Abstract
Endospores of proteolytic type B Clostridium botulinum TMW 2.357 and Bacillus amyloliquefaciens TMW 2.479 are currently described as the most high-pressure-resistant bacterial spores relevant to food intoxication and spoilage in combined pressure-temperature applications. The effects of combined pressure (0.1 to 1,400 MPa) and temperature (70 to 120°C) treatments were determined for these spores. A process employing isothermal holding times was established to distinguish pressure from temperature effects. An increase in pressure (600 to 1,400 MPa) and an increase in temperature (90 to 110°C) accelerated the inactivation of C. botulinum spores. However, incubation at 100°C, 110°C, or 120°C with ambient pressure resulted in faster spore reduction than treatment with 600 or 800 MPa at the same temperature. This pressure-mediated spore protection was also observed at 120°C and 800, 1,000, or 1,200 MPa with the more heat-tolerant B. amyloliquefaciens TMW 2.479 spores. Inactivation curves for both strains showed a pronounced pressure-dependent tailing, which indicates that a small fraction of the spore populations survives conditions of up to 120°C and 1.4 GPa in isothermal treatments. Because of this tailing and the fact that pressure-temperature combinations stabilizing bacterial endospores vary from strain to strain, food safety must be ensured in case-by-case studies demonstrating inactivation or nongrowth of C. botulinum with realistic contamination rates in the respective pressurized food and equipment.
High hydrostatic pressure (HHP) is a powerful tool for inactivating food pathogens and spoilage organisms and thus achieving food safety, with flavors, colors, and nutrients being affected to a lesser extent than in conventional heat treatments (6, 28). It is generally assumed that the introduction of HHP to a heat-mediated inactivation process enhances the inactivation of bacterial endospores compared to heat treatment alone and that the rise of pressure or temperature will result in accelerated and steady inactivation. This is of particular interest because bacterial endospores are not inactivated by pressure treatment at ambient temperature, and endospores of the genera Bacillus and Clostridium tolerate pressures over 1,000 MPa at 25°C (28). For inactivation of these organisms, combinations of high pressure and heat are required (27).
Changes in pressure are thermodynamically coupled to changes in temperature, and the adiabatic heating of samples during compression makes it difficult to distinguish pressure effects on bacterial spores from temperature effects. Spores in a pressure-treated sample inevitably experience a dynamic temperature curve, which depends on the pressure difference, the compression rate, and the heat transfer rate from the sample to the pressure vessel. A nearly constant temperature during pressure holding times can be achieved by minimizing or eliminating heat transfer throughout the process (adiabatic treatments). With the aim of a fast, energy-saving process for the inactivation of bacterial endospores, instrumentation for adiabatic processes at 500 to 700 MPa has been developed (9).
Pressure treatment opens channels of Bacillus subtilis spores that permit the release of dipicolinic acid (DPA) from the spores (20), which results in activation of the germination pathway (20, 33) at ambient temperature. Such germinating spores are sensitive to pressure at ambient temperature. However, germination is not induced in all spores of a population (17, 18), and therefore repeated pressurization cycles fail to achieve commercial sterility of foods. The inactivation of spores from B. subtilis and Bacillus licheniformis by HHP at temperatures of >70°C follows a two-stage mechanism that does not involve spore germination. Instead, pressure causes DPA release and a concomitant loss of heat resistance. For the inactivation of such spores, Margosch el al. (19) proposed a “hit and wait” strategy, where DPA is released from the cell by a short HHP pulse at high temperature and spores are thermally inactivated upon pressure release. An explanation for the high HHP tolerance of bacterial endospores is their ability to retain DPA during pressure treatments (19), possibly as a result of a different spore composition or structure.
While HHP inactivation of bacterial endospores has been studied with Bacillus spp. and Clostridium sporogenes, only a few reports are available on the pressure resistance of Clostridium botulinum spores. At pH values of >4.5, spores of C. botulinum can germinate and produce the neurotoxin that causes botulism; therefore, their absence or inability to germinate determines the safety of low-acid foods. Spore counts of heat-resistant C. botulinum type A were not reduced >3 log following treatments with 827 MPa and 75°C (19, 23). Margosch et al. (19) determined the pressure resistance of toxigenic C. botulinum and Bacillus cereus as well as the food spoilers B. subtilis, B. licheniformis, Bacillus amyloliquefaciens, Bacillus smithii, and Thermoanaerobacterium thermosaccharolyticum. They followed a “worst-case scenario,” employing the most resistant types of spores from the most pressure-resistant strain to identify within their selection spores of B. amyloliquefaciens TMW 2.457 as the most pressure-resistant spores, followed by those of C. botulinum TMW 2.357. The heat resistance of the spores did not correlate with their pressure resistance, and a high level of variation of pressure and heat resistance within spores of various strains of C. botulinum was observed. A 5- to 6-log reduction of C. botulinum spores was observed at 600 MPa and 110 to 116°C, but it was not possible to distinguish between temperature and pressure effects on spore inactivation because of the temperature fluxes during compression and pressure holding times.
The aim of this study was to determine the effects of combined pressure and temperature on C. botulinum TMW 2.357 and B. amyloliquefaciens TMW 2.479, which, to our knowledge, are the most pressure-resistant spores described in the literature (19). Pressure/temperature combinations of 0.1 to 1,400 MPa and 70 to 120°C were investigated using an experimental design allowing isothermal pressure holding times and thus distinguishing between temperature and pressure effects on spore inactivation.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of spore suspensions.
The bacterial strains used in this study were proteolytic Clostridium botulinum TMW 2.357, REB 89 type B (obtained from the Institut für Medizinische Mikrobiologie und Infektionsepidemologie, Leipzig, Germany), and Bacillus amyloliquefaciens TMW 2.479, Fad 82, isolated from ropy bread by Röcken and Spicher (25) and reclassified as B. amyloliquefaciens based on its 16S rRNA sequence and randomly amplified polymorphic DNA patterns (data not shown). C. botulinum was grown anaerobically in RCM broth (Merck, Darmstadt, Germany), and B. amyloliquefaciens was grown aerobically in ST1 broth (Merck, Darmstadt, Germany) at 30°C. Spores were prepared by plating aliquots of 0.1 ml from fresh overnight cultures on agar plates at the temperatures noted above. B. amyloliquefaciens was sporulated aerobically on ST1 agar supplemented with 10 mg liter−1 MnSO4 · H2O. C. botulinum was sporulated anaerobically on WSH agar as described by Margosch et al. (18). WSH agar was made as follows: to 1 liter of soil extract prepared according to the method of Gams et al. (11) were added 20 g of meat extract, 3 g of yeast extract, 0.5 g of cysteine-HCl, 5 g of CaCO3, and egg whites from 1.5 fresh eggs, and the pH was adjusted to 7.0. The agar plates were incubated for 5 days for the bacilli and 10 days for the clostridia. Preparations showed between 90 and 99% phase-bright spores by phase-contrast microscopy. Spores were collected from the plates by flooding the surface of the culture with 10-ml aliquots of cold sterile distilled water. After being harvested, the spore suspensions were washed four times by centrifugation at 3,000 × g for 15 min at 5°C, resuspended in sterile distilled water, and stored at −80°C until use. Between the second and third wash cycle, the suspensions were pasteurized at 80°C for 10 min.
Determination of cell counts.
Cell counts of the Bacillus strains were determined on ST1 agar. Appropriate dilutions were plated using a spiral plater (IUL, Königswinter, Germany), and plates were incubated aerobically for 36 h at 30°C. Cell counts of C. botulinum were determined on RCM agar. Appropriate dilutions were pipetted into petri dishes and mixed with agar, and plates were incubated anaerobically for 36 h at 30°C.
Pressure treatment of spores with isothermal holding times.
Samples were pressurized in Tris-His buffer (THB; 10 mM Tris-HCl, 20 mM histidine-HCl) adjusted to pH 4.0, 5.15, or 6.0. Di-2-ethyl-hexyl sebacate (Sigma, Munich, Germany) was used as pressure transmission fluid, because in ultrahigh pressure-temperature ranges (1.4 GPa and 120°C), no phase transition was observed. Also, this fluid was a better stabilizer for the packing system of the piston than alternatively tested mixtures of petroleum and extraction naphtha or hexane, pentane, and ethanol tested as pure liquids or in mixtures with petroleum (3). The pressurization medium was inoculated with spores to a spore count of 5.0 × 107 to 4.5 × 108 spores ml−1. A volume of 150 μl of the spore suspension was transferred to a polypropylene tube with an internal diameter of 1 mm, which was sealed on two sides and stored on ice until treatment. The temperatures used were 70 to 120°C, and the pressure ranged from 600 to 1,400 MPa. After decompression, the sample tubes were stored on ice until determination of plate counts. For each experiment, an untreated sample running through the same temperature protocol was used as a control to determine the initial number of spores.
Close-to-isothermal conditions were achieved essentially as described by Ardia et al. (3) by using a high-pressure-equipment microsystem (Unipress, Warsaw, Poland). The high-pressure vessel, with a volume of approximately 150 μl, was placed into a heating-cooling block and heated at the same rate as the increase of the temperature of the sample by adiabatic compression. The temperature of the pressure vessel was controlled through a pressure-resistant shielded K-type thermocouple which was installed at the internal surface of the heating-cooling block, directly in contact with the pressure cell. The entire high-pressure cycle was controlled by software-based pressure and temperature controllers. The initial temperature was calculated by software written by Ardia et al. (3) on the basis of the adiabatic heating profiles of water, the requested processing pressure, and the temperature. The heat of compression of the pressure-transmitting medium (di-2-ethyl-hexyl sebacate) was taken into account while calculating the correct preheating temperature. A maximum compression/decompression rate of approximately 70 MPa/s was used. Since the time for compression even up to 1,400 MPa was <20 s and because this block reproduced the increase of temperature caused through adiabatic heating, adiabatic conditions and isothermal holding times could be simulated.
Heat treatment of spores.
Spore suspensions in THB were prepared as noted above and transferred to glass capillaries with internal and external diameters of 1.12 and 1.47 mm, respectively, and a length of 10.8 mm. The glass capillaries were heat sealed, placed in an oil bath maintained at 100, 110, or 120°C for up to 64 min, and rapidly cooled on ice. The cell counts of heat-treated spore suspensions were determined as described above.
Experimental error.
The experimental errors of duplicate independent pressure or heat treatments and cell count determinations were 0.5 log cycles or less.
Generation of p-T diagram of spore inactivation.
A pressure-temperature (p-T) diagram with isokineticity lines is the most concise way of presenting the combined effects of pressure and temperature. Isokineticity lines indicate all pressure/temperature combinations for a given treatment time and reduction level (e.g., 5 log cycles) that meet these conditions. They are derived from kinetic analysis of the experimental inactivation data.
From inactivation experiments in close-to-isobaric and -isothermal situations, rate constants (k) are regressively obtained by fitting nth-order kinetics (equation 1) to the survival data. This allows an appropriate description of curves which show significant deviations from log-linear behavior, as follows:
 |
(1) |
where t is time (min), N is the number of survivors at time t (CFU/ml), N0 is the initial spore count (CFU/ml), k is the rate constant (min−1), and n is the reaction order.
The most critical part in this analysis is the identification of a unifying reaction order (n) which matches the kinetic curves of all p-T conditions of the available data set. This is usually done by minimizing the cumulative standard error of fit over a range of reaction orders (from 1.0 to 1.8), i.e., averaging the predictive error in all individual kinetics.
Upon fixing the reaction order, the rate constant (k) is left as the only parameter that has to be obtained regressively (Table Curve 2D v4.0 statistical package; Systat Software Inc., Richmond, CA). Since the obtained rate constant (k) depends on pressure and temperature only, a functional relationship can easily be found. Empirical equations have often been suggested (3, 4, 16), and in this situation, equation 2 sufficiently fits the data, as follows:
 |
(2) |
The generation of isokineticity lines is done by replacing k in equation 1 by the pressure-temperature relation of equation 2. Using p as an independent variable and setting the reduction rate (N/N0) and treatment time t as constants, the obtained equation can be solved for T.
RESULTS
Effect of pressurization with isothermal holding times on bacterial endospores.
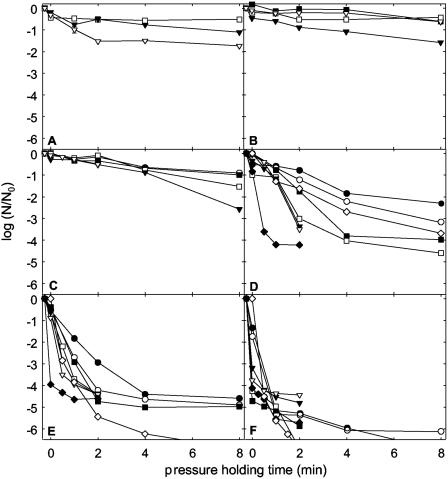
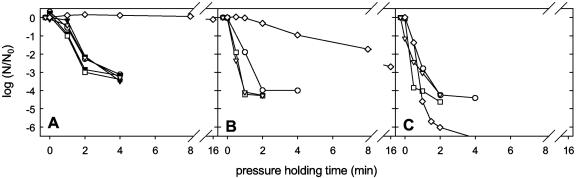
Spores of C. botulinum TMW 2.357 and B. amyloliquefaciens TMW 2.479 were used to investigate the effects of combined pressure (0.1 to 1,400 MPa) and temperature (70 to 120°C) treatments with isothermal holding times on spore inactivation. The experimental setup enabled the determination of up to 6.5 log cycles of inactivation. The inactivation kinetics of WSH spores of C. botulinum TMW 2.357 at temperatures ranging from 70 to 120°C and ambient pressure or pressures ranging from 600 to 1,400 MPa are displayed in Fig. 1. At 70°C, pressurization with 1,200 MPa resulted in 1.7 log cycles of inactivation after 8 min of pressure holding time. Increasing the temperature to 80 and 90°C increased the inactivation effect of the treatments only slightly (Fig. 1B and C). In contrast, pressurization at 100°C accelerated spore inactivation compared to treatments at 90°C or below. At 100°C and 1,400 MPa, a 4.2-log-cycle reduction was achieved after 1 min of pressure holding time. Remarkably, incubation at ambient pressure and 100°C resulted in faster spore reduction than treatment at 600 or 800 MPa. This pressure-mediated protection was also observed at 110 and 120°C. Furthermore, a strong tailing was observed for pressurized samples at 100°C and 110°C and was also noticeable at 120°C. At 100, 110, and 120°C, about 1 spore in 104, 105, and 106 spores, respectively, was resistant to pressure treatments. In contrast, treatment with 110 or 120°C at 0.1 MPa did not show any measurable tailing and led to spore inactivation to below the detection limit.
FIG. 1.
Log spore counts (N) of C. botulinum TMW 2.357 spores after combined pressure/temperature treatments with isothermal holding times in THB (pH 5.15). Spore counts are depicted relative to the spore counts of untreated samples (N0). (A) 70°C; (B) 80°C; (C) 90°C; (D) 100°C; (E) 110°C; (F) 120°C. The pressure level was 600 MPa (•), 800 MPa (○), 900 MPa (▪), 1,000 MPa (□), 1,100 MPa (▾), 1,200 MPa (▿), 1,400 MPa (⧫), or 0.1 MPa (◊). Lines dropping below the x axis indicate spore counts below the detection limit [log(N/N0) = −6.5].
Kinetic analysis of spore inactivation.
To exemplarily demonstrate spore stabilization, a kinetic analysis of the inactivation of C. botulinum endospores was performed. Rate constants were determined (data not shown), revealing a reaction order of 1.35. The strong tailing of the survival kinetics could accurately be reproduced by the chosen reaction order.
The parameters of the p-T function of ln(k) (equation 2) were as follows: A0, 2.465 ± 6.242; A1, −0.023 ± 0.009; A2, −0.149 ± 0.091; A3, 2.259 × 10−5 ± 0.554 × 10−5; A4, 1.462 × 10−3 ± 0.368 × 10−3; A5, 1.798 × 10−4 ± 0.839 × 10−4; and A6, −1.806 × 10−7 ± 0.511 × 10−7.
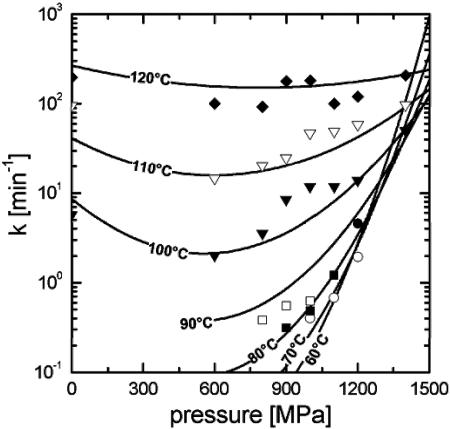
A graphical representation of equation 2 is shown in Fig. 2. In this case, k is plotted versus pressure at constant temperatures. A minimum in k versus pressure at higher temperatures (100 to 120°C) becomes visible after isokineticity lines are generated from equation 2, and this minimum can be located at approximately 600 MPa. At temperatures lower than 100°C, little or no spore inactivation was observed at ambient pressure, but spores were inactivated by 1 to 2 log cycles at 1 to 1.2 GPa.
FIG. 2.
Pressure dependence of inactivation rate constant (k) of C. botulinum TMW 2.357 at 60°C (•), 70°C (○), 80°C (▪), 90°C (□), 100°C (▾), 110°C (▿), and 120°C (⧫).
The inactivation kinetics of the B. amyloliquefaciens TMW 2.479 spores with isothermal holding times at temperatures ranging from 100 to 120°C and pressures ranging from 800 to 1,200 MPa are shown in Fig. 3. Spore inactivation at 100 or 110°C could be accelerated through pressure application, but at 120°C, pressure-mediated protection was also observed. Likewise, a strong tailing was observed for pressure applications at 100, 110, and 120°C. In contrast to the results for C. botulinum TMW 2.357, pressure levels between 800 and 1,200 MPa showed almost no variation in inactivation effects.
FIG. 3.
Log spore counts (N) of B. amyloliquefaciens TMW 2.479 spores after combined pressure/temperature treatments with isothermal holding times in THB (pH 5.15). Spore counts are depicted relative to the spore counts of untreated samples (N0). (A) 100°C; (B) 110°C; (C) 120°C. The pressure level was 800 MPa (○), 900 MPa (▪), 1,000 MPa (□), 1,100 MPa (▾), 1,200 MPa (▿), or 0.1 MPa (◊). Lines dropping below the x axis indicate spore counts below the detection limit [log(N/N0) = −6.5].
These results reveal a principal analogy with specific differences in the behaviors of spores of C. botulinum TMW 2.357 and B. amyloliquefaciens TMW 2.479 with respect to their resistance to combined pressure/temperature treatments (compare Fig. 1 and 3). The spores of these organisms can be stabilized at specific pressure/temperature combinations compared to heat treatment alone. On the other hand, the stabilizing parameter combinations are different for the two strains. As a consequence, B. amyloliquefaciens TMW 2.479 spores were much more resistant at any temperature and 0.1 MPa and at any pressure at 120°C than C. botulinum spores. However, for isothermal pressure treatments at 100°C or 110°C, the resistance of C. botulinum spores matched or exceeded that of B. amyloliquefaciens spores as a result of the pressure-mediated spore protection of C. botulinum in this parameter range.
DISCUSSION
It is a generally reported principle that HHP strongly and steadily accelerates the inactivation of bacterial spores while simultaneously bringing down the number of resistant survivors (1, 12, 13, 14, 21, 22, 23, 24, 26). In this communication, we report on the stabilization of bacterial endospores by specific pressure/temperature combinations and the occurrence of resistant fractions which are not observed with treatments at the respective temperature alone. Literature data on spore inactivation are difficult to compare because the temperature dynamics during treatments are not reported or not reproducible with different instrumentation because of variations with respect to the dimension of the pressure vessel, the compression rate, and the use of internal insulation or external thermostats to minimize or maximize, respectively, heat transfer. For the experiments described here, we used an experimental design that enabled the determination of spore inactivation after isothermal pressure holding times in a buffer system that is essentially pressure independent. C. botulinum produces the most potent bacterial toxin upon spore germination and cell growth. Therefore, the behavior of its endospores must be considered in the design of HHP equipment and treatments aiming to improve the safety of low-acid canned foods.
Pressure-mediated spore stabilization.
In contrast to previous investigations, the effects of combined pressure/temperature treatments on spores of B. amyloliquefaciens TMW 2.479 and C. botulinum TMW 2.357 were determined with isothermal holding times. In this way, it was possible to investigate temperature and pressure effects on inactivation independently. The investigated ranges of pressure and temperature were enhanced to levels of up to 1,400 MPa and 120°C, respectively, to inactivate B. amyloliquefaciens TMW 2.479 and C. botulinum TMW 2.357 endospores, which were proposed as target organisms for food spoilage and food safety in pressure-treated foods (19). An increase of pressure (600 to 1,400 MPa) and an increase in temperature (90 to 110°C) accelerated the inactivation of C. botulinum TMW 2.357 spores, in accordance with results from nonisothermal treatments (18, 19, 23). However, the use of isothermal conditions allowed us to identify parameter combinations in which HHP stabilizes the bacterial endospore against lethal temperatures. Sharma et al. (29) microscopically observed Escherichia coli cells at up to 1.6 GPa pressure and postulated that life may exist in so far undescribed extremes. Our finding of surviving bacterial endospores upon treatment at 120°C and 1.4 GPa pressure adds the idea of pressure stabilization of living systems at high temperatures.
p-T diagram of spore inactivation.
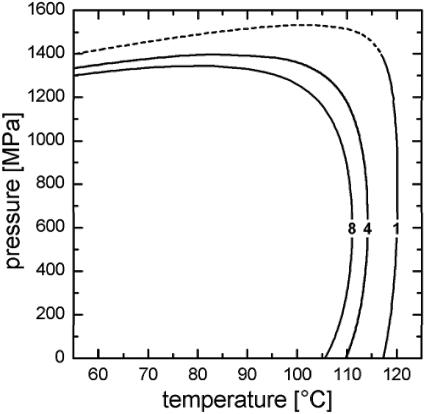
The kinetic analysis enabled the generation of a p-T diagram, which shows those conditions that bring about a 5-log reduction in survivor count at the indicated isobaric and isothermal treatment times (Fig. 4). The reduction of 5 log was chosen because most of the inactivation experiments did not result in higher levels.
FIG. 4.
Pressure-temperature isokineticity diagram for 5-log reduction of C. botulinum TMW 2.357 after 1, 4, and 8 min.
For processing times as short as about 1 to 5 min, the effect of pressure on the lethal action of the combined p-T treatment is marginal unless the pressure exceeds 1,400 MPa. The sharp breaks at higher pressures, where the isokineticity lines are tilted to the left, are within a domain that has not been investigated experimentally. Nevertheless, the similarity of the isokineticity curve for 8 min supports the validity of the extrapolation to higher pressure levels.
For the lower-pressure domain, equation 2 predicts a concave shape for the isokineticity lines. This supports the interpretation of pressure stabilization against heat inactivation since higher temperatures are required to kill 5 log cycles at, e.g., 600 MPa, than at ambient pressure.
Whenever the mechanism of pressure-mediated heat tolerance of the bacterial endospore remains unknown, spore stabilization under specific parameter combinations is in apparent analogy with pressure stabilization observed with proteins. The denaturation of proteins and impairment of membrane functionality have been discussed as reasons for the inactivation of microorganisms (32). For many proteins, it has been described that pressure induces structural changes and denaturation, which follow elliptic pressure/temperature phase diagrams with areas of pressure stabilization of a protein at a denaturing temperature. An overview is given by Smeller (31). This behavior may also account for the stabilization of endospores with specific pressure/temperature combinations. Still, for the complexity of a bacterial endospore, the behavior of a single protein can hardly be determined or described as determinative for cell death unless it is required for a vital function or is abundant, e.g., as a structural or protective component.
Pressure-resistant fractions within the spore population.
Tailing of the inactivation curves indicates that a small fraction of the spore population is highly pressure resistant and that the spore population is heterogenous. Tailing caused by resistant fractions was also noticed in other studies with various types of spores (5, 7, 8, 14, 17, 23). Although spore population heterogeneity is currently a favored explanation, it has also been reported that pressure treatment can be causative for a small percentage of survivors (15).
Limits in identification of the most pressure-resistant spore formers.
The inactivation behavior of B. amyloliquefaciens TMW 2.479 spores with isothermal holding times differed strongly in value from that of spores of C. botulinum TMW 2.357, although similar principles apparently apply. Pressure levels between 800 and 1,200 MPa showed almost no variations in effect with respect to faster spore reduction. This was also found for spores of T. thermosaccharolyticum TMW 2.299 in nonisothermal treatments (data not shown), and others have described similar behavior (8, 14). As a consequence, the degree of resistance of spores to combined pressure/temperature treatment is not fixed when different equipment and pressure-temperature regimens are applied. Since the rearrangement of one parameter can lead to another “most resistant” target strain, it is difficult, if not impossible, to suggest a general target organism for the pressure processing of low-acid foods, as proposed by Margosch et al. (19) for nonisothermal treatments. Following our observations, the requirements for the approach of Sizer et al. (9, 30), suggesting the development of a high-pressure 12-D concept in analogy to thermal inactivation, cannot be met because the postulates have not even been proven for thermal treatments.
Impact on food safety concepts.
Current food safety concepts employ a 12-D concept based on data from Esty and Meyer (10) and the assumption that the D121°C value of C. botulinum is 0.204 min or less. To ensure the inactivation of these spores and those of the more heat-tolerant spoilage organisms, a 5-D concept with respect to food-spoiling C. sporogenes is employed in industrial practice, equivalent to treatments of 5 min at 121°C. However, the D120°C value for thermal inactivation of C. botulinum TMW 2.359 was determined to be 1.2 min (R. Wittmann and W. Hennlich, Fraunhofer Institut Verfahrenstechnik und Verpackung, Freising, Germany, personal communication). Based on the D120°C value of the most heat-resistant spores of C. botulinum TMW 2.359, treatments equivalent to 5 min at 121°C (F5) will result in de facto 4-D and 2-D reductions of such spores in a “worst-case scenario.”
Since the 12-D thermal process has a long history of safe use and because botulism from commercially canned foods has been virtually eliminated since the implementation of these regulations (2), the 4-D reduction of the most resistant spores apparently is sufficient to consider canned foods safe. Such a 4-D reduction is achieved in less than 2 min by combined pressure-temperature processes when treatments at 100, 110, and 120°C are combined with pressures of ≥1,400, 800, and 600 MPa, respectively. Thus, even if there is a theoretical risk of highly pressure- and temperature-tolerant spores of C. botulinum stemming from soil, pressure processing appears to be a suitable process to reduce contamination in the same dimension as conventional heat treatment. However, in contrast to temperature treatments, a resistant fraction of spores is observed after combined pressure-temperature applications, namely, in isothermal treatments, that cannot be inactivated by prolonged treatment times. Thus, realistic contamination rates must be considered in risk assessments in case-by-case studies with the respective food and equipment.
Acknowledgments
We thank Arne C. Rodloff for providing the strain of Clostridium botulinum.
The Deutsche Bundesstiftung Umwelt is acknowledged for financial support (grant 13053/20).
REFERENCES
- 1.Ananta, E., V. Heinz, O. Schlüter, and D. Knorr. 2001. Kinetic studies on high-pressure inactivation of Bacillus stearothermophilus spores suspended in food matrices. Innovative Food Sci. Emerg. Technol. 2:261-272. [Google Scholar]
- 2.Anonymous. 2003. Scientific criteria to ensure safe food. National Academic Press, Washington, D.C. [PubMed]
- 3.Ardia, A., D. Knorr, G. Ferrari, and V. Heinz. 2004. Kinetic studies on combined high-pressure and temperature inactivation of Alicyclobacillus acidoterrestris spores in orange juice. Appl. Biotechnol. Food Sci. Policy 1:169-172. [Google Scholar]
- 4.Buckow, R., V. Heinz, and D. Knorr. 2005. Two fractional models for evaluating the activity of glucoamylase from Aspergillus niger under combined pressure and temperature conditions. Food Bioprod. Process. 83:1-9. [Google Scholar]
- 5.Cerf, O. 1977. Tailing of survival curves of bacterial spores. J. Appl. Bacteriol. 42:1-19. [DOI] [PubMed] [Google Scholar]
- 6.Cheftel, J. C., and J. Culioli. 1997. Effects of high pressure on meat: a review. Meat Sci. 46:211-236. [DOI] [PubMed] [Google Scholar]
- 7.Cléry-Barraud, C., A. Gaubert, P. Masson, and D. Vidal. 2004. Combined effects of high hydrostatic pressure and temperature for inactivation of Bacillus anthracis spores. Appl. Environ. Microbiol. 70:635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford, Y. J., E. A. Murano, D. G. Olson, and K. Shenoy. 1996. Use of high pressure and irradiation to eliminate Clostridium sporogenes spores in chicken breast. J. Food Prot. 59:711-715. [DOI] [PubMed] [Google Scholar]
- 9.de Heij, W. B. C., L. J. M. M. van Schepdael, R. Moezelaar, and R. W. van den Berg. 2003. Sterilization by high hydrostatic pressure: increasing efficiency and product quality by improved temperature control, p. 367-370. In R. Winter (ed.), Advances in high pressure bioscience and biotechnology II. Springer Verlag, Berlin, Germany.
- 10.Esty, J. R., and K. F. Meyer. 1922. The heat resistance of the spores of Bacillus botulinus and allied anaerobes. J. Infect. Dis. 31:650-663. [Google Scholar]
- 11.Gams, W., E. S. Hoekstra, and A. Aptroot. 1998. CBS course of mycology. Centraalbureau voor Schimmelcultures, Baarn, The Netherlands.
- 12.Hayakawa, I., T. Kanno, K. Yoshiyama, and Y. Fujio. 1994. Oscillatory compared with continuous high pressure sterilization of Bacillus stearothermophilus spores. J. Food Sci. 59:164-167. [Google Scholar]
- 13.Hayakawa, I., T. Kanno, M. Tomita, and Y. Fujio. 1994. Application of high pressure for spore inactivation and protein denaturation. J. Food Sci. 59:159-163. [Google Scholar]
- 14.Lee, S. Y., R. H. Dougherty, and D. H. Kang. 2002. Inhibitory effects of high pressure and heat on Alicyclobacillus acidoterrestris spores in apple juice. Appl. Environ. Microbiol. 68:4158-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig, H., K. G. Werner, E. Schattmann, and M. Schauer. 2002. High pressure induced alterations in morphology and cell characteristics of the bacterium Bacillus thuringiensis, p. 303-310. In R. Hayashi (ed.), Trends in pressure bioscience and biotechnology. Elsevier Science B.V., Amsterdam, The Netherlands.
- 16.Ly-Nguyen, B., A. Van Loey, C. Smout, I. Verlent, T. Duvetter, and M. Hendrickx. 2003. Effect of mild-heat and high-pressure processing on banana pectin methylesterase: a kinetic study. J. Agric. Food Chem. 51:7974-7979. [DOI] [PubMed] [Google Scholar]
- 17.Mallidis, C. G., and D. Drizou. 1991. Effect of simultaneous application of heat and pressure on the survival of bacterial spores. J. Appl. Bacteriol. 71:285-288. [DOI] [PubMed] [Google Scholar]
- 18.Margosch, D., M. G. Gänzle, M. A. Ehrmann, and R. F. Vogel. 2004. Pressure inactivation of Bacillus endospores. Appl. Environ. Microbiol. 70:7231-7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margosch, D., M. A. Ehrmann, M. G. Gänzle, and R. F. Vogel. 2004. Comparison of pressure and heat resistance of Clostridium botulinum and other endospores in mashed carrots. J. Food Prot. 67:2530-2537. [DOI] [PubMed] [Google Scholar]
- 20.Paidhungat, M., B. Setlow, B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 68:3172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raso, J., G. Barbosa-Cánovas, and B. G. Swanson. 1998. Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J. Appl. Microbiol. 85:17-24. [DOI] [PubMed] [Google Scholar]
- 22.Raso, J., M. M. Góngora-Nieto, G. V. Barbosa-Cánovas, and B. G. Swanson. 1998. Influence of several environmental factors on the initiation of germination and inactivation of Bacillus cereus by high hydrostatic pressure. Int. J. Food Microbiol. 44:125-132. [DOI] [PubMed] [Google Scholar]
- 23.Reddy, N. R., H. M. Solomon, R. C. Tetzloff, E. J. Rhodehamel, V. M. Balasubramaniam, and S. Palaniappan. 2003. Inactivation of Clostridium botulinum type A spores by high-pressure processing at elevated temperatures. J. Food Prot. 66:1402-1407. [DOI] [PubMed] [Google Scholar]
- 24.Reddy, N. R., H. M. Solomon, G. A. Fingerhut, E. J. Rhodehamel, V. M. Balasubramaniam, and S. Palaniappan. 1999. Inactivation of Clostridium botulinum type E spores by high pressure processing. J. Food Saf. 19:277-288. [Google Scholar]
- 25.Röcken, W., and G. Spicher. 1993. Fadenziehende Bakterien—Vorkommen, Bedeutung Gegenmassnahmen. Getreide Mehl Brot 47:30-35. [Google Scholar]
- 26.Rovere, P., S. Gola, A. Maggi, N. Sacamuzza, and L. Miglioli. 1998. Studies on bacterial spores by combined high pressure-heat treatments: possibility to sterilize low acid foods, p. 354. In N. S. Isaacs (ed.), High pressure food science, bioscience and chemistry. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 27.Sale, J. H., G. W. Gould, and W. A. Hamilton. 1970. Inactivation of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:323-334. [DOI] [PubMed] [Google Scholar]
- 28.San Martín, M. F., G. V. Barbosa-Cánovas, and B. G. Swanson. 2002. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 42:627-645. [DOI] [PubMed] [Google Scholar]
- 29.Sharma, A., J. H. Scott, G. D. Cody, M. L. Fogel, R. M. Hazen, R. J. Hemley, and W. T. Huntress. 2002. Microbial activity at gigapascal pressures. Science 295:1514-1516. [DOI] [PubMed] [Google Scholar]
- 30.Sizer, C. E., V. M. Balasubramaniam, and E. Ting. 2002. Validating high-pressure processes for low-acid foods. Food Technol. 56:36-42. [Google Scholar]
- 31.Smeller, L. 2002. Pressure-temperature phase diagrams of biomolecules. Biochim. Biophys. Acta 1595:11-29. [DOI] [PubMed] [Google Scholar]
- 32.Vogel, R. F., M. A. Ehrmann, M. G. Gänzle, C. Kato, C. Scheyhing, A. Molina-Gutierrez, H. M. Ulmer, and R. Winter. 2003. Advances in high pressure response of lactic acid bacteria, p. 249-254. In R. Winter (ed.), High pressure bioscience and biotechnology II. Springer Verlag, Berlin, Germany.
- 33.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]