Abstract
In previous studies workers determined that two lactic acid bacterium isolates, Lactococcus lactis subsp. lactis C-1-92 and Enterococcus durans 152 (competitive-exclusion bacteria [CE]), which were originally obtained from biofilms in floor drains, are bactericidal to Listeria monocytogenes or inhibit the growth of L. monocytogenes both in vitro and in biofilms at 4 to 37°C. We evaluated the efficacy of these isolates for reducing Listeria spp. contamination of floor drains of a plant in which fresh poultry is processed. Baseline assays revealed that the mean numbers of Listeria sp. cells in floor drains sampled on six different dates (at approximately biweekly intervals) were 7.5 log10 CFU/100 cm2 for drain 8, 4.9 log10 CFU/100 cm2 for drain 3, 4.4 log10 CFU/100 cm2 for drain 2, 4.1 log10 CFU/100 cm2 for drain 4, 3.7 log10 CFU/100 cm2 for drain 1, and 3.6 log10 CFU/100 cm2 for drain 6. The drains were then treated with 107 CE/ml in an enzyme-foam-based cleaning agent four times in 1 week and twice a week for the following 3 weeks. In samples collected 1 week after CE treatments were applied Listeria sp. cells were not detectable (samples were negative as determined by selective enrichment culture) for drains 4 and 6 (reductions of 4.1 and 3.6 log10 CFU/100 cm2, respectively), and the mean numbers of Listeria sp. cells were 3.7 log10 CFU/100 cm2 for drain 8 (a reduction of 3.8 log10 CFU/100 cm2), <1.7 log10 CFU/100 cm2 for drain 1 (detectable only by selective enrichment culture; a reduction of 3.3 log10 CFU/100 cm2), and 2.6 log10 CFU/100 cm2 for drain 3 (a reduction of 2.3 log10 CFU/100 cm2). However, the aerobic plate counts for samples collected from floor drains before, during, and after CE treatment remained approximately the same. The results indicate that application of the two CE can greatly reduce the number of Listeria sp. cells in floor drains at 3 to 26°C in a facility in which fresh poultry is processed.
Controlling the widely distributed psychrotrophic organism Listeria monocytogenes in food processing facilities has been a formidable challenge for the entire food industry, from the smallest food processor to the largest. Besides this pathogen's widespread occurrence in nature, it is nonfastidious, grows at refrigeration temperatures, and can form or coexist in protective biofilms (1, 2, 7, 15, 16). Floor drains in food processing facilities are a particularly important niche for the persistence of listeriae and can be a point of contamination in the processing plant environment and possibly in food products.
Decontaminating floor drains of Listeria sp. is especially challenging because when entrapped in a biofilm, Listeria sp. is afforded unusual protection against disinfectants and treatments available to control pathogens on environmental surfaces (7, 9, 14, 17, 18). Once attached, the cells may produce multicellular biofilms that are resistant to disinfection and from which cells can become detached and contaminate food products. The establishment of biofilms by pathogenic bacteria in floor drains in food processing plants is believed to protect against effective cleaning regimens and to reduce or minimize the efficacy of bactericidal treatments. Studies have indicated that L. monocytogenes growing within mixed-microflora biofilms in food processing environments can be a major source of contamination (1, 9, 20).
Promising in vitro results have been obtained in previous studies with two competitive-exclusion bacteria (CE), Lactococcus lactis subsp. lactis C-1-92 and Enterococcus durans 152, for inhibition of the growth of L. monocytogenes in culture media and in biofilms at temperatures ranging from 4 to 37°C (22). The objective of this study was to evaluate the efficacy of these CE in controlling listerial growth and possibly eliminating the pathogen in floor drains at a wide range of temperatures in a facility in which fresh poultry is processed, including under refrigeration conditions.
MATERIALS AND METHODS
Processing plant.
The poultry processing plant had two processing lines; each line processed 140 birds per min, and the plant had the capacity to process 400,200 birds per day. Approximately 268,800 birds were processed during a normal day with two shifts.
Floor drain selection.
Based on the direction of flow of the drains, the fluid flow rate, the fluid volume, the drain size, the temperatures of rooms, and the occurrence and persistence of Listeria sp., six floor drains for CE treatment and an untreated control were identified. Drains 1 and 2 were open trenches (height, 25 cm; width, 30 cm) and were comprised of concrete with fiberglass covers. These drains were located close to poultry meat cutting lines at mean temperatures of 16.8 and 11.5°C, respectively, and largely contained liquid drippings from meat, including meat that had fallen on the floor. Drains 1 and 2 were typically filled to 10 to 30% of capacity during operation. Drain 3 was an open trench (height, 25 cm; width, 30 cm) comprised of concrete with metal edging and a metal cover. This drain was located in the middle of a high-traffic area at a mean temperature of 15.1°C and was typically 20 to 40% full during operation. Drains 4 and 6 were open trenches (height, 20 cm; width, 30 cm) and were comprised of fiberglass. They were located near poultry meat packing areas at mean temperatures of 2.8 and 3.8°C, respectively, and were typically filled to 5 to 20% of capacity during operation. Drain 8 was an open trench (height, 150 cm; width, 90 cm) and was comprised of concrete with a fiberglass cover. This drain was near a liver and lung removal line at a mean temperature of 26.1°C and contained blood and meat debris. It was typically filled with liquid to 15 to 20% of capacity.
Sampling.
Using sterile gloves, an 18-oz. sterile “speci-sponge” (3.8 by 7.6 cm; Nasco Laboratory, Fort Atkinson, WI) was used to wipe an area that was ca. 10 by 10 cm at each sampling location. Five locations, including the inside (bottom) of the drain, the right side of the drain, the left side of the drain, the bottom side of the trench cover, and the floor within 30 cm of the drain, were sampled for each floor drain. Each sponge was placed in a Whirl-Pak bag and kept at 5°C for 2 to 14 h until the sample was assayed.
Enumeration of Listeria sp. and L. monocytogenes.
Ten milliliters of 0.1% peptone was added to each bag, and the sponge and peptone were blended with a stomacher blender (Seward Medical, London, United Kingdom) at 150 rpm for 1 min. The fluid was serially diluted (1:10) in 0.1% peptone (Becton Dickinson Microbiology Systems, Sparks, MD), and 0.1 ml from each dilution tube was plated in duplicate on the surface of modified Oxford medium (MOX) (Oxoid, Ogdensburg, NY) plates. When Listeria sp. was not detected by the direct plating method, the broth plus the sponge was selectively enriched in 225 ml Fraser broth (Becton Dickinson Microbiology Systems) for 24 h at 37°C. Then 1 ml of the broth was serially diluted (1:10) in 0.1% peptone to obtain 10−8 CFU/ml, and 0.1 ml of each dilution was surface plated on MOX plates in duplicate. The MOX plates were incubated at 37°C for 48 h, and typical black colonies were counted as presumptive Listeria sp. colonies. Colonies counted as Listeria sp. colonies were randomly selected and transferred to MOX plates, and they were confirmed to be Listeria sp. by biochemical tests (API 20I miniaturized diagnostic test; bioMérieux Vitek, Hazelwood, MO) and a lateral flow latex agglutination assay (Listeria Rapid Test; Oxoid, Ogdensburg, NY) and to be L. monocytogenes by an enzyme-linked fluorescent immunoassay with an automated VIDAS instrument (miniVIDAS; bioMérieux Vitek, Hazelwood, MO) and by a PCR assay (Qualicon BAX system; DuPont, Wilmington, DE).
Determination of aerobic plate counts (APCs).
Serial dilutions (duplicate 0.1-ml portions of each dilution) of the samples described above were surface plated on plate count agar plates (Becton Dickinson Microbiology Systems) and incubated at 30°C for 72 h for enumeration.
Preparation of competitive-exclusion bacteria.
Each CE strain, L. lactis subsp. lactis C-1-92 or E. durans 152, was grown individually in 250 ml of Lactobacillus MRS broth (Becton Dickinson Microbiology Systems) at 32°C for 24 h. Cells were sedimented by centrifugation at 10,000 × g for 20 min at 4°C. The bacteria were resuspended in 25 ml of MRS broth at a density of ca. 109 CFU/ml, serially diluted (1:10) in 0.1% peptone, and plated on MRS agar and tryptic soy agar in duplicate for bacterial counting.
Application of competitive-exclusion bacteria to floor drains.
The CE treatment was performed within 20 min after routine sanitation procedures in the plant were completed. Two enzyme-based cleaning products, DY-GEST I (Ecolab, St. Paul, MN) and DY-GEST II (Ecolab), were used to apply the CE treatment. This treatment was routinely used with the cleaning products as part of the plant's routine sanitation procedures; however, the drains were not included as part of the daily routine sanitation procedures. When used under pressure, the cleaning agents formed a foam that remained for about 15 min. The DY-GEST preparation was prepared by mixing in a bottle 20 ml of each product with 3.78 liters of tap water. The two CE isolates (25 ml each) were added to a tank foaming dispenser (model 925916; Lafferty Equipment Manufacturing Co., North Little Rock, AR), followed by the ca. 4 liters of diluted DY-GEST preparation. The tank foaming dispenser was connected to pressurized air, and foam was applied to each floor drain. Approximately 107 CE/ml in the foam formula was applied in order to cover all locations in the floor drain following sanitation four times during the first week of application (Monday through Thursday). After this, the CE treatment was applied twice a week (Tuesday and Thursday) for the following 3 weeks. Samples were collected once a week during the 5 weeks after the CE treatment was initiated. After this, samples were collected every other week or once a month for up to 18 weeks following the last CE treatment.
Statistical analysis.
The least-square means of Listeria sp. counts and APCs at each sampling location before and after CE treatment in each floor drain were determined using the general linear model of the Statistical Analyses System procedure (SAS Institute, Cary, NC). The values used for statistical analysis when bacteria were detected and not detected by selective enrichment in each floor drain were 1.6 and 0 log10 CFU/100 cm2, respectively. The criterion used for significance for differences was P < 0.05 for all assays.
RESULTS
Baseline determinations revealed that the mean Listeria sp. counts in floor drains sampled at six different times (2-week intervals) before treatment with CE were 7.5 log10 CFU/100 cm2 for drain 8, 4.9 log10 CFU/100 cm2 for drain 3, 4.4 log10 CFU/100 cm2 for drain 2, 4.1 log10 CFU/100 cm2 for drain 4, 3.7 log10 CFU/100 cm2 for drain 1, and 3.6 log10 CFU/100 cm2 for drain 6 (Table 1). This included Listeria sp. counts in drains after routine cleaning and sanitation treatment of processing equipment and facilities were performed (10-week sample) (Fig. 1 to 5). The results revealed that Listeria sp. counts for drain samples collected after plant sanitation treatments were not significantly different from Listeria sp. counts for drains before processing equipment and facilities were cleaned and sanitized.
TABLE 1.
Listeria sp. counts for floor drain samples collected before and after CE treatment
| Time | Location |
Listeria sp. count (log10 CFU/100 cm2) for floor drainsa |
|||||
|---|---|---|---|---|---|---|---|
| Drain 1 | Drain 3 | Drain 4 | Drain 6 | Drain 8 | Drain 2 (control)b | ||
| Before CE treatmentc | Bottom side of cover | 3.3 ± 0.8 | 4.2 ± 1.2 | 4.2 ± 1.1 | 4.2 ± 1.2 | 7.6 ± 1.1 | 4.2 ± 1.1 |
| Right side of drain | 3.9 ± 0.7 | 5.0 ± 0.9 | 4.3 ± 0.8 | 3.5 ± 1.9 | 7.6 ± 0.8 | 4.6 ± 0.7 | |
| Left side of drain | 4.0 ± 1.0 | 4.4 ± 1.3 | 3.4 ± 1.1 | 3.2 ± 1.5 | 8.2 ± 0.5 | 4.1 ± 0.6 | |
| Inside of drain | 3.5 ± 0.8 | 5.4 ± 1.3 | 4.5 ± 1.1 | 3.6 ± 1.3 | 7.8 ± 0.9 | 5.4 ± 1.4 | |
| Floor within ca. 30 cm of drain | 3.6 ± 0.9 | 5.3 ± 0.6 | 4.3 ± 1.4 | 3.3 ± 1.3 | 6.1 ± 1.9 | 3.8 ± 1.0 | |
| After CE treatmentd | Bottom side of cover | 1.8 ± 0.4e | 1.9 ± 0.3e | 1.9 ± 0.3e | 2.1 ± 0.4e | 4.0 ± 2.0e | 3.6 ± 0.3f |
| Right side of drain | 2.0 ± 0.5e | 2.1 ± 0.8e | 1.7 ± 0.1e | 1.9 ± 0.7e | 3.9 ± 2.0e | 4.0 ± 0.6f | |
| Left side of drain | 2.0 ± 0.6e | 2.3 ± 0.6e | 2.1 ± 1.2e | 1.9 ± 0.4e | 3.9 ± 2.2e | 4.6 ± 0.7f | |
| Inside of drain | 2.0 ± 0.5e | 2.8 ± 1.4e | 1.7 ± 0.1e | 2.0 ± 0.7e | 3.7 ± 1.8e | 5.5 ± 1.2f | |
| Floor within ca. 30 cm of drain | 1.9 ± 0.5e | 2.1 ± 0.6e | 2.1 ± 0.9e | 1.9 ± 0.6e | 3.5 ± 0.8e | 4.1 ± 1.5f | |
The temperatures at drains 1, 3, 4, 6, 8, and 2 were 16.8 ± 2.6, 15.1 ± 2.3, 2.8 ± 1.2, 3.8 ± 1.1, 26.1 ± 1.2, and 11.5 ± 2.7°C (means ± standard deviations), respectively.
This drain served as the control and was not treated with CE.
Each drain was sampled in four locations and assayed for Listeria sp. every 2 weeks for 10 weeks before CE treatment. The values are the means ± standard deviations for six samplings.
Each drain was sampled in four locations and assayed for Listeria sp. every week for 5 weeks following CE treatment. The values are the means ± standard deviations for five samplings.
The Listeria sp. counts in drains after CE treatment were significantly different (P < 0.05) than the Listeria sp. counts in drains before CE treatment.
The Listeria sp. counts in drains after CE treatment were not significantly different (P > 0.05) than the Listeria sp. counts in drains before CE treatment.
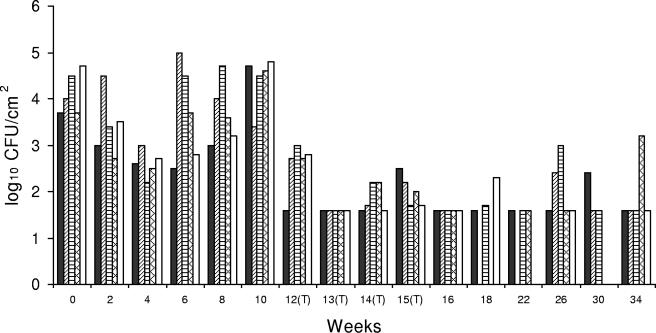
FIG. 1.
Listeria sp. counts in concrete, open-trench floor drain 1, which was located near poultry meat cutting lines and contained liquid drippings from meat, including meat that had fallen on the floor. The drain was typically filled to 10 to 30% of capacity during operation. ▪, bottom side of cover; ▨, right side of drain; ▤, left side of drain; ▩, inside of drain; □, floor within ca. 30 cm of the drain. (T), CE treatment applied.
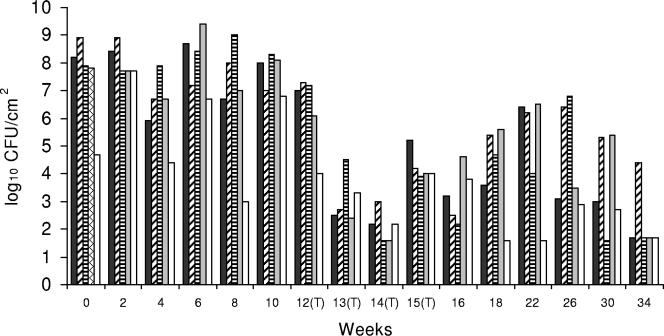
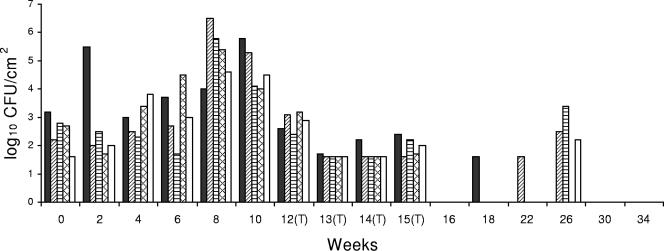
FIG. 5.
Listeria sp. counts in concrete, open-trench floor drain 8, which was located near a liver and lung removal line at a mean temperature of 26.1°C and contained blood and meat debris. This drain was typically filled with liquid to 15 to 20% of capacity. ▪, bottom side of cover; ▨, right side of drain; ▤, left side of drain; ▩, inside of drain; □, floor within ca. 30 cm of the drain. (T), CE treatment applied.
Drain 8 had considerably higher initial Listeria sp. counts than the other drains, and there was only a modest reduction in the Listeria sp. counts following the first week of treatment. However, Listeria sp. counts were dramatically reduced in all locations following the second and third weeks of treatment. The mean Listeria sp. count following CE treatment at weeks 12 to 16 was 3.8 log10 CFU/100 cm2 (Fig. 5), which indicated that there was a 3.7-log10 CFU/100 cm2 reduction after CE treatment (P < 0.05) (Table 1).
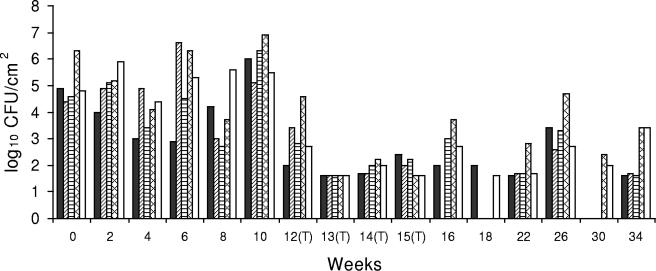
A significant reduction in Listeria sp. counts was also observed for drain 3 in the second week after CE treatment, and Listeria sp. was not detectable by a direct plating method at all sites. The mean Listeria sp. count at weeks 12 to 16 was 2.2 log10 CFU/100 cm2, which indicated that there was a 2.7-log10 CFU/100 cm2 reduction after the CE treatments (P < 0.05) (Table 1). However, the Listeria sp. counts in some locations of drain 3 were highly variable at later sampling times (Fig. 2).
FIG. 2.
Listeria sp. counts in concrete, open-trench floor drain 3, which was located in the middle of a high-traffic area and was typically filled to 20 to 40% of capacity during operation. ▪, bottom side of cover; ▨, right side of drain; ▤, left side of drain; ▩, inside of drain; □, floor within ca. 30 cm of the drain. (T), CE treatment applied.
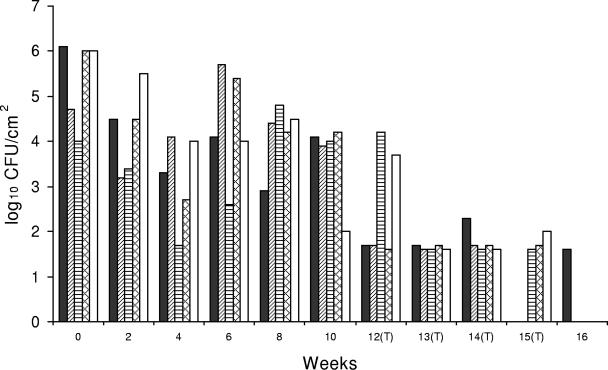
The Listeria sp. counts at most locations in drain 4 were reduced after CE treatment was initiated (first week), and the mean Listeria count was 1.9 log10 CFU/100 cm2, which indicated that there was a 2.2-log10 CFU/100 cm2 reduction after CE treatments (P < 0.05) (Table 1). One week following the conclusion of CE treatments (week 16) Listeria sp. was detectable (at the minimum level of detection) in only one drain location and was not detectable at the other three sampling sites (Fig. 3). Sampling in this drain was discontinued after this because of construction at this location of the plant.
FIG. 3.
Listeria sp. counts in fiberglass, open-trench floor drain 4, which was located near a poultry meat packing area and was typically filled to 5 to 20% of capacity during operation. ▪, bottom side of cover; ▨, right side of drain; ▤, left side of drain; ▩, inside of drain; □, floor within ca. 30 cm of the drain. (T), CE treatment applied.
At all sites in drain 1 the Listeria sp. counts were greatly reduced the second week following the sixth CE treatment, and Listeria sp. was undetectable by a direct plating method. The mean Listeria sp. count at weeks 12 (first week of CE treatments) to 16 (fourth week of CE treatments) was 1.9 log10 CFU/100 cm2 (Fig. 1), which indicated that there was a 1.8-log10 CFU/100 cm2 reduction after CE treatments (P < 0.05) (Table 1). This trend was consistent for most locations in drain 1 until the study was terminated (week 34) (Fig. 1).
The mean Listeria sp. count for drain 6 at weeks 12 to 16 was 2.0 log10 CFU/100 cm2 after CE treatment, which indicated that there was a 1.6-log10 CFU/100 cm2 reduction after CE treatment (P < 0.05) (Table 1). Following CE treatment either the Listeria sp. counts at most locations in the drain were substantially reduced or Listeria sp. was undetectable, and this response continued for at least 18 weeks after the last treatment (Fig. 4).
FIG. 4.
Listeria sp. counts in fiberglass, open-trench floor drain 6, which was located near a poultry meat packing area and was typically filled to 5 to 20% of capacity during operation. ▪, bottom side of cover; ▨, right side of drain; ▤, left side of drain; ▩, inside of drain; □, floor within ca. 30 cm of the drain. (T), CE treatment applied.
The listerial counts in control floor drain 2 that was not treated with CE were not significantly different throughout the study (P > 0.05) (Table 1). Most of the mean APCs for the drains before, during, and after CE treatment were not significantly different (P > 0.05) (Table 2); the few exceptions included drain 3 during CE treatment and drain 4 after the CE treatment regimen was completed.
TABLE 2.
Aerobic plate counts for drain samples collected before, during, and after CE treatment
| Time | Location | Aerobic plate count (log10 CFU/100 cm2) for drains |
||||
|---|---|---|---|---|---|---|
| Drain 1 | Drain 3 | Drain 4 | Drain 6 | Drain 8 | ||
| Before CE treatmenta | Bottom side of cover | 8.4 ± 1.0 | 8.3 ± 0.6 | 8.7 ± 1.0 | 9.1 ± 0.6 | 10.0 ± 0.8 |
| Right side of drain | 8.5 ± 0.5 | 8.6 ± 0.6 | 8.6 ± 0.3 | 9.3 ± 0.6 | 9.6 ± 0.4 | |
| Left side of drain | 8.7 ± 0.3 | 8.5 ± 0.6 | 8.3 ± 0.7 | 9.1 ± 0.2 | 9.6 ± 1.0 | |
| Inside of drain | 8.7 ± 0.8 | 9.4 ± 0.3 | 8.8 ± 0.6 | 8.4 ± 0.4 | 9.9 ± 0.4 | |
| Floor within ca. 30 cm of drain | 8.4 ± 0.7 | 9.0 ± 0.8 | 8.1 ± 1.2 | 9.5 ± 0.6 | 9.0 ± 0.7 | |
| During CE treatmentb | Bottom side of cover | 8.4 ± 0.9e | 7.3 ± 0.7d | 9.1 ± 0.6e | 9.9 ± 0.6e | 9.3 ± 0.4e |
| Right side of drain | 8.4 ± 1.4e | 8.0 ± 0.6e | 8.8 ± 0.9e | 9.5 ± 0.8e | 8.8 ± 0.5d | |
| Left side of drain | 8.0 ± 0.7e | 7.4 ± 0.7d | 8.5 ± 1.5e | 9.4 ± 0.8e | 9.1 ± 0.5e | |
| Inside of drain | 8.1 ± 0.8e | 7.4 ± 0.8d | 8.6 ± 0.7e | 9.0 ± 1.2e | 9.0 ± 0.9e | |
| Floor within ca. 30 cm of drain | 7.8 ± 0.3e | 8.1 ± 0.6d | 9.2 ± 0.5e | 9.2 ± 0.7e | 8.5 ± 0.4e | |
| After CE treatmentc | Bottom side of cover | 8.8 ± 1.1e | 8.0 ± 0.9e | 9.6 ± 0.5e | 8.2 ± 0.4d | 9.6 ± 1.3e |
| Right side of drain | 8.3 ± 1.3e | 8.5 ± 1.3e | 7.1 ± 0.3d | 8.7 ± 0.9e | 9.2 ± 1.4e | |
| Left side of drain | 8.9 ± 1.4e | 8.3 ± 1.6e | 6.7 ± 0.4d | 7.9 ± 0.9d | 8.2 ± 1.3d | |
| Inside of drain | 8.7 ± 1.5e | 8.2 ± 1.0d | 7.6 ± 0.5d | 8.4 ± 0.8e | 9.8 ± 0.7e | |
| Floor within ca. 30 cm of drain | 8.3 ± 1.3e | 9.0 ± 0.8e | 7.7 ± 0.2e | 8.4 ± 1.2d | 9.0 ± 1.6e | |
Each drain was sampled in four locations and assayed for APCs every 2 weeks for 10 weeks before CE treatment. The values are the means ± standard deviations for six samplings.
Each drain was sampled in four locations and assayed for APCs every week for 4 weeks during CE treatment. The values are the means ± standard deviations for four samplings.
Each drain was sampled in four locations and assayed for APCs six times at 2- to 4-week intervals for up to 19 weeks after the last CE treatment was applied. The values are the means ± standard deviations for six samplings.
The APCs were significantly different (P < 0.05) from the APCs before CE treatment.
The APCs were not significantly different (P > 0.05) from the APCs before CE treatment.
A total of 184 Listeria sp. isolates from drains were tested further for identification of L. monocytogenes. The results revealed that 89 of the 184 isolates (48.4%) were L. monocytogenes isolates.
DISCUSSION
Our results revealed that the Listeria sp. counts in all five drains in rooms at different temperatures were substantially reduced following CE treatment. The efficacy of the treatment appeared to be influenced by room temperature, foot traffic, the composition of the drain material, and the amount of fluid flowing through the drain. The greatest reduction in Listeria sp. counts occurred in drain 6 (>3.6-log10 CFU/100 cm2 reduction to an undetectable level at week 16), which was comprised of a fiberglass material, was located in a low-temperature room (3.8°C), usually contained less than 20% liquid, and was not in a high-traffic area. Substantial reductions in Listeria sp. counts occurred within 1 week after only four CE treatments were applied. In contrast, drain 8, which was in a warm room (mean temperature, 26.1°C) and a dirty environment with frequent inflow of blood and poultry debris and which was comprised of concrete, required ca. 3 weeks of CE treatments until there was a substantial reduction (3.7 log10 CFU/100 cm2) in the Listeria sp. counts.
We used an enzyme-foam-based cleaner to apply the CE to the drains. The advantage of the foam cleaner was that it provided more exposure time (minimum, 15 min) for CE to be in contact with the drain surface. Data obtained from inoculation studies revealed that CE strains survived well in the foam cleaner and that there was no change in CE counts during 24 h of exposure (data not shown).
Our results revealed that application of the two CE strains in the floor drains did not substantially change the total countable bacterial population during or after the treatment compared with the APCs of samples collected before CE treatment. However in some locations (especially drains 3 and 4) the APCs were less during and after treatment than before treatment. CE may have colonized the drains and not only reduced Listeria sp. populations but also subsequently formed their own biofilms and controlled bacterial populations that were sensitive to the antagonistic metabolites of the CE (1, 2, 6, 19).
The mechanism by which the CE reduce Listeria sp. counts has not been elucidated; however, we have previously reported that both CE isolates produce antimicrobials that are active against L. monocytogenes (22). L. lactis subsp. lactis C-1-92 possesses genes for nisin expression. Nisin is approved as a food preservative in many countries and has antimicrobial activity against L. monocytogenes. Direct use of nisin in food products for control of Listeria may increase the likelihood of Listeria adaptation to nisin and subsequent tolerance to this bacteriocin. Although there is a possibility that listeriae in floor drains could develop tolerance to nisin following repeated exposure to L. lactis C-1-92, the likelihood is reduced by the inclusion of E. durans 152, which produces a different antimicrobial(s) than nisin (22).
The antimicrobial activity of lactic acid bacteria and their metabolites against L. monocytogenes has been well documented (3-5, 8, 10-13, 21). However, the application of these bacteria to floor drains in food processing facilities to control L. monocytogenes has not been reported previously. Our study revealed that application of two CE isolates can greatly reduce the numbers of Listeria sp. and L. monocytogenes cells in floor drains at 3 to 26°C in a facility in which fresh poultry is processed.
Acknowledgments
We thank Kristen Bray, Rhonda Howell, and Kellie McKoon for their technical assistance.
This study was supported in part by grants from the State of Georgia's Traditional Industries Program for Food Processing and the American Meat Institute Foundation.
REFERENCES
- 1.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton, J. W., P. S. Steward, and E. P. Freenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 3.Delves-Broughton, J. 1990. Nisin and its uses as a food preservative. Food Technol. 44:100-117. [Google Scholar]
- 4.De Vuyst, L., R. Callewaert, and K. Crabbe. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 5.Fernández, L., M. L. Marín, S. Langa, R. Martín, C. Reviriego, A. Fernández, M. Olivares, J. Xaus, and J. M. Rodríguez. 2004. A novel genetic label for detection of specific probiotic lactic acid bacteria. Food Sci. Technol. Int. 10:101-108. [Google Scholar]
- 6.Goeres, D. M., L. R. Loetterle, M. A. Hamilton, R. Murga, D. W. Kirby, and R. M. Donlan. 2005. Statistical assessment of a laboratory method for growing biofilms. Microbiology 151:757-762. [DOI] [PubMed] [Google Scholar]
- 7.Jams, L. S., and L. M. Larry. 1986. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl. Environ. Microbiol. 51:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janes, M. E., R. Nannapaneni, and M. G. Johnson. 1999. Identification and characterization of two bacteriocin-producing bacteria isolated from garlic and ginger root. J. Food Prot. 62:899-904. [DOI] [PubMed] [Google Scholar]
- 9.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 10.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, T. Abuladze, M. Yang, R. Safner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv, W., W. Cong, and Z. Cai. 2004. Nisin production by Lactococcus lactis subsp. lactis under nutritional limitation in fed-batch culture. Biotechnol. Lett. 26:235-238. [DOI] [PubMed] [Google Scholar]
- 12.Matsusaki, H., N. Endo, K. Sonomoto, and A. Ishizaki. 1996. Lantibiotic nisin Z fermentative production by Lactococcus lactis IO-1—relationship between production of the lantibiotic and lactate and cell growth. Appl. Microbiol. 45:36-40. [DOI] [PubMed] [Google Scholar]
- 13.McLean, N. W., and I. J. Rosenstein. 2000. Characterization and selection of a Lactobacillus species to re-colonize the vagina of women with recurrent bacterial vaginosis. J. Med. Microbiol. 49:543-552. [DOI] [PubMed] [Google Scholar]
- 14.Milohanic, E., R. Jonquières, P. Glaser, P. Dehoux, C. Jacquet, P. Berche, P. Cossart, and J.-L. Gaillard. 2004. Sequence and binding activity of the autolysin-adhesin Ami from epidemic Listeria monocytogenes 4b. Infect. Immun. 72:4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk, I. R., G. M. Cook, B. C. Monk, and P. J. Bremer. 2004. Morphotypic conversion in Listeria monocytogenes biofilm formation: biological significance of rough colony isolates. Appl. Environ. Microbiol. 70:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nightingale, K. K., Y. H. Schukken, C. R. Nightingale, E. D. Fortes, A. J. Ho, Z. Her, Y. T. Grohn, P. L. McDonough, and M. Wiedmann. 2004. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 70:4458-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taormina, P. J., and L. R. Beuchat. 2001. Survival and heat resistance of Listeria monocytogenes after exposure to alkali and chlorine. Appl. Environ. Microbiol. 67:2555-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman, H. M., C. J. M. Koning, L. Mulder, F. M. Rombouts, and A. C. Beynen. 2004. Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int. J. Food Microbiol. 96:219-233. [DOI] [PubMed] [Google Scholar]
- 19.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penadés, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tompkin, R. B. 2002. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 65:709-725. [DOI] [PubMed] [Google Scholar]
- 21.Turcott, C., C. Lacroix, E. Kheadr, L. Grignon, and I. Fliss. 2004. A rapid turbidometric microplate bioassay for accurate quantification of lactic acid bacteria bacteriocins. Int. J. Food Microbiol. 90:283-293. [DOI] [PubMed] [Google Scholar]
- 22.Zhao, T., M. P. Doyle, and P. Zhao. 2004. Control of Listeria monocytogenes in a biofilm by competitive-exclusion microorganisms. Appl. Environ. Microbiol. 70:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]