Abstract
A laboratory-scale model system was developed to investigate the transport mechanisms involved in the horizontal movement of bacteria in overland flow across saturated soils. A suspension of Escherichia coli and bromide tracer was added to the model system, and the bromide concentration and number of attached and unattached E. coli cells in the overland flow were measured over time. Analysis of the breakthrough curves indicated that the E. coli and bromide were transported together, presumably by the same mechanism. This implied that the E. coli was transported by advection with the flowing water. Overland-flow transport of E. coli could be significantly reduced if the cells were preattached to large soil particles (>45 μm). However, when unattached cells were inoculated into the system, the E. coli appeared to attach predominantly to small particles (<2 μm) and hence remained unattenuated during transport. These results imply that in runoff generated by saturation-excess conditions, bacteria are rapidly transported across the surface and have little opportunity to interact with the soil matrix.
The transport of microorganisms from agricultural land into waterways can have detrimental effects on water quality and human health (10, 18, 28, 29). To develop robust strategies to control the passage of microorganisms to waterways, a good understanding of the transport mechanisms involved is required. In a study of pig slurry flowing down a slope, it was reported that all of the bacteria were absorbed and retained by the soil under relatively dry antecedent conditions (9). In contrast, studies with saturated soils often show poor removal of bacteria (4, 5). This can be attributed, in part, to a lack of infiltration, which limits interaction of runoff with the soil matrix (1, 3).
A further important factor in determining bacterial transport in overland flow is the state in which the bacteria occur, with different transport mechanisms postulated for bacteria attached to soil particles, present in flocs of cells, or occurring as single cells (11, 16, 28). It has been calculated that bacteria would need to be attached to soil particles >63 μm in diameter to settle out of overland flow and would need to be in large flocs >500 μm in diameter before they could be filtered out by grasses (11). In studies with artificial cowpats under simulated rainfall conditions, it was found that Escherichia coli cells were not eroded in flocs and that only 8% were attached to particles (21). These findings imply that E. coli eroded from cowpats during rainfall events would, under saturation-excess conditions, be rapidly transported over the soil surface unless it can attach to large soil particles during the transport process.
There have been numerous studies on the attachment of bacteria to particles in marine waters, but only a small number with freshwater systems (8). In storm water from urban land, fecal indicator bacteria were adsorbed predominantly to fine clay particles (<2 μm) (6). In another study with urban storm water, 83% of fecal coliforms were observed to remain in suspension after 4 h in a static column, and a similar percentage were able to pass through a 5-μm filter (24). In a groundwater system, the number of bacteria attached to particles has been reported to range from 0 to 100% (17). It has been observed that flocs are an important transport mechanism for fine particles in river systems and that bacteria were an important component of these flocs (8). In studies of floc size, it was found that more than 90% of the fecal coliforms in lake water passed through a 5-μm filter (12). These studies of freshwater systems suggest that if bacteria are attached to particles in freshwater, they are attached predominantly to small particles. However, it is possible that bacteria attached to larger particles are rapidly removed from the water column, leaving behind unattached cells and cells attached to small particles.
In this study we created a model lab-scale runoff system to study the mechanisms involved in the horizontal transport of E. coli under saturation-excess flow conditions, with a particular focus on the potential for E. coli to attach to soil particles during transport.
MATERIALS AND METHODS
Runoff experiments.
A series of runoff experiments was conducted, whereby a spike of E. coli and bromide tracer was added to water flowing horizontally over and through sterile soil held within a small box. The resulting runoff was collected and analyzed for tracer (Br) concentration and the numbers of attached and unattached E. coli cells. Table 1 contains a summary of the combination of the variables tested and the number of experiments conducted. Briefly, the soil boxes were held on either a 5 or a 15% slope. In most experiments, water was pumped onto the top of the soil boxes at a flow rate of either 0.6 or 2.0 ml s−1 to generate runoff. To determine if the extra soil particles in the runoff led to increased attachment, in some experiments (conducted at the 0.6 ml s−1 flow rate only), additional sterile soil particles were added to the water to create a slurry which was pumped onto the top of the soil boxes (Table 1). The E. coli and bromide tracer was inoculated into the flowing water (or slurry) at the top of the soil boxes. Two different E. coli isolates were used in the inocula: a laboratory strain (ATCC 25922) and a recent environmental isolate from cow feces (22). The lab isolate was always inoculated from a broth solution, and therefore the cells were not attached to soil particles in the inoculum. In contrast, the environmental isolate was used in the inoculum as both unattached cells and cells preattached to soil particles, as described below (Table 1).
TABLE 1.
Summary of treatments used in the runoff experiments
| Flow rate (ml s−1) | Slope (%) | Presence of additional soil in runoff water (slurry) | No. of expts performed with:
|
||
|---|---|---|---|---|---|
| Lab isolate | Environmental isolate
|
||||
| Unattached | Unattached | Preattached | |||
| 0.6 | 5 | No | 2 | 2 | 2 |
| 5 | Yes | 2 | 2 | 0 | |
| 15 | No | 2 | 2 | 2 | |
| 15 | Yes | 2 | 2 | 0 | |
| 2.0 | 5 | No | 2 | 2 | 2 |
| 15 | No | 2 | 2 | 2 | |
To prepare the unattached cell suspensions used in this study, the two isolates were revived from cryopreservation (Microbank; Pro-Lab Diagnostics, Richmond Hill, Canada) on tryptic soy agar (TSA) (Difco) plates before a single colony was inoculated into tryptic soy broth (Difco) and grown overnight for 16 to 18 h at 35°C to a concentration of approximately 109 cells ml−1. Overnight broths were diluted to approximately 107 cells ml−1 in a 10,000-ppm KBr solution. Suspensions of cells attached to soil particles (environmental isolate only) were also prepared using an up-flow reactor as described below.
One local silt loam soil was used in this study, the Waikiwi soil (NZ classification, typic firm brown soil) (15). The soil boxes, made of stainless steel, were 30 cm long, 5 cm wide, and 6 cm deep. One end had a collection trough recessed 1 cm from the top for collecting the runoff. The soil was packed into the boxes to a depth of 5 cm before each entire box was sterilized by autoclaving at 121°C for 25 min. The reverse osmosis (RO) water was pumped onto the soil surface at the upper end of the soil box by using a variable-speed peristaltic pump until the soil boxes had filled with water and runoff occurred. For the experiments where additional soil particles (slurry) were added to the RO water, 10 g (wet weight) of sterile soil was added to 1 liter of RO water and mixed in a blender (KB290; Kambrook, Oakleigh, Victoria, Australia) on low for 1 min. The resulting soil slurry was kept in suspension with a mechanical stirrer before being pumped onto the top of the soil boxes.
Once runoff began, flushing of water through the soil boxes was continued for an additional 20 to 30 min prior to the addition of the bromide tracer and bacteria in the inoculum. A 1-ml inoculum of the suspension was pipetted into the flowing water at the top of the soil box, and the runoff water was sampled immediately and at subsequent time intervals. For each experiment, a total of 15 runoff water samples were collected, as a composite sample of either 10, 15, or 20 seconds in duration in a series of increasing time intervals, so that all of the runoff from the first minute was collected. Thereafter, sampling intervals were gradually increased until the last sample was collected after 20 min. For each runoff water sample, the E. coli in a 5-ml subsample was separated into attached and unattached fractions by using a Nycodenz buoyant-density centrifugation technique previously described (21). Briefly, a sample of bacteria and soil particles was centrifuged on a Nycodenz cushion (density of 1.3 g ml−1) and any bacteria recovered from below the cushion was deemed to have been attached to a dense particle (21). The number of E. coli cells in each fraction was estimated using the drop plate technique (2) on TSA. The bromide concentration in the sample was determined using a selective ion probe (model no. 9435BN; Orion Research Incorporated, Boston, Mass.).
For each experiment, the E. coli concentration in the inoculum was determined by the drop plate technique on TSA plates. For experiments which used E. coli that was preattached to soil particles in the inoculum, the attached and unattached fractions were estimated as described in “Up-flow reactor” below.
Attachment of E. coli to soil particles in a static system.
Waikiwi soil was dried, sieved through a 2-mm sieve, rewet to approximately 60% moisture, and sterilized by autoclaving at 121°C for 15 min. Overnight broths of the E. coli were diluted in sterile water to approximately 107 cells ml−1, and 0.1 ml of the resulting solution was then inoculated into 0.5 g of sterile soil and mixed by tapping the tube on a bench until the soil had absorbed the droplet. After 30 min of contact time, the attached and unattached fractions of E. coli in the soil were separated by the buoyant-density separation technique and the percentage of cells in each of the two fractions was estimated using the drop plate technique.
Up-flow reactor.
To generate samples in which a consistently high percentage of E. coli cells was attached to large soil particles, an up-flow reactor was developed. This consisted of a 10-ml disposable pipette tip held upright in a lab clamp with the large opening at the top. The reactor was half filled with sterile soil, and an overnight culture (tryptic soy broth) of the E. coli isolate (approximately 109 cells ml−1) was added to the base of the reactor (pipette tip) at a flow rate of 0.25 ml s−1, with the excess culture overflowing to waste at the top of the reactor. This resulted in a vertical velocity at the top of the reactor of 1.6 mm s−1. Only particles with a settling rate greater than 1.6 mm s−1 remained in the reactor, corresponding to a soil particle size greater than 45 μm in diameter (27). After 15 to 20 min of up-flowing, the culture was replaced with a solution of 10,000 ppm KBr for a further 20 min. During this time, unattached E. coli cells were flushed out of the reactor. The KBr solution was added to create a sample that contained both attached cells and bromide tracer for use in subsequent runoff experiments. Duplicate 1-ml subsamples were collected from the up-flow reactor, and the numbers of cells in the attached and unattached fractions were determined as previously described.
Data analysis.
To allow for differences in the E. coli concentrations in the inocula, both the E. coli and bromide concentrations measured in the runoff (Cr) were normalized by dividing these values by the concentrations in the inoculum (Ci). Summary data of each experiment were calculated from the bromide and E. coli breakthrough curves by determining the time and normalized concentration (Cr/Ci) of the peak of each breakthrough curve. For each individual runoff sample from each experiment, the relative bacterium-to-bromide (RB) ratio was calculated by dividing the E. coli concentration (Cr/Ci) by the bromide concentration (Cr/Ci) as previously described (14). The RB ratio indicates the proportion of the bacteria transported relative to a conservative tracer which allows for dispersion and dilution of the breakthrough curves, which vary between experiments. The RB summary data for each experiment were calculated as the average RB ratio for five data points centered on the peak of the E. coli breakthrough curve. The summarized data were analyzed by comparing contrasting batches of experiments with analysis of variance using GENSTAT version 7.
RESULTS
Runoff experiments.
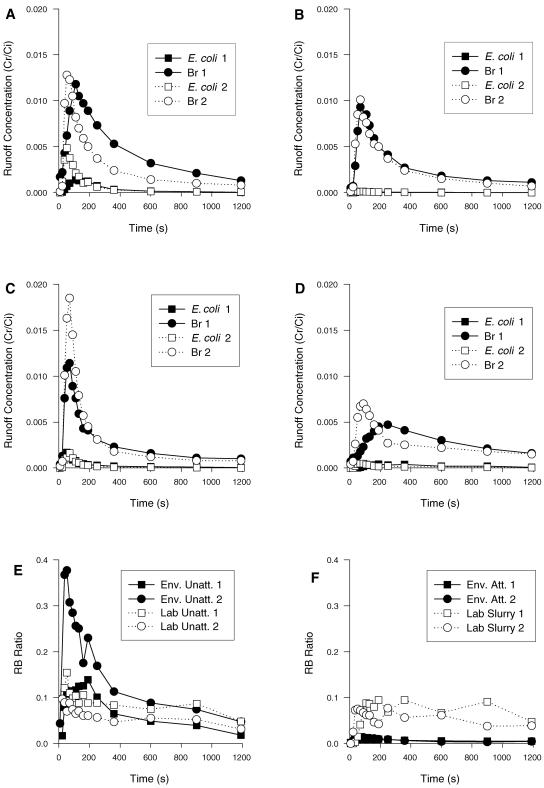
Typical breakthrough curves obtained from the runoff experiments are shown in Fig. 1. While the concentration of the environmental isolate in the runoff was less than the bromide concentration, the time of the arrival of the peak appeared to be the same (Fig. 1A). This suggests that unattached cells were being transported by the same mechanism as bromide (i.e., advection). In contrast, the preattached cells of the environmental isolate (from the up-flow reactor) had very low concentrations compared to the bromide although the peak times of arrival were similar (Fig. 1B). Experiments with the lab isolate showed that the concentration of E. coli was again less than that of bromide and that E. coli concentrations appeared to be intermediate between the unattached and preattached environmental isolate (Fig. 1C). When additional soil particles (slurry) were added to the flowing water, there was a delay in the arrival of the peak concentrations for both E. coli and bromide indicating that the water flow was slower, although for each individual experiment, E. coli and bromide peaks still coincided (Fig. 1D). When the RB ratios were calculated for all experiments, the RB ratio was always less than 1 and showed a pattern of peak RB ratio at the same time as E. coli breakthrough and tapering off (Fig. 1E and F). On average, the RB ratio for the environmental isolate was greater than the RB ratio for the lab isolate (Fig. 1E). In contrast, the RB ratio for the preattached environmental isolate was much smaller than that observed for the unattached isolates (Fig. 1E and F). Despite the delay in the arrival of peaks for the unattached cells transported in the slurry, there appeared to be no difference in the RB ratio compared to that for the unattached cells transported in water (Fig. 1E and F).
FIG. 1.
E. coli and bromide breakthrough curves from selected runoff experiments conducted at a flow rate of 0.6 ml s−1 and slope of 5%. (A) Unattached environmental isolate transported in water; (B) environmental isolate preattached to soil particles transported in water; (C) unattached lab isolate transported in water; (D) unattached lab isolate transported in the slurry; (E) RB ratios plotted against time for the breakthrough curves from panels A and C; (F) RB ratios plotted against time for the breakthrough curves from panels B and D. Env. Unatt., unattached environmental isolate; Lab Unatt., unattached lab isolate; Env. Att., preattached environmental isolate.
For all experiments, the timing of breakthrough curves for E. coli and bromide coincided. A regression line for the relationship between the times of arrival of the bromide and E. coli peak concentrations on a log-log graph had a slope of 0.98 and an R2 of 0.99, which was statistically significant (P < 0.0001) (data not shown). The mean percentages of E. coli cells attached to soil particles in runoff ranged from 18 to 50%, with an overall mean of 32% (data not shown). There were no significant differences in the percentages of cells attached in runoff between any of the variables tested. The addition of extra soil particles to the model system (slurry) did not increase the percentage of E. coli cells attached in runoff. Also, there were no trends between percentage attached and time through the breakthrough curve. In light of this result, all further statistical analysis of the E. coli data from the runoff experiments was conducted on the total E. coli concentration (attached plus unattached fractions).
Overall, there were no significant differences between the different slopes and/or flow rates used in the experiments. This suggests that, for the model system used in this study, these variables have little effect on E. coli or bromide transport in the runoff.
Comparison of summary data from the preattached versus unattached environmental isolates showed no significant difference between the times or concentrations of bromide peaks or the times (only) of the E. coli peaks. This illustrated that the pattern of water flow was relatively consistent between experiments. However, there was a significant difference between the E. coli peak concentrations (P < 0.001) and the RB ratios (P < 0.001). The mean peak E. coli concentrations and RB ratios were 0.0005 and 0.05, respectively, for the preattached inocula and 0.003 and 0.32, respectively, for the unattached inocula. These results support the hypothesis that bacterial cells attached to large soil particles are transported less than unattached cells. The patterns of both the peak concentrations and the RB ratios showed that the preattached cells were transported at a rate of 16% (0.05/0.32) of that of the unattached cells.
There was a significant difference (P < 0.01) between RB ratios calculated for lab (0.17) and environmental (0.32) isolates. This indicated that the environmental isolate was attenuated less than the lab isolate. However, there was no significant difference between the times or peak concentrations for either the bromide or the E. coli breakthrough curves. This highlights the additional power of the RB ratio analysis, as it can remove some of the variability between individual experiments. A good illustration of this is shown in Fig. 1D, where the breakthrough curves of the duplicate experiments had distinctly different shapes but the RB ratios were similar (Fig. 1F).
Addition of extra soil particles to the flowing water (slurry) slowed the transport of both bromide and bacteria compared to their transport in water (Fig. 1D). This time delay resulted in increased dispersion, which reduced peak concentrations. Statistically, there was a significant difference (P < 0.05) between the times and peak concentrations for both the bromide and the E. coli breakthrough curves. This effect is most likely caused by the additional soil particles blocking soil pores and causing water to follow a more torturous pathway. Visually, the cloudiness of the runoff samples was much less apparent than that of the input slurry, indicating that most of the added soil particles had been trapped in the soil boxes. Despite this delayed transport through the model system, there was no significant difference between the RB ratios for the experiments using water or slurry as the transport medium (Fig. 1). Therefore, the transport of the cells was not affected by additional soil particles, indicating that the cells were not attaching to large soil particles in the water flow. This was further supported by analysis for attached E. coli cells in the runoff: samples from the slurry experiments consistently produced a larger “pellet” after centrifugation, indicating that there were more soil particles in the runoff from the slurry experiments than in the runoff from the water experiments. However, the percentages of E. coli cells attached to these particles were similar in both matrices.
Attachment of E. coli to soil particles in a static system.
In a static system, the two E. coli isolates consistently showed contrasting abilities to attach to the Waikiwi soil. Analysis of six batches of experiments conducted with the lab isolate found a mean attachment of 24%. In contrast, analysis of 11 batches of experiments with the environmental isolate resulted in a mean attachment of 81%. The difference between these means was statistically significant (P < 0.001).
Up-flow reactor.
The up-flow reactor was used to generate inocula for runoff experiments where bacteria would be consistently attached to soil particles in high numbers in a solution of 10,000 ppm KBr. Three duplicate analyses of the lab isolate found that 79% of the cells attached and that the total E. coli numbers in the reactors ranged from 4 × 106 to 1 × 107 CFU ml−1. In contrast, the environmental isolate consistently produced greater cell concentrations in the reactor, showing that more cells were retained, and this was reflected in a higher percentage of cells attached to particles. Twelve duplicate analyses of the environmental isolate found that 95% of the cells were attached to particles and that the total E. coli numbers in the reactors ranged from 1.7 × 107 to 7.7 × 107 CFU ml−1.
DISCUSSION
Previously published studies on the transport of bacteria through soil have focused on the vertical movement of bacteria through soil columns. The key findings of these soil column experiments have been that the breakthrough curves for bacteria are very different from those of a conservative chemical tracer, thereby implying that different transport mechanisms are involved (19, 20, 26). In our studies of the horizontal transport of E. coli, the most striking result is the very similar shapes and timings of the E. coli and bromide breakthrough curves. This finding suggests that the bacteria and bromide are transported by similar mechanisms. In our study, water ran through the small soil boxes in order to simulate saturation-excess overland flow prior to the addition of the E. coli-bromide mixture into the flowing system. It is likely that a large proportion of the inoculum moved rapidly over the soil surface, with little interaction with the soil matrix (1). This rapid movement is reflected in the short arrival time of breakthrough, the peak taking as little as 15 seconds to arrive at the higher flow rate of 2 ml s−1, equivalent to an average velocity of 20 mm s−1.
The strong similarity between the E. coli and bromide breakthrough curves enabled us to use the RB ratio (14) to remove some of the variability between different soil boxes and increase the power of our analysis. If E. coli were transported in a manner identical to bromide, then the RB ratio plotted against time would be a flat line with a value of 1. Our results showed that the RB ratio was always less than 1 and tended to decrease on the tail of breakthrough curves. This indicated that E. coli was retained more than bromide and that the bromide concentrations remained higher on the tail of the breakthrough curves (Fig. 1). These observations are consistent with the bromide diffusing into and out of the soil matrix while any E. coli that moved into the soil matrix was likely to remain trapped (23, 25).
Comparison of the preattached (from the up-flow reactor) and unattached inocula of the environmental isolate showed that there was a significant difference between the peak E. coli concentration and the RB ratio. These data show that cells preattached to large soil particles were trapped in soil boxes. While many researchers have proposed that the attachment of bacteria to soil particles decreases cell transport (10, 16, 28, 29), we believe that the current study is the first to demonstrate this experimentally. There were two interesting observations of the transport of preattached cells: (i) a comparison of the RB ratios and (ii) the timing of the breakthrough curve peak. If we assume that only 5% of E. coli cells in the preattached inocula were unattached, then we would expect a similar proportion of the E. coli cells to be transported in the overland flow and collected in the runoff. However, comparing the RB ratios of the preattached versus unattached inocula found that the measured difference in transport was 16%, indicating that more preattached E. coli cells were transported than expected. It is likely that some of the E. coli cells were only weakly bound and therefore disassociated from the large particles during transport; this is supported by the analysis that showed that a large proportion of E. coli cells in the runoff was unattached. Surprisingly, for the environmental isolate, there was no significant difference in percentage of cells attached to particles in the runoff between preattached and unattached treatments. Scanning electron microscope analysis (data not shown) of samples of the attached fraction from the runoff samples revealed that the E. coli cells were associated predominantly with particles smaller than the bacteria. While these small particles (with bacteria attached) were dense enough to move through the Nycodenz layer that was used to separate the attached and unattached bacteria, it appears these small particles did not affect E. coli transport under the runoff conditions generated in this study (11).
It could be argued that our model system would have produced less erosion of soil particles into runoff than a more typical raindrop impact-driven system (7). We therefore conducted experiments with additional soil particles (slurry) added into the runoff system to test this. However, when the RB ratios were compared, there was no significant difference between the slurry and water runoff experiments. There was also no significant difference between the percentages of E. coli attached to particles in runoff. This is despite the large increase in the number of soil particles transported in slurry samples. This result would imply that the bacteria are predominantly interacting with and attaching to small soil particles (fines) that are also being carried along in the flowing water. If the E. coli cells being transported in the slurry had attached to large soil particles, they would have been removed from the runoff (as in the preattached experiments) and the resulting RB ratio would have been smaller. This also supports our hypothesis that, in runoff generated by saturation-excess conditions, E. coli cells are transported in overland flow where the bacteria interact predominantly with small soil particles in the flow rather than with large particles in the soil matrix.
When the rates of transport of the two E. coli isolates were compared, there was a significant difference in the RB ratios. In studies of the transport of bacteria through soil columns by using 19 different isolates, it was found that there were “markedly different degrees of transport,” with variability both between and within species (13). Our observation of different RB ratios (0.32 versus 0.17) was therefore not unexpected. Since the lab isolate was transported to a lesser degree than the environmental isolate, it is tempting to conclude that the lab isolate had a greater tendency to attach to soil particles. However, this conclusion is not supported by other observations. First, if the lab isolate had a greater attachment to soil particles that reduced its transport, then we would also expect to see greater attachment to the small soil particles in runoff. Second, the results from runoff experiments contradict our results from the static attachment test and up-flow reactor study. This highlights the need for caution when comparing the behavior of bacteria in a static system with that in a flowing system.
Using our model runoff system, we have demonstrated that E. coli cells attached to large soil particles are transported less than unattached cells. However, in flowing systems it appears that the attachment of E. coli cells to soil particles may not have a large effect on their overall transport since (i) under saturation-excess runoff conditions, E. coli cells appear to be rapidly transported by advection with the water flow and have little opportunity to interact with the soil matrix and (ii) attachment of E. coli in the overland flow was predominantly to small soil particles that did not settle.
This work from a laboratory-based study provides direction for designing experiments investigating the transport of bacteria on a larger scale. The implication that arises from the results is that bacteria moving in overland flow are unlikely to be removed by attachment to soil particles. Techniques to enhance the removal of E. coli from runoff therefore need to focus on increasing infiltration of the bacteria into the soil matrix. This approach appears to have a greater potential to trap bacteria.
Acknowledgments
This work was funded in part by the New Zealand government through the Foundation for Research, Science and Technology, grant number C10X0320. Richard Muirhead received a Ph.D. scholarship from the University of Otago.
Thanks to Roger Littlejohn of AgResearch for the statistical analysis.
REFERENCES
- 1.Ahuja, L. R., A. N. Sharpley, M. Yamamoto, and R. G. Menzel. 1981. The depth of rainfall-runoff-soil interaction as determined by 32P. Water Resour. Res. 17:969-974. [Google Scholar]
- 2.Barboas, H. R., M. F. A. Rodrigues, C. C. Campos, M. E. Chaves, I. Nunes, Y. Juliano, and N. F. Novo. 1995. Counting of viable cluster-forming and non cluster-forming bacteria: a comparison between the drop and spread plate methods. J. Microbiol. Methods 22:39-50. [Google Scholar]
- 3.Burns, D. A., and L. Nguyen. 2002. Nitrate movement and removal along a shallow groundwater flow path in a riparian wetland within a sheep-grazed pastoral catchment: results of a tracer study. N. Z. J. Mar. Freshw. Res. 36:371-385. [Google Scholar]
- 4.Collins, R., A. Donnison, C. Ross, and M. McLeod. 2004. Attenuation of effluent-derived faecal microbes in grass buffer strips. N. Z. J. Agric. Res. 47:565-574. [Google Scholar]
- 5.Crane, S. R., J. A. Moore, M. E. Grismer, and J. R. Miner. 1983. Bacterial pollution from agricultural sources: a review. Trans. Am. Soc. Agric. Eng. 26:858-866. [Google Scholar]
- 6.Davies, C. M., and H. J. Bavor. 2000. The fate of stormwater-associated bacteria in constructed wetland and water pollution control pond systems. J. Appl. Microbiol. 89:349-360. [DOI] [PubMed] [Google Scholar]
- 7.Davies, C. M., C. M. Ferguson, C. Kaucner, M. Krogh, N. Altavilla, D. A. Deere, and N. J. Ashbolt. 2004. Dispersion and transport of Cryptosporidium oocysts from fecal pats under simulated rainfall events. Appl. Environ. Microbiol. 70:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droppo, I. G., and E. D. Ongley. 1994. Flocculation of suspended sediment in rivers of southeastern Canada. Water Res. 28:1799-1809. [Google Scholar]
- 9.Entry, J. A., R. K. Hubbard, J. E. Thies, and J. J. Fuhrmann. 2000. The influence of vegetation in riparian filterstrips on coliform bacteria. I. Movement and survival in water. J. Environ. Qual. 29:1206-1214. [Google Scholar]
- 10.Ferguson, C., A. M. de Roda Husman, N. Altavilla, D. Deere, and N. Ashbolt. 2003. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Tech. 33:299-361. [Google Scholar]
- 11.Fiener, P., and K. Auerswald. 2003. Effectiveness of grassed waterways in reducing runoff and sediment delivery from agricultural watersheds. J. Environ. Qual. 32:927-936. [DOI] [PubMed] [Google Scholar]
- 12.Gannon, J. J., M. K. Busse, and J. E. Schillinger. 1983. Fecal coliform disappearance in a river impoundment. Water Res. 17:1595-1601. [Google Scholar]
- 13.Gannon, J. T., U. Mingelgrin, M. Alexander, and R. J. Wagenet. 1991. Bacterial transport through homogeneous soil. Soil Biol. Biochem. 23:1155-1160. [Google Scholar]
- 14.Harvey, R. W., and S. P. Garabedian. 1991. Use of colloid filtration theory in modelling movement of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 25:178-185. [Google Scholar]
- 15.Hewitt, A. E. 1998. New Zealand soil classification, 2nd ed. Manaaki Whenua Press, Lincoln, New Zealand.
- 16.Jamieson, R., R. Gordon, D. Joy, and H. Lee. 2004. Assessing microbial pollution of rural surface waters: a review of current watershed scale modeling approaches. Agric. Water Manag. 70:1-17. [Google Scholar]
- 17.Mahler, B. J., J.-C. Personne, G. F. Lods, and C. Drogue. 2000. Transport of free and particulate-associated bacteria in karst. J. Hydrol. 238:179-193. [Google Scholar]
- 18.Mawdsley, J. L., R. D. Bardgett, R. J. Merry, B. F. Pain, and M. K. Theodorou. 1995. Pathogens in livestock waste, their potential for movement through soil and environmental pollution. Appl. Soil Ecol. 2:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLeod, M., J. Aislabie, J. Ryburn, A. McGill, and M. Taylor. 2003. Microbial and chemical tracer movement through two Southland soils, New Zealand. Aust. J. Soil Res. 41:1163-1169. [Google Scholar]
- 20.McLeod, M., J. Aislabie, J. Ryburn, and A. McGill. 2004. Microbial and chemical tracer movement through granular, ultic and recent soils. N. Z. J. Agric. Res. 47:557-563. [Google Scholar]
- 21.Muirhead, R. W., R. P. Collins, and P. J. Bremer. 2005. Erosion and subsequent transport state of Escherichia coli from cowpats. Appl. Environ. Microbiol. 71:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muirhead, R. W., R. P. Littlejohn, and P. J. Bremer. 2004. Evaluation of the effectiveness of a commercially available defined substrate medium and enumeration system for measuring Escherichia coli numbers in faeces and soil samples. Lett. Appl. Microbiol. 39:383-387. [DOI] [PubMed] [Google Scholar]
- 23.Scheibe, T. D., and B. D. Wood. 2003. A particle-based model of size or anion exclusion with application to microbial transport in porous media. Water Resour. Res. 39:3.1-3.10. [Google Scholar]
- 24.Schillinger, J. E., and J. J. Gannon. 1985. Bacterial adsorption and suspended particles in urban stormwater. J. Water Pollut. Control Fed. 57:384-389. [Google Scholar]
- 25.Sirivithayapakorn, S., and A. Keller. 2003. Transport of colloids in saturated porous media: a pore-scale observation of the size exclusion effect and colloid acceleration. Water Resour. Res. 39:11.1-11.11. [Google Scholar]
- 26.Smith, M. S., G. W. Thomas, R. E. White, and D. Ritonga. 1985. Transport of Escherichia coli through intact and disturbed soil columns. J. Environ. Qual. 14:87-91. [Google Scholar]
- 27.Tanner, C. B., and M. L. Jackson. 1947. Nomographs of sedimentation times for soil particles under gravity or centrifugal acceleration. Proc. Soil Sci. Soc. Am. 12:60-65. [Google Scholar]
- 28.Tyrrel, S. F., and J. N. Quinton. 2003. Overland flow transport of pathogens from agricultural land receiving faecal wastes. J. Appl. Microbiol. 94:87s-93s. [DOI] [PubMed] [Google Scholar]
- 29.Unc, A., and M. J. Goss. 2004. Transport of bacteria from manure and protection of water resources. Appl. Soil Ecol. 25:1-18. [Google Scholar]