH.
The pHs of triplicate samples were recorded using a pH meter (Sentron Titan pH meter) equipped with a Lancefet probe inserted directly into cross-sectioned samples of sausages. Triplicate measurements were taken and averaged. Water activity was analyzed in triplicate (Novasina AW Sprint TH 500 aw-measuring unit; Axair AG, Pfäffikon, Switzerland).
(ii) Determination of percent fat in sausages.
The Soxhlet method (3) was used for crude fat determination. Three replicates of each treatment of salami were placed in the freeze-dryer for 72 h. Five grams of freeze-dried salami was placed into thimbles and covered with glass wool. Thimbles were placed inside the extracting tubes. Flat-bottom flasks (250 ml) containing boiling chips were treated at 125°C for 30 min in a drying oven. Subsequently, the flasks were cooled inside a desiccator and then weighed on an analytical balance. One hundred milliliters of hexane was added to each flask. The extracting tubes were assembled with the flasks, and the Soxhlet system was refluxed for approximately 16 h. Subsequently, flasks were evaporated to dryness in a heating mantle and then placed inside the oven for 1 h at 100°C. Following heating, flasks were placed in a desiccator for 1 h and then weighed. The fat content in the salami was calculated using the following equation: percent crude fat = (grams crude fat/grams sample) × 100.
Dry fermented sausage manufacture for sensory evaluation.
Three separate 10-kg batches of salami were prepared using the same formulation and manufacturing conditions as used for the salami pathogen inoculation experiment, but no E. coli O157:H7 was used. To two batches of salami either 1.11% or 1.66% (wt/wt) microcapsules (containing 45.16 mg of AIT/g of microcapsules) were added to yield 500 or 750 ppm AIT in the sausages. A third batch, identified as control salami, did not have any microencapsulated AIT added. Sausages containing 1,000 ppm AIT were not included in the sensory study because preliminary tests indicated that these samples had a bitter, strong flavor which could generate bias against the other AIT-treated samples among panelists.
Sensory evaluation.
A consumer taste panel was used to compare the sensory quality of sausages containing different levels of microencapsulated AIT with that of control salami. A group of 51 untrained volunteers who consumed fermented sausages at least four times a year comprised the sensory panel. The sensory analysis facility in the Department of Food Science was used, and panelists made their evaluations in private sensory booths. Panelists were given three slices of numerically identified, blindly coded sausage samples from the different treatments (control, AIT at 500 ppm, and AIT at 750 ppm) and asked to evaluate them for overall acceptability, appearance, flavor, and texture on a nine-point Hedonic scale, with 9 being extremely good and 1 being extremely poor (33). On the questionnaire, panelists were asked whether they regularly consumed spicy food and whether they would buy any of the tested sausage samples.
Statistical analysis.
Data were analyzed by the general linear models procedure, using the Statistical Analysis System (SAS) software program, version 8.1 (SAS Institute Inc., Cary, NC). Mean differences were compared using a Tukey test at the 95% significance level (P < 0.05).
RESULTS AND DISCUSSION
Effect of microencapsulated AIT on starter culture performance and on pH and aw changes of dry fermented sausages.
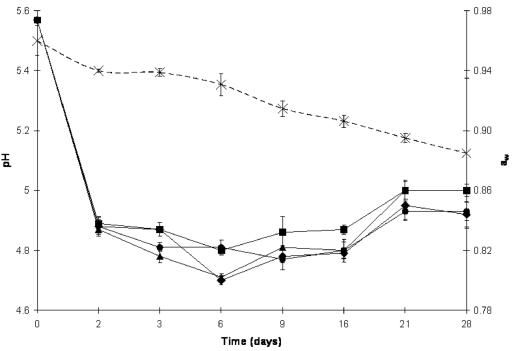
AIT levels ranging from 500 ppm to 1,000 ppm did not significantly affect (P > 0.05) the acidification performance of Pediococcus pentosaceus compared with the control during salami fermentation. All salami treatments achieved the guidelines for fermentation safety (degree-hour factor) established by Agriculture and Agri-Food Canada (1); that is, the time above 15°C required to reach pH 5.3 was <65 h. The pHs of the different sausage treatments dropped initially from 5.57 to less than 4.89 within 48 h of fermentation at 26 to 24°C. pH values remained steady until the later phases of drying at 16 days, where a slight increase in the pH was observed (Fig. 1). This is regarded as a normal increase in pH as a consequence of production of ammonia and buffering peptides by bacterial metabolism (10).
FIG. 1.
Changes in pH and aw of salami during fermentation (first 3 days) and drying (next 25 days) after treatment with different concentrations of microencapsulated AIT. ♦, control salami pH; •, salami pH with 500 ppm of AIT; ▴, salami pH with 750 ppm of AIT; ▪, salami pH with 1,000 ppm of AIT; ×, aw averages for all treatments. Error bars represent standard deviations.
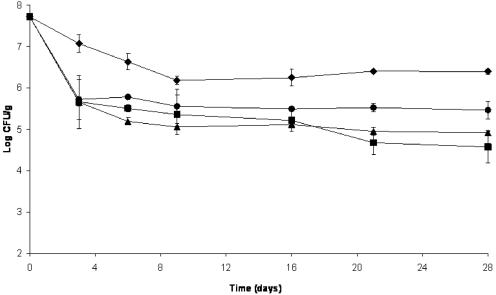
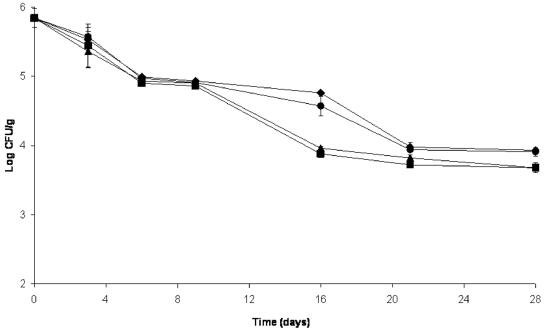
The level of P. pentosaceus decreased from 7.72 to 6.39 log10 CFU/g in the control salami by the end of drying. Initial numbers of P. pentosaceus were significantly reduced to levels close to 5.7 log10 CFU/g in all treatments containing AIT after 3 days of fermentation and thereafter decreased slowly until levels reached from 5.7 to 4.6 log10 CFU/g at the end of drying (Fig. 2). This reduction in the number of these gram-positive bacteria by AIT was expected on the basis of previous evaluations (2, 21), which were confirmed by our preliminary results (data unpublished). S. carnosus numbers decreased by ≤2 log CFU/g at the end of drying in all treatments (Fig. 3). A reduction in the number of Staphylococcus cells has been reported by several researchers (12, 18), and it is considered normal in the processing of dry fermented sausage.
FIG. 2.
Effect of different concentrations of microencapsulated AIT on the number of Pediococcus pentosaceus cells during fermentation (first 3 days) and drying (next 25 days) of salami. ♦, control salami; •, salami with 500 ppm of AIT; ▴, salami with 750 ppm of AIT; ▪, salami with 1,000 ppm of AIT. Organisms were recovered on MRS agar incubated anaerobically at 45°C. Error bars represent standard deviations.
FIG. 3.
Effect of different concentrations of microencapsulated AIT on the number of Staphylococcus carnosus cells during fermentation (first 3 days) and drying (next 25 days) of salami. ♦, control salami; •, salami with 500 ppm of AIT; ▴, salami with 750 ppm of AIT; ▪, salami with 1,000 ppm of AIT. Organisms were recovered on mannitol salt agar incubated at 37°C. Error bars represent standard deviations.
The aw of control sausage dropped from 0.960 ± 0.010 at the beginning of fermentation to 0.885 ± 0.050 by the end of 28 days of processing. No significant differences in aw were found among the control salami and the three treatments containing 500, 750, or 1,000 ppm of AIT, and therefore the mean aw value is presented for all treatments (Fig. 1).
Antimicrobial effects of microencapsulated AIT on E. coli O157:H7 inoculated in dry fermented sausages.
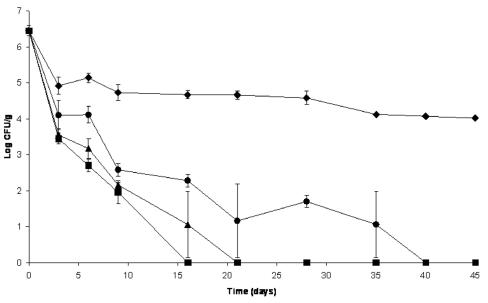
The antimicrobial effects of microencapsulated AIT on E. coli O157:H7 inoculated at 6.45 log10 CFU/g in dry fermented sausage are shown in Fig. 4. At these E. coli O157:H7 numbers, 1,000 and 750 ppm of AIT in the salami batter reduced E. coli O157:H7 to undetectable levels (<1 CFU/25 g meat) after 16 and 21 days of processing, respectively. In dry fermented sausages treated with 500 ppm of AIT, E. coli O157:H7 numbers were reduced (P < 0.05) by 4.75 log10 CFU/g at the end of 28 days of processing. An extension of the drying time showed that after 40 days, E. coli O157:H7 numbers were reduced by 6.5 log10 CFU/g in sausage samples containing 500 ppm AIT. E. coli O157:H7 was able to survive 45 days of processing in the untreated salami, with a reduction in its numbers of only 2.4 log10 CFU/g. E. coli O157:H7 not only showed its high acid tolerance but also survived exposure to an aw level of 0.87.
FIG. 4.
Survival of Escherichia coli O157:H7 treated with different concentrations of microencapsulated AIT during fermentation (first 3 days) and extended drying (next 42 days) of salami. ♦, control salami; •, salami with 500 ppm of AIT; ▴, salami with 750 ppm of AIT; ▪, salami with 1,000 ppm of AIT. Organisms were recovered on CT-SMAC agar incubated at 37°C. IMS was performed for each presumptively negative sample after 24 h of enrichment in TSB at 37°C. Error bars represent standard deviations.
The antimicrobial effect of gaseous AIT against E. coli O157:H7 has been broadly studied in meat systems; however, there is not much information about the antimicrobial potency of this compound used in its liquid or microencapsulated liquid state. Chacon et al. (6) successfully used microencapsulated AIT powder for decontamination of E. coli O157:H7 from ground beef. They found that 2,828 ppm of encapsulated AIT reduced E. coli O157:H7 by 2.7 log10 CFU/g in inoculated ground beef stored at 4°C for 18 days. High concentrations of AIT (close to 5,000 ppm) were needed to eliminate initial E. coli O157:H7 levels of 8 log10 CFU/g from the ground meat. In the present experiments it was found that 1,000 ppm of microencapsulated AIT was able to eliminate 6.45 log10 CFU/g E. coli O157:H7 from sausages in 16 days. It is probable that factors such as the presence of ≥3% NaCl, a pH of <5.3, lower aw (0.88, compared with 0.96 in ground beef), ≤200 ppm nitrite, and a higher storage temperature (13°C) may have contributed to the greater effectiveness of AIT against E. coli O157:H7 in the sausages than in ground beef. In addition, the antimicrobial activity of microencapsulated AIT may be enhanced by the higher fat concentrations in the fermented sausages, which were 33 to 35%, in comparison with the <5% fat in the ground beef. Hasegawa et al. (15) reported similar augmentation of AIT antimicrobial activity when they challenged Vibrio parahaemolyticus in tuna containing 20.8 or 0.04% fat. They found that V. parahaemolyticus was inhibited significantly better in fatty than in lean tissue suspensions, perhaps as a consequence of partial AIT dissolution in the fatty acids of the tuna meat. Nadarajah (25) reported that the high levels of cis-oleic acid in beef fat may complex with the AIT molecules, producing a reduction in volatilization of AIT. Additionally, the high fat (low moisture) content of sausages has been reported to significantly reduce survival of E. coli O157:H7 in such products (13).
A large reduction of E. coli O157:H7 numbers occurred during the first 3 days of fermentation in all treatments as a consequence of the rapid drop in pH. However, the reduction of E. coli O157:H7 viability continued progressively in treatments containing microencapsulated AIT until the organism was eliminated. This extended reduction was explained by Chacon et al. (6) as a result of the continued AIT release from microcapsules in the presence of elevated relative humidity.
Effects of microencapsulated AIT on the total bacterial numbers in dry fermented sausages.
The effect of microencapsulated AIT on the total numbers of viable bacteria in dry fermented sausages was minimal. Numbers of organisms recovered from treatments showed the same profiles as recorded for P. pentosaceus in Fig. 2. In the untreated control, the total bacterial numbers decreased from an initial level of 7.9 log10 CFU/g to 6.9 log10 CFU/g at 28 days of processing. This reduction in the total number could be related in part to the reduction of E. coli O157:H7 in the control treatment but is more likely related to a gradual reduction of P. pentosaceus viability during sausage maturation, since the latter organism was the predominant component of the microflora. In treatments containing microencapsulated AIT, the total bacterial numbers decreased to levels ranging from 5.2 to 6.0 log10 CFU/g at 28 days of processing, and these levels were lower than those in the control without AIT. It is notable that gram-positive lactic acid bacteria are the predominant component of the microflora recovered from fermented sausages on nonselective media (17) and that gram-positive bacteria, including the lactic acid bacteria, are more AIT tolerant than gram-negative bacteria (30, 32). In the present work, while AIT reduced the viability of the Pediococcus starter culture to some extent, there was no significant effect on the rate of meat acidulation (which is primarily due to lactic acid production by this component of the starter culture mixture).
Sensory evaluation.
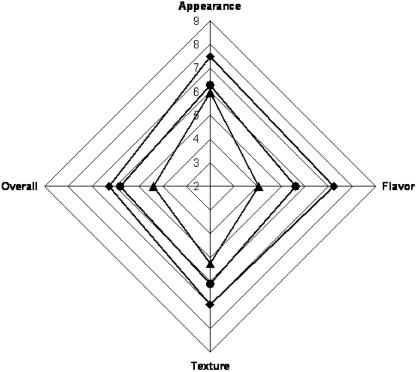
Fermented sausages containing 1,000 ppm AIT were prescreened and found to have a strong and somewhat bitter mustard flavor. To avoid introducing bias in tests, sausages with 1,000 ppm AIT were not presented to panelists. The addition of microencapsulated AIT at 500 or 750 ppm significantly affected the sensory attributes (flavor, appearance, texture, and overall impression) of dry fermented sausages measured (Fig. 5). The appearance of the control salami received a significantly (P < 0.05) better score than that of those treated with 500 or 750 ppm of AIT, probably because of slight yellow color development and more brittle texture in some AIT-treated samples. Texture differences, which were apparent in samples treated with 750 ppm AIT but absent from sausages treated with 500 ppm AIT, were probably due to the inclusion of 1.69% (wt/wt) gum acacia in the former samples. The appearance of the control salami was scored by the panelist as “like moderately,” while the appearance of the salami from the AIT treatments was scored as “like slightly.” Significant differences were found in the flavor, texture, and overall impression of all sausages tested, where the scores for the control and the 500 ppm AIT and 750 ppm AIT treatments were “like moderately,” “like slightly,” and “dislike slightly,” respectively. Ninety percent of the panelists ate spicy food at least four times per year. This preference for spicy food had a positive impact on the acceptability of samples containing microencapsulated AIT at the lowest concentration (which yielded a slightly spicy sensation). Clark (7) noted that allyl isothiocyanate has been used as a flavor fortifier when a sharp spice effect is needed to simulate the mustard or horseradish taste. The spicy effect in treatments containing 500 ppm was acceptable to the panelists, and 41% indicated that they would buy this product. However, only 13% of the panelists indicated that they would buy dry salami containing 750 ppm AIT.
FIG. 5.
Sensory analysis of dry fermented sausages at the end of ripening (nine-point Hedonic scale, where 9 and 1 represent “extremely good” and “extremely poor,” respectively, and a score of 6 represents “like slightly”). ♦, control salami; •, salami with 500 ppm of AIT; ▴, salami with 750 ppm of AIT.
Microencapsulated AIT did not affect meat acidulation by the starter cultures used in the manufacture of dry fermented sausages. AIT at 1,000 and 750 ppm eliminated >6 log10 CFU/g of E. coli O157:H7 in sausages after 16 and 21 days of processing, respectively. The 5-log10-unit reduction of E. coli O157:H7 necessary to validate the process of dry fermented sausage production (28) was nearly achieved (reduction of 4.75 log10 CFU/g) at 28 days of processing using 500 ppm AIT, and this treatment was successful in eliminating >6 log10 CFU/g E. coli O157:H7 at day 40. It is possible that microencapsulated AIT at <500 ppm could eliminate 5 log10 CFU/g E. coli O157:H7 if sausages were dried for ≥40 days at 13°C. The total bacteria enumerated were mainly lactic acid bacteria, which were slightly inhibited at the end of drying by the AIT-containing microcapsules.
Sausages containing 500 ppm AIT were the most acceptable AIT-treated sausages as assessed by the sensory panel, and these sausages could be marketed as a safe specialty product such as a hot salami. Sausages treated with 750 ppm of AIT had a strong (pungent) flavor and odor which were unacceptable to many of the panelists.
Acknowledgments
We acknowledge R. Buffo and D. Ryland for assistance with microcapsule preparation methods and sensory analysis, respectively. We also thank G. Graumann for technical help.
This project was made possible by a strategic grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) and by funds from Piller's Sausages and Delicatessens, Waterloo, ON, Canada.
REFERENCES
- 1.Agriculture and Agri-Food Canada. 1992. Manual of procedures for use in registered establishments, p. 76A-76P. Meat Hygiene Division, Agriculture and Agri-Food Canada, Ottawa, Canada.
- 2.Ahn, E., J. Kim, and D. Shin. 1999. Antimicrobial effects of allyl isothiocyanate on several microorganisms. Korean J. Food Sci. Technol. 31:206-211. [Google Scholar]
- 3.Association of Official Analytical Chemists. 1975. Official methods of analysis, 12th ed., p. 417-427. Association of Official Analytical Chemists, Washington, D.C.
- 4.Centers for Disease Control and Prevention. 1993. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1992-1993. Morb. Mortal. Wkly. Rep. 42:258-263. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1995. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. Morb. Mortal. Wkly. Rep. 44:57-160. [PubMed] [Google Scholar]
- 6.Chacon, P. A., R. A. Buffo, and R.A. Holley. 2006. Inhibitory effects of microencapsulated allyl isothiocyanate (AIT) against Escherichia coli O157:H7 in refrigerated, nitrogen packed, finely chopped beef. Int. J. Food Microbiol. 107:231-237. [DOI] [PubMed] [Google Scholar]
- 7.Clark, G. 1992. Allyl isothiocyanate. Perf. Flav. 17:107-109. [Google Scholar]
- 8.Clydesdale, F. 1999. Isothiocyanates. Crit. Rev. Food Sci. Nutr. 39:245-257. [Google Scholar]
- 9.Delaquis, P., and P. Sholberg. 1997. Antimicrobial activity of gaseous allyl isothiocyanate. J. Food Prot. 60:943-947. [DOI] [PubMed] [Google Scholar]
- 10.Demeyer, D., P. Vandekerckhove, and R. Moermans. 1979. Compounds determining pH in dry sausages. Meat Sci. 3:161-167. [DOI] [PubMed] [Google Scholar]
- 11.Duffy, L., F. Grau, and P. Vanderlinde. 2000. Acid resistance of enterohaemorrhagic and generic Escherichia coli associated with food-borne disease and meat. Int. J. Food Microbiol. 60:83-89. [DOI] [PubMed] [Google Scholar]
- 12.Erkkila, S., M. Venalainen, S. Hielm, E. Petaja, E. Puolanne, and T. Mattila-Sandholm. 2000. Survival of Escherichia coli O157:H7 in dry sausage fermented by probiotic lactic acid bacteria. J. Sci. Food Agric. 80:2101-2104. [Google Scholar]
- 13.Faith, N., R. Wierzba, A. Ihnot, A. Roering, T. Lorang, C. Kaspar, and J. Luchansky. 1998. Survival of E. coli O157:H7 in full- and reduced-fat pepperoni after manufacture of sticks, storage of slices at 4°C or 21°C under air and vacuum, and baking of slices on frozen pizza at 135, 191 and 246°C. J. Food Prot. 61:383-389. [DOI] [PubMed] [Google Scholar]
- 14.Glass, K., J. Loeffelholz, J. Ford, and M. Doyle. 1992. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Appl. Environ. Microbiol. 58:2513-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa, N., Y. Matsumoto, A. Hoshino, and K. Iwashita. 1999. Comparison of effects of Wasabica japonica and allyl isothiocyanate on the growth of four strains of Vibrio parahaemolyticus in lean and fatty tuna meat suspensions. Int. J. Food Microbiol. 49:27-34. [DOI] [PubMed] [Google Scholar]
- 16.Hinkens, J., N. Faith, T. Lorang, P. Bailey, D. Buege, C. Kasper, and J. Luchansky. 1996. Validation of pepperoni processes for control of Escherichia coli O157:H7. J. Food Prot. 59:1260-1266. [DOI] [PubMed] [Google Scholar]
- 17.Holley, R. A., P. A. Jui, M. Wittmann, and P. Kwan. 1988. Survival of S. aureus and S. typhimurium in raw ripened dry sausages formulated with mechanically separated chicken meat. Fleischwirtschaft 68:194-201. [Google Scholar]
- 18.Hughes, M., J. Kerry, E. Arendt, P. Kenneally, P. McSweeney, and E. O'Neill. 2002. Characterization of proteolysis during ripening of semi-dry fermented sausages. Meat Sci. 62:205-216. [DOI] [PubMed] [Google Scholar]
- 19.Kanemaru, K., and T. Miyamoto. 1990. Inhibitory effects on the growth of several bacteria by brown mustard and allyl isothiocyanate. Nippon Shokuhin Kogyo Gakkaishi 37:823-829. [Google Scholar]
- 20.Kim, Y., C. Morr, and T. Schenz. 1996. Microencapsulation properties of gum arabic and several food proteins: liquid orange oil emulsion particles. J. Agric. Food Chem. 44:1308-1313. [Google Scholar]
- 21.Kyung, K., and H. Fleming. 1997. Antimicrobial activity of sulfur compounds derived from cabbage. J. Food Prot. 60:67-71. [DOI] [PubMed] [Google Scholar]
- 22.Lahti, E., T. Johansson, T. Honkanen-Buzalski, P. Hill, and E. Nurmi. 2001. Survival and detection of Escherichia coli O157:H7 and Listeria monocytogenes during the manufacture of dry sausage using two different starter cultures. Food Microbiol. 18:75-85. [Google Scholar]
- 23.Mead, P., and P. Griffin. 1998. Escherichia coli O157:H7. Lancet 352:1207-1212. [DOI] [PubMed] [Google Scholar]
- 24.Muthukumarasamy, P., J. Han, and R. Holley. 2003. Bactericidal effects of Lactobacillus reuteri and allyl isothiocyanate on Escherichia coli O157:H7 in refrigerated ground beef. J. Food Prot. 66:2038-2044. [DOI] [PubMed] [Google Scholar]
- 25.Nadarajah, D. 2003. M.S. thesis. Lethality of Escherichia coli O157:H7 in hamburger treated with purified allyl isothiocyanate and mustard flour. University of Manitoba, Winnipeg, Canada.
- 26.Nadarajah, D., J. H. Han, and R. A. Holley. 2005. Inactivation of Escherichia coli O157:H7 in packaged ground beef by allyl isothiocyanate. Int. J. Food Microbiol. 99:269-279. [DOI] [PubMed] [Google Scholar]
- 27.Nissen, H., and A. Holck. 1998. Survival of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella kentucky in Norwegian fermented, dry sausage. Food Microbiol. 15:273-279. [Google Scholar]
- 28.Reed, C. 1995. Challenge study—Escherichia coli O157:H7 in fermented sausage. Letter to plant managers, 28 April 1995. U.S. Department of Agriculture, Food Safety and Inspection Service, Washington, D.C.
- 29.Riley, L., R. Remis, S. Helgerson, H. McGee, J. Wells, B. Davis, R. Hebert, E. Olcott, L. Johnson, N. Hargrett, P. Blake, and M. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 30.Shofran, B., S. Purrington, F. Breidt, and H. Fleming. 1998. Antimicrobial properties of sinigrin and its hydrolysis products. J. Food Sci. 63:621-624. [Google Scholar]
- 31.Tarr, P., T. Besser, D. Hancock, W. Keene, and M. Goldoft. 1997. Verotoxigenic Escherichia coli infection: U.S. overview. J. Food Prot. 60:1466-1471. [DOI] [PubMed] [Google Scholar]
- 32.Ward, S., P. Delaquis, R. Holley, and G. Mazza. 1998. Inhibition of spoilage and pathogenic bacteria on agar and pre-cooked roast beef by volatile horseradish distillates. Food Res. Int. 31:19-26. [Google Scholar]
- 33.Watts, B., G. Ylimaki, L. Jeffery, and L. Elias. 1989. Basic sensory methods for food evaluation, p. 59-104. International Development Research Centre, Ottawa, Canada.
- 34.Yu, C., and C. Chou. 1998. Fate of Escherichia coli O157:H7 in Chinese-style sausage subjected to different packaging and storage conditions. J. Sci. Food Agric. 78:486-490. [Google Scholar]
- 35.Zadik, P., P. Chapman, and C. Siddons. 1993. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157:H7. J. Med. Microbiol. 39:155-158. [DOI] [PubMed] [Google Scholar]