Abstract
A physiological, unbalanced model is presented that explicitly describes growth of the marine cyanobacterium Trichodesmium sp. at the expense of N2 (diazotrophy). The model involves the dynamics of intracellular reserves of carbon and nitrogen and allows the uncoupling of the metabolism of these elements. The results show the transient dynamics of N2 fixation when combined nitrogen (NO3−, NH4+) is available and the increased rate of N2 fixation when combined nitrogen is insufficient to cover the demand. The daily N2 fixation pattern that emerges from the model agrees with measurements of rates of nitrogenase activity in laboratory cultures of Trichodesmium sp. Model simulations explored the influence of irradiance levels and the length of the light period on fixation activity and cellular carbon and nitrogen stoichiometry. Changes in the cellular C/N ratio resulted from allocations of carbon to different cell compartments as demanded by the growth of the organism. The model shows that carbon availability is a simple and efficient mechanism to regulate the balance of carbon and nitrogen fixed (C/N ratio) in filaments of cells. The lowest C/N ratios were obtained when the light regime closely matched nitrogenase dynamics.
In marine waters, nitrogen generally controls primary production (16). In such environments, N2-fixing organisms have a competitive advantage over organisms that rely on the availability of combined nitrogen. In the tropical oceans, N2-fixing cyanobacteria can be extremely abundant in surface waters and account for a considerable input of combined nitrogen into the upper mixed layer (11, 28), with a strong impact on local community production (9).
At ocean basin scales, N2-fixing cyanobacteria affect the coupling of C-N-P cycles and contribute considerably to the net oceanic sequestration of atmospheric carbon dioxide (28). For instance, a global-scale estimate made by Lee et al. (31) of net CO2 fixation in the absence of measurable nitrate led to the conclusion that 20 to 40% of the total new primary production in tropical and subtropical oceans could be attributed to N2-fixing organisms. The quantitative impact of N2-fixing organisms on nutrient cycling in the oceans and on the global carbon budget is widely acknowledged (14, 16, 26, 36, 51). Biogeochemical models have been developed to offer a dynamic view of biological and biochemical systems. At global scales, these models aim to provide estimates of the main oceanic biogeochemical fluxes, such as total, new, and regenerated production (17). Others describe the global nitrogen cycle at the ecosystem level (18, 25, 35). At smaller scales, phytoplankton growth models describe time-dependent changes in biomass or numbers as a function of one or several limiting factors, thereby offering support to hypotheses about biological and physiological processes. However, these models do not account for the physiological and regulatory mechanisms of N2 fixation in cyanobacteria, since there is still very little knowledge about the factors that control them.
In this paper we focus on the marine nonheterocystous cyanobacterium Trichodesmium sp., identified as one of the most significant N2-fixing organisms in oceans (6). Because nitrogenase, the enzyme complex responsible for the reduction of N2, is very sensitive to inactivation by O2, cyanobacteria have evolved various strategies to separate N2 fixation from O2-generating photosynthesis (2, 4, 21). It has been hypothesized that Trichodesmium separates N2 fixation spatially from oxygenic photosynthesis, allowing it to fix N2 during the day (1). In this respect, Trichodesmium has adopted a protective strategy similar to that of heterocystous cyanobacteria (4). Since C/N ratios in natural populations of Trichodesmium remain relatively constant (10), it is expected that Trichodesmium will show balanced growth.
This paper presents a model designed to assess the mechanisms that control physiological processes, in particular primary production. This model explicitly describes unbalanced growth and N2 fixation in Trichodesmium spp. and their control by environmental factors. Different numerical simulations were performed under various conditions to test the effects of light and nutrient availability on growth and N2 fixation under both transient and steady-state conditions. We aimed to pinpoint (i) whether light intensity and the temporal distribution of light would have an effect on the pattern and rate of N2 fixation and (ii) the analysis of the role of carbon availability on N2 fixation. We also analyze, in qualitative terms, the role of the nitrogen supply in (i) the interaction between nitrogen limitation and N2 fixation and (ii) N2 fixation dynamics. The results of these simulations are compared to measurements of nitrogenase activity carried out on exponentially growing cultures of Trichodesmium.
MATERIALS AND METHODS
Laboratory experiments.
Trichodesmium sp. strain IMS101 was grown at 27°C in modified YCBII medium (12) in 250-ml Erlenmeyer flasks under a 12-h light/12-h dark (L12) regime in an incubator without shaking. YCBII medium was modified by the addition of 15.9 g liter−1 Na2CO3 and 1.6 × 10−9 M Na2SeO3 and was devoid of combined nitrogen. The pH of the medium was ∼8.2. Light was provided by cool white 15-W fluorescent tubes at an incident photon irradiance of 70 μmol · m−2 · s−1. The cultures were swirled daily by hand in order to prevent the formation of large aggregates or wall growth. N2 fixation was monitored by using the acetylene reduction technique. Only exponentially growing cultures were used for our experiments. Samples of 50 ml of culture were filtered on a GF/F glass fiber filter (diameter, 47 mm) under a moderate vacuum. Subsequently, the filter was placed in the incubation chamber for an online acetylene reduction assay (47). Filter incubation was preferred to liquid incubation, because it has been shown to be superior (47). The gas flow over the filter with Trichodesmium was 2 liters · h−1 and was composed of 20% O2, 70% N2, and 10% C2H2. The incubation chamber was kept at 25°C and at the same light regime and photon flux density as in the culture. The gas flow was in line with a gas chromatograph (Chrompack-CP9000; Varian, The Netherlands) equipped with a flame ionization detector and a 500-μl sample loop for automated injections. The conditions of the gas chromatograph and other details were those described by Staal et al. (47).
N2 fixation model. (i) Internal pools.
Depending on their structure, growth models can or cannot represent fluctuations in cellular composition and thus the adaptation of the organism to its environment. The Trichodesmium N2 fixation model is a physiological growth model that explicitly includes reserves of carbon and nitrogen whose dynamics are unbalanced. The model describes the interaction between nitrogen and carbon metabolism and emphasizes the biotic and abiotic conditions that control N2 fixation rates. The model is described below, and a flow diagram is given in Fig. 1. The model variables are listed in Table 1; see Table 2 for the model formulation, Table 3 for the modeled processes, and Table 4 for parameter values.
FIG. 1.
Schematic representation of the four compartments in the model and their related mass fluxes. Solid arrows, C flux; dashed arrows, N flux; dotted arrows, losses due to mortality (M) and maintenance costs (R). The units (C and/or N) used to describe the content of each compartment are circled.
TABLE 1.
Model variables
| Variable | Unita | Description |
|---|---|---|
| State variables | ||
| Csm | mmol C · m−3 | Concentration of small metabolites in Trichodesmium |
| Cr | mmol C · m−3 | Concentration of carbon reserves in Trichodesmium |
| Cs | mmol C · m−3 | Concentration of the structural and functional biomass in Trichodesmium |
| Nr | mmol N · m−3 | Concentration of nitrogen as first N products and N reserve in Trichodesmium |
| Nit | mol N · mol C−1 · h−1 | Nitrogenase activity |
| N1 | mmol N · m−3 | Nitrate concentration in the environment |
| N2 | mmol N · m−3 | Ammonium concentration in the environment |
| Det | mmol C · m−3 | Detritus |
| Dt | h−1 | Dilution rate (turbidostat only) |
| Ordinary variables | ||
| N | mmol N · m−3 | Total dissolved inorganic nitrogen (DIN) in the culture medium |
| Ctot | mmol C · m−3 | Total Trichodesmium carbon |
| Ns | mmol N · m−3 | Cs expressed as nitrogen |
| Ntot | mmol N · m−3 | Total Trichodesmium nitrogen |
| C:N | mol C · mol N−1 | C/N ratio of Trichodesmium |
| pCsm | Proportion of carbon in the small-metabolite pool Csm with regard to total cellular carbon | |
| pCr | Proportion of carbon stored in Cr with regard to total cellular carbon | |
| pCs | Proportion of carbon in the structural biomass (Cs) with regard to total cellular carbon | |
| pNr | Proportion of nitrogen stored in Nr with regard to total cellular nitrogen (expressed as carbon units) | |
| at | g Chl · m−3 | Trichodesmium chlorophyll content |
| aC | g Chla · g C−1 | Chla/C ratio of Trichodesmium |
| Ce | mol C · mol C−1 | Excess of Csm over the cellular quota QC |
| lC | Limitation factor due to the availability of carbon in Csm | |
| Ne | mol N · mol N−1 | Excess of N reserves over the cellular quota QN |
| IN | Limitation factor due to the availability of nitrogen in Nr | |
| el | m−1 | Light extinction coefficient |
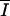
|
μmol phot · m−2 · s−1 | Average irradiance in the culture |
phot, photons.
TABLE 2.
Model formulation
| Variable | Equation |
|---|---|
| Rate of change of the state variablesa | |
| dCsm/dt | +P + g2 (Cr) − g3 (Csm) − G − (m + rb) × Csm − ra − Csm × Dt |
| dCr/dt | +g3 (Csm) − g2 (Cr) − (m + rb) × Cr − Cr × Dt |
| dCs/dt | +G − (m + rb) × Cs − Cs × Dt |
| dNr/dt | +F + U1 + U2 − G × (N:C)Cs − (m + rb) × Nr − Nr × Dt |
| dNit/dt | +g4 − g8 − Nit × Dt |
| dN1/dt | −U1 − N1 × Dt |
| dN2/dt | −U2 − N2 × Dt |
| dDet/dt | +m × Ctot − Det × Dt |
| dDt/dt |
c13 × Dt × (Ieq −  ) ) |
| Ordinary variables | |
| N | N1 + N2 |
| Ctot | Csm + Cr + Cs + Nr/(N:C)Nr |
| Ns | Cs × (N:C)Cs |
| Ntot | Ns + Nr |
| C:N | Ctot/Ntot |
| pCsm | Csm/Ctot |
| pCr | Cr/Ctot |
| pCs | Cs/Ctot |
| pNr | [Nr/(N:C)Nr]/Ctot |
| at | Cs × (N:C)Cs × aN |
| aC | at/(Ctot × 12) |
| Ce | Csm/Ctot − QC |
| lC | Ce2/(kC2 + Ce2) |
| Ne | Nr/Ntot − QN |
| lN | Ne1.2/(kN1.2 + Ne1.2) |
| el | c9 + c10 × at + c11 × atc12 |
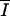
|
I/zm × {[−1/el] × [exp(−el × zm) − 1]} |
When the model is run as a continuous culture, the dilution rate becomes a constant: Dt = D.
TABLE 3.
Modeled processes
| Process | Equation | Unit | Description |
|---|---|---|---|
| P |
g1 ( ) × (1 − lC) × aC × 12 × Cs ) × (1 − lC) × aC × 12 × Cs
|
mmol C · m−3 · h−1 | Actual gross photosynthesis rate |
g1(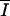 ) ) |
Pm × [1 − exp(−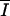 /Ik)] × exp(− /Ik)] × exp(−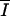 /βl) /βl) |
mol C · g Chla−1 · h−1 | P-I curve (41) |
| g2(Cr) | c1 × Cr | mmol C · m−3 · h−1 | Carbon flux from Cr to Csm (catabolism) |
| g3(Csm) | c2 × lC × Cs | mmol C · m−3 · h−1 | Carbon flux from Csm to Cr (storage) |
| g4 | c3 × MIN {[1 − g5(N1)], [1 − g6(Ne)]} × g7(Ce) | mol N · mol C−1 · h−1 · h−1 | Synthesis of nitrogenase activity |
| g5(N1) | N1/(N1 + kd) | Limitation due to presence of nitrate | |
| g6(Ne) | Ne6/(kN6 + Ne6) | Limitation due to Ne | |
| g7(Ce) | Ce6/(kCn6 + Ce6) | Limitation due to Ce | |
| g8 | c4 × g9(Nit) × g7(Ce) | mol N · mol C−1 · h−1 · h−1 | Breakdown of nitrogenase activity |
| g9(Nit) | Nit/(Nit + c5) | Decay scaled to nitrogenase activity | |
| F | Nit × (1 − lN) × g10(Ce) × Cs | mmol N · m−3 · h−1 | Actual N2 fixation rate |
| g10(Ce) | Ce6/(kCf6 + Ce6) | Limitation due to availability of DIN | |
| U1 | c6 × lC × g11(N) × (1 − lN) × Cs × N1/N | mmol N · m−3 · h−1 | Actual nitrogen uptake rate |
| U2 | c6 × lC × g11(N) × (1 − lN) × Cs × (1 − N1/N) | mmol N · m−3 · h−1 | Actual ammonium uptake rate |
| g11(N) | N/(N + kd) | Limitation due to availability of DIN | |
| G | gm × MIN(lC, lN) × Cs | mmol C · m−3 · h−1 | Actual gross production of structural biomass Cs |
| ra1 | if N ≤ 10−5, c7; otherwise, c8 + (c7 − c8) × (1 − N1/N) | Minimum fraction of the gross growth (Cs production) lost as respiration activity | |
| ra | ra1 + (ra2 − ra1) × [1 − g11(N)] × G | mmol C · m−3 · h−1 | Actual loss due to respiration activity |
TABLE 4.
Parameter values
| Parameter | Value | Unita | Description |
|---|---|---|---|
| Photosynthesis | |||
| Pm | 3.1438 | mol C · g Chla−1 · h−1 | Maximum gross photosynthesis rate in Trichodesmium (from Kana [27]) |
| aN | 1.5 | g Chl · mol N−1 | Chlorophyll a-to-nitrogen ratio |
| Ik | 100 | μmol phot · m−2 · s−1 | Saturation irradiance for photosynthesis |
| β1 | 350 | μmol phot · m−2 · s−1 | Photoinhibition coefficient for photosynthesis |
| c1 | 0.12 | h−1 | Catabolism rate of Cr |
| c2 | 0.2 | h−1 | Maximum carbon storage rate |
| kC | 0.06 | mol C · mol C−1 | Half-saturation Csm/Ctot ratio for carbon storage |
| N2 fixation and uptake | |||
| c3 | 10 × gm × 24−1 | mol N · mol C−1 · h−2 | Maximum rate of increase of nitrogenase activity related to the maximum specific growth rate (gm) |
| c4 | 8 × gm × 24−1 | mol N · mol C−1 · h−2 | Maximum rate of decay of nitrogenase activity related to the maximum specific growth rate (gm) |
| c5 | 0.0005 | mol N · mol C−1 · h−1 | Coefficient of the decay function g8 |
| c6 | 0.0272 | mol N · mol C−1 · h−1 | Maximum carbon-specific rate of nitrogen uptake |
| kCn | 0.045 | mol C · mol C−1 | Half-saturation Csm/Ctot ratio for the allocation of carbon to nitrogenase synthesis |
| kCf | 0.05 | mol C · mol C−1 | Half-saturation Csm/Ctot ratio for the allocation of carbon to N2 fixation |
| kd | 0.5 | mmol N · m−3 | Half-saturation constant for DIN uptake |
| kN | 0.1 | mol N · mol N−1 | Half-saturation Nr/Ntot ratio for nitrogen storage |
| Growth | |||
| gm | 0.9 × 24−1 | h−1 | Maximum specific growth rate of Trichodesmium |
| (N:C)Nr | 0.4 | mol N · mol C−1 | N-to-C ratio in nitrogen reserves (Nr) |
| (N:C)Cs | 0.3 | mol N · mol C−1 | N-to-C ratio in structural biomass (Cs) |
| QC | 0.03 | mol C · mol C−1 | Minimum cellular C quota (Csm-to-Cs ratio) |
| QN | 0.00 | mol N · mol N−1 | Minimum cellular N quota (Nr-to-Cs ratio) |
| c7 | 0.4 | Highest value in the range of the minimum respiration activity | |
| c8 | 0.2 | Lowest value in the range of the minimum respiration activity | |
| ra2 | 0.45 | Maximum fraction of the gross growth (Cs production) lost as respiration activity | |
| rb | 0.02 × 24−1 | h−1 | Rate of the maintenance cost |
| m | 0.002 × 24−1 | h−1 | Natural mortality rate |
| Forcing functions | |||
| I | 120.0 | μmol phot · m−2 · s−1 | Incident solar irradiance during light hours |
| Ieq | Variable | μmol phot · m−2 · s−1 | Avg irradiance in the culture at the dynamic equilibrium |
| c9 | 0.04 | m−1 | Background light extinction due to suspended material |
| c10 | 0.0088 | Extinction parameter | |
| c11 | 0.054 | Extinction parameter | |
| c12 | 0.667 | Extinction parameter | |
| zm | 1 | m | Maximum depth of the water column |
| D | 0.0085 | h−1 | Dilution rate in the culture |
| c13 | 0.001 | Adjustment coefficient for the dilution rate in the turbidostat |
phot, photons.
The cellular carbon components are decomposed as described by Lancelot and Billen (29). Cellular carbon is represented by the pool of low-molecular-weight carbon molecules (photosynthate) (Csm), the internal carbon reserves (glycogen) (Cr), and the structural biomass (Cs). Csm has a short turnover time, allowing fast changes of process rates. In the model this pool served as the intermediate for all metabolic processes. The Csm consumption terms can be either (i) storage in the form of carbon reserves (Cr), (ii) incorporation in structural biomass (Cs), (iii) dissimilation, or (iv) consumption for maintenance. An exchange thus occurs between the intracellular carbon reserves (Cr) and Csm. Similarly, internal storage of nitrogen (Nr) is included in the model in order to account for temporal variations in N2 fixation. This nitrogen reserve groups glutamine (the early product of N2 fixation [7]) and the pool of nitrogen reserve components (e.g., cyanophycin and phycobiliproteins). This pool represents intracellular nitrogen available for the synthesis of structural biomass (Cs) (structural carbon and nitrogen). The uptake of external combined nitrogen also feeds into the nitrogen reserve pool.
The structural biomass (Cs) pool sets the maximum rates of all processes. The uncoupling between carbon and nitrogen metabolism allows processes to be modulated (limited or inhibited) by both the carbon status and the nitrogen status of cells (Ce and Ne, respectively), which represent the availability of these two elements for metabolism. Carbon status is calculated as the relative proportion of the photosynthate (Csm) to total carbon, excluding the minimum cellular carbon quota; nitrogen status is the relative proportion of the nitrogen reserves (Nr) to total N, excluding the minimum cellular nitrogen quota (Table 2). Hence, growth can occur independently of the periods of photosynthesis, provided that sufficient nitrogen and carbon are available to cells. The limitation functions (lC and lN) associated with the internal states follow a type III (sigmoid) response (Table 2). Inhibition by internal status is expressed as 1 minus sigmoid.
(ii) Formulations of photosynthesis and growth.
The carbon-specific photosynthesis rate (P) depends on the chlorophyll a (Chla) content. Light dependence [g1(I)] is described using the exponential saturation function from reference 41, including a photoinhibition term. The actual photosynthesis rate is further limited by the extent of carbon reserves in cells (1 − lC). The maximum rate of photosynthesis, Pm (expressed as mol of C · g of Chla−1 · h−1), was recalculated from Kana's measurements (27) on Trichodesmium thiebautii. The rate of carbon storage in Cr (g3) is assumed to be proportional to the carbon status following a saturation function. The consumption rate of this reserve (g2) is linearly related to the reserve concentration (Cr). Growth (G) is the production of structural biomass at the expense of photosynthate and nitrogen reserves. We considered a maximum theoretical specific growth rate of Trichodesmium of 0.9 day−1. The actual growth rate results from the limitation by carbon and nitrogen internal states, following Liebig's law of the minimum. Phosphorus is not limiting in our model, and the effect of temperature is not taken into account.
(iii) Effects of nitrogen substrates and associated growth costs.
Trichodesmium is a facultative diazotroph and can take up combined inorganic nitrogen compounds (38). In the model, combined nitrogen is preferred over N2, a condition based on the absence or lower rates of N2 fixation when Trichodesmium is grown on NH4+ or NO3− (24). The uptake and assimilation of combined nitrogen are described by one equation, in which the rates of consumption of NH4+ (U1) and NO3− (U2) are proportional to their concentrations. The rate of consumption follows Michaelis-Menten kinetics and is modulated by the depletion of internal nitrogen reserves. As Stephens et al. (49) proposed, we simulate the potential activity of N2 fixation as the result of a balance between an increase (g4) and a decrease (g8) in the potential activity rather than as a turnover of the enzyme itself. Nitrogenase activity is dependent on N2, ATP, and reducing equivalents. The increase in the potential rate of N2 fixation is assumed to be controlled by the nitrogen (g6) and energy status of the cells. It is also sensitive to the concentration of NO3− (40). The actual rate of N2 fixation results from carbon availability and the cellular nitrogen status as well as the potential rate of nitrogenase activity.
ATP pools are not modeled sensus stricto; the energy gained from photosynthesis is represented as the accumulation of fixed carbon. Thus, the energy for any metabolic process is provided by the dissimilation of the carbon reserves. For the model, we assume that N2 fixation is limited by electrons originating from the mobilization of the carbon metabolites (Csm). Respiration accounts for differences in energy costs that result from the use of different nitrogen sources. When growth cannot be sustained by the uptake of combined nitrogen, N2 fixation is induced in order to meet nitrogen demand. The transition to N2 fixation will moderately increase the energy cost for N assimilation, which is reflected in a higher rate of respiration. The higher energy cost of growth on NO3− than on NH4+ is also taken into account. The minimum maintenance rate increases when the concentration of NH4+ decreases.
(iv) Physical setting.
The modeled system can be run either as a well-stirred continuous culture or as a turbidostat. We emphasize that the term chemostat does not apply here, since that term usually refers to cultures in steady state controlled by a growth-limiting nutrient (often but not exclusively nitrogen) and requires a continuous light regime. In this study a light-dark regime was applied, and Trichodesmium growth was light limited and not nutrient limited.
Biomass generates an extinction of light (eI). Hence, the higher the biomass, the lower the resultant average irradiance (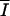 ) within the vessel becomes, lowering the specific photosynthesis rate. Biomass is considered to be well mixed and as a result homogeneously distributed. Growth will be light limited at the equilibrium state. Dilution removes part of the biomass and prevents culture senescence, since light is never completely extinguished. When the model reaches equilibrium, the daily average of all the specific rates becomes constant and the daily net specific growth rate equals the dilution rate. To qualify this equilibrium, we do not use here the term “steady state,” which refers to a perfect constancy of variables in time. In the model, the light regime induces a periodicity in the metabolism, and at equilibrium, variables are still fluctuating within 24 h but their daily average is constant from one day to another. Such an equilibrium is called “dynamic equilibrium.” In this model, the dynamic equilibrium is determined by light conditions and by the dilution rate. When the model is run as a turbidostat, the dilution rate becomes a state variable that fluctuates until the average irradiance (
) within the vessel becomes, lowering the specific photosynthesis rate. Biomass is considered to be well mixed and as a result homogeneously distributed. Growth will be light limited at the equilibrium state. Dilution removes part of the biomass and prevents culture senescence, since light is never completely extinguished. When the model reaches equilibrium, the daily average of all the specific rates becomes constant and the daily net specific growth rate equals the dilution rate. To qualify this equilibrium, we do not use here the term “steady state,” which refers to a perfect constancy of variables in time. In the model, the light regime induces a periodicity in the metabolism, and at equilibrium, variables are still fluctuating within 24 h but their daily average is constant from one day to another. Such an equilibrium is called “dynamic equilibrium.” In this model, the dynamic equilibrium is determined by light conditions and by the dilution rate. When the model is run as a turbidostat, the dilution rate becomes a state variable that fluctuates until the average irradiance ( ) in the culture reaches a chosen value, Ieq (Table 2). Ieq characterizes the light regime reached in the turbidostat at the equilibrium.
) in the culture reaches a chosen value, Ieq (Table 2). Ieq characterizes the light regime reached in the turbidostat at the equilibrium.
(v) Implementation.
The model is formulated as a system of ordinary differential equations. It is implemented in the FORTRAN language, in the FEMME simulation environment (44), running on Windows. The model is available for download from the FEMME website (http://www.nioo.knaw.nl/cemo/femme/).
Simulations.
The model was run as a continuous culture and as a turbidostat. In the continuous-culture runs, the daily average of the net specific growth rate equaled the dilution rate when the model reached the dynamic equilibrium. The turbidostat allowed better control of the light intensity in the culture. In each turbidostat simulation, the average desired irradiance in the culture at equilibrium (Ieq) was set as a parameter. The dilution rate was not constant in these experiments but changed at each time step, resulting in a constant daily average of the biomass concentration that created the light conditions in the vessel equal to Ieq.
(i) Transient behavior experiments.
The first two simulations consisted of a well-mixed continuous culture in which the initial concentration of combined nitrogen in the culture vessel was 10 μM and the initial total biomass was 6.8 mmol of C · m−3. The medium supply did not contain combined nitrogen. The culture was exposed to a constant incident photon irradiance of 120 μmol · m−2 · s−1 during the light period.
In the L12 simulation, the on-off light rhythm applied was 12 h of light and 12 h of darkness (hence, the daily average was 60 μmol · m−2 · s−1). The model was first run as a continuous culture with a dilution rate set to 0.0085 h−1. Then, under the same conditions, the model was run as a turbidostat, with an Ieq of 12.5 μmol · m−2 · s−1 during the light period.
In the L16 simulation, the length of the light period was changed to 16 h of light and 8 h of darkness. The same incident photon irradiance was applied (120 μmol · m−2 · s−1 during the 16 h of light), and the model was run as a turbidostat, with an Ieq of 9.375 μmol · m−2 · s−1 during the light period. The total average daily light dose experienced by the turbidostat culture at the dynamic equilibrium was then the same as that in the turbidostat culture in the L12 simulation.
(ii) Equilibrium state analysis.
One hundred simulations were performed in order to investigate the influence of the average light intensity in the culture vessel on the stoichiometry of cellular carbon and nitrogen. The model was run as a turbidostat, and Ieq was uniformly varied between predefined ranges.
Series 1 consisted of 100 simulations similar to the L12 experiments (i.e., under a 12-h light/12-h dark cycle) with Ieq values ranging from 5 to 100 μmol · m−2 · s−1. The incident photon irradiance applied during the light phase was again constant in all runs (120 μmol · m−2 · s−1); the total daily light dose remained the same.
Series 2 consisted of 100 simulations to analyze the coinfluence of the average light intensity and the length of the light period on cell metabolism. Both Ieq (5 to 100 μmol · m−2 · s−1) and the length of the light period (8 h to 18 h) were changed during these simulations, while the incident photon irradiance applied during the light phase was again constant in all runs (120 μmol · m−2 · s−1).
RESULTS
Figure 2 displays experimental data; Fig. 3 shows simulation results with the model run as a continuous culture; and Fig. 4 to 6 show results of the simulation with the model run as a turbidostat.
FIG. 2.
Measurement of nitrogenase activity in Trichodesmium sp. strain IMS101 over 24 h.
FIG. 3.
Simulation of the successive use of different nitrogen sources in a continuous culture of Trichodesmium sp. strain IMS101 grown under a regime consisting of 12 h of light and 12 h of darkness. Shown are the concentration of DIN in the medium, the DIN uptake rate, and the N2 fixation rate. Horizontal black bars on the x axis show the dark period.
FIG. 4.
L12 (left panels) and L16 (right panels) turbidostat experiments. Shown are daily dynamics when the model has reached the dynamic equilibrium, for simulations run with a regime consisting of 12 h of light and 12 h of darkness (L12) or 16 h of light and 8 h of darkness (L16). (a) Availability of carbon reserves, and synthesis and decay of nitrogenase activity. (b) Nitrogenase activity, rate of N2 fixation, and availability of nitrogen reserves. (c) Resultant average irradiance (Ieq) in the vessel and C/N ratio. Horizontal black bars represent the duration of the dark period.
FIG. 6.
Combined influences of the average irradiance (Ieq) in the culture and the length of the light period at the dynamic equilibrium (series 2). Daily averages of variables at the equilibrium state are plotted as a function of the resultant daily average irradiance in the vessel. Lines represent changes in the daily average C/N ratio for a given light period: 8 h (L8), 12 h (L12), or 16 h (L16). Note that the x axis gives the irradiance (Ieq) averaged over 24 h.
Dynamics of nitrogenase activity.
Figure 2 depicts the daily pattern of nitrogenase activity in a culture of Trichodesmium sp. strain IMS101, measured by the online acetylene reduction technique. Nitrogenase activity was observed essentially during the light period of the light-dark cycle. The rate of N2 fixation increased throughout the first half of the light period and reached its maximum 4 to 5 h after the onset of the light period. During the second half of the light period, activity decreased steadily, and activity quickly ceased when it became dark.
Transient dynamics of nitrogen concentration, rate of N2 fixation, and consumption of nitrogen in a simulated experiment (L12 experiment).
The model behaved qualitatively in the same way whether it was run in the light-limited continuous-culture mode or in the turbidostat mode. This was true for the transient phase as well as for the equilibrium phase (data not shown). This was because the system was limited by light in both modes. The only difference was the resultant light intensity in the culture vessel. The results of the L12 continuous culture run are depicted in Fig. 3. The dilution experiment exhibited a progressive shift from growth at the expense of combined nitrogen to diazotrophic growth when the external combined nitrogen levels fell below the semisaturation concentration for nitrogen uptake. Nitrogen uptake and N2 fixation occurred during the transition period. When available, combined nitrogen was preferred. Subsequently, when uptake decreased, N2 fixation became progressively more important (Fig. 3). During this transient phase, both dissolved inorganic nitrogen (DIN) uptake and N2 fixation were strongly stimulated when the light was switched on. A gradual and slight decrease in DIN uptake was then observed during the light phase (due to inhibition by the nitrogen reserves [Nr]), while N2 fixation continued to increase due to the increasing potential activity of the nitrogenase. After the light was switched off, DIN uptake, which is dependent on energy from the catabolism of carbon reserves, dropped to half the value obtained during the light period, while N2 fixation dropped to zero because nitrogenase broke down during the night. Several days were required for the population to deplete the DIN from the environment, because the inoculum was small.
Influence of the photoperiod on the daily pattern (L12 versus L16 run).
In the turbidostat as well as in the continuous-culture experiment, the model displayed a daily pattern of N2 fixation with increasing activity during the first half of the light period and a decrease during the second half. The daily behavior at equilibrium is depicted comparatively for the two light regimes in Fig. 4. Nitrogenase synthesis was activated at the beginning of the day by the depletion of the nitrogen reserves (Fig. 4a and b). When energy became available, the rate of N2 fixation increased, and so did the nitrogen reserves (Fig. 4b). Eventually, the cellular nitrogen status caused a decay of active nitrogenase in the middle of the light period, thus decreasing the N2 fixation potential (Fig. 4a). N2 fixation continued during the rest of the light period but decreased (i) because the “synthesis” of nitrogenase decreased as the internal nitrogen reserves became replete during the course of the day and (ii) due to the decay of nitrogenase. The decay of nitrogenase is proportional to the nitrogenase activity as well as to the shortage of available carbohydrates in cells (Fig. 4a). When the light was switched off, carbon (Csm) was no longer supplied by photosynthesis and net consumption led to a decrease of the available Csm reserves.
The resultant average irradiance in the vessel chosen in L16 was such that the total daily average light experienced by colonies was the same as that in the L12 turbidostat. Due to the lower average irradiance (Ieq) experienced by colonies in the L16 culture, a lower carbon-specific maximum rate of Cs synthesis was observed. However, photosynthesis occurred for a longer period, so that the daily net Cs synthesis rate was higher in L16. As a result, L16 showed higher total intracellular carbon and nitrogen contents (in structural biomass as well as in reserves) than L12 (data not shown).
Figure 4 shows that N2 fixation activity occurred as long as the culture was exposed to light. Biomass-specific nitrogenase activity still showed a maximum approximately halfway into the light period, but this maximum value was lower than that in the L12 experiment. The daily N2 fixation rate was higher in L16 than in L12. Cells in L16 accumulated more carbon and nitrogen than cells in L12. However, proportionally, more carbon was stored in Cr and Csm reserves in L16, while more was allocated to Nr and Cs in L12. As a result, the C/N ratio observed in L16 was somewhat higher than that in L12.
Influence of the available irradiance on cell metabolism (series 1).
In the series 1 runs, the average irradiance (Ieq) in the culture at the equilibrium was set and the dilution rate progressively changed until the amount of biomass gave the desired value Ieq. Beyond a critical dilution rate, which equals the maximum growth rate, washout occurs. An unbalanced growth rate was achieved by changes in the cellular carbon and nitrogen contents, which were expressed as the relative proportions of the different molecules (Table 2). Thus, we define the relative proportion of structural carbon, pCs, as its content relative to total intracellular carbon: pCs = [Cs]/Ctot (Table 2). The relative content of the carbon reserve, pCr, was calculated in the same way. The relative proportion of stored nitrogen, pNr, is the content of Nr, expressed as carbon, versus total carbon content. In Fig. 5 the daily average of different variables at equilibrium is plotted as a function of the daily average photon flux density (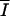 ). Because at dynamic equilibrium the biomass dilution equaled the net growth rate, a higher imposed irradiance provoked a higher growth rate and therefore higher dilution rates at equilibrium (Fig. 5a). With increasing irradiances, more carbon was fixed and stored in Csm and Cr. Hence, the proportion of carbon storage (pCsm and pCr) increased, while the proportion of carbon in structural biomass (Cs) decreased (Fig. 5b and c). N2 fixation is regulated by the availability of Csm and therefore increased with light, as did the proportion of nitrogen stored (Nr) (Fig. 5c). Although growth decreased the cellular carbon content (because of losses due to carbon dissimilation), carbon assimilation increased faster than nitrogen assimilation. As a result, the C/N ratio increased with the average irradiance (Fig. 5a).
). Because at dynamic equilibrium the biomass dilution equaled the net growth rate, a higher imposed irradiance provoked a higher growth rate and therefore higher dilution rates at equilibrium (Fig. 5a). With increasing irradiances, more carbon was fixed and stored in Csm and Cr. Hence, the proportion of carbon storage (pCsm and pCr) increased, while the proportion of carbon in structural biomass (Cs) decreased (Fig. 5b and c). N2 fixation is regulated by the availability of Csm and therefore increased with light, as did the proportion of nitrogen stored (Nr) (Fig. 5c). Although growth decreased the cellular carbon content (because of losses due to carbon dissimilation), carbon assimilation increased faster than nitrogen assimilation. As a result, the C/N ratio increased with the average irradiance (Fig. 5a).
FIG. 5.
Overview of 100 simulations similar to the L12 experiments (series 1), except that the dilution rate changes. The daily averages of variables at the equilibrium state are plotted as a function of the daily average irradiance in the vessel. (a) Average dilution rate and C/N ratio. (b) pCs and pCr. (c) pCsm and pNr. Rates are given per volume of medium.
Coinfluence of the average irradiance in the culture and the duration of the light period (series 2).
Figure 6 displays the fluctuations of the C/N ratio for different light periods. Three lines were drawn that represent, respectively, L8, L12, and L16. These curves show that higher C/N ratios resulted from the extra carbon assimilated during longer day lengths. This phenomenon is attributed to the saturation in the photosynthesis-light relationship. In this experiment, the total daily amount of light in the culture vessel (Ieq × day length) was equal in all runs. Hence, when the light period was short, light intensity was high. This resulted in a maximum rate of photosynthesis, but not all energy was used. In contrast, when the light period was long, incident irradiance was lower and photosynthesis was submaximal. Last, the relative increase in the C/N ratio with irradiance is slightly higher at long day lengths (Fig. 6). For instance, the C/N ratio at 34.8 μmol · m−2 · s−1 is 113.8% of the value at 3.7 μmol · m−2 · s−1 in L8, while it is 119% of that value in L16.
DISCUSSION
Little is known about the regulatory mechanisms of N2 fixation in Trichodesmium. Nitrogenase concentrations in populations are rarely quantified, and even when they are, the active and inactive forms of this enzyme complex are not easily distinguished from each other (12, 22, 54). Furthermore, the number of N2-fixing cells in a trichome of Trichodesmium is mostly unknown. Therefore, it seems almost impossible to quantify the nitrogenase content or its potential activity in individual nitrogen-fixing cells. Due to these uncertainties, nitrogenase content is not a suitable state variable for the model. Therefore, we chose to model nitrogenase activity (potential and actual rates of N2 fixation) rather than the enzyme content. N2 fixation is an energy-costly process and therefore is not preferred when combined nitrogen is sufficiently available. As emphasized by Gallon (21), use of N2 instead of DIN entails some extra costs, associated with (i) the synthesis of nitrogenase and (ii) the maintenance of anaerobic or microaerobic conditions to protect nitrogenase from inactivation by O2. High respiration rates have been observed in Trichodesmium populations during periods of N2 fixation (4, 8, 27, 42), and it has been suggested that additional O2-consuming reactions are enhanced when N2 fixation and photosynthesis occur simultaneously (2). In addition, combined nitrogen availability in the environment seems to elicit a down-regulation of the synthesis and activity of nitrogenase (40). A representation of intracellular reserves of both carbon and nitrogen is essential in order to accurately describe the dynamics of N2 fixation and its regulation by the environment. To date, different physiologically based models already describe the uncoupling of carbon and nitrogen assimilation in the phytoplankton. In such unbalanced models, internal reserves consisting of either energy reserves (carbon) or intracellular nitrogen are included as an intermediate between photosynthesis and growth (15, 29, 50). These formulations have proved their accuracy in models related to cultures (23) as well as to natural environments (19, 30, 45, 52). Stephens et al. (49) devised a model that simulates the interaction between the different nitrogen sources in the nonheterocystous diazotrophic cyanobacterium Gloeothece. In this model, uncoupling of carbon and nitrogen assimilation was achieved through the description of the intracellular concentration of a nitrogen reserve (glutamine) and included the description of nitrogenase activity. Thus, in the Gloeothece model, the changes in the C/N ratio were driven by fluctuations of the glutamine pool (nitrogen reserve), while the accumulation of storage carbon (and hence energy reserves) was not explicitly included. Therefore, the representation of the interactions led to a complex set of equations. In order to be able to more easily interpret the analysis of the model behavior and to consider N2 fixation from a mechanistic point of view, we have chosen to use a simpler formulation. We included decomposition of cellular carbon components as proposed by Lancelot and Billen (29) in which an intermediary pool of low-molecular-weight carbon molecules (Csm), the product of photosynthesis, is described in addition to the internal reserves of carbon. The Csm pool is an important component of the model, since its rapid turnover allows fast fluctuations of the cellular processes. Added to that description, an internal storage of nitrogen reserves was included to account for N2 fixation. Hence, the simulated mechanisms include a down-regulation of active nitrogenase by available combined nitrogen in the environment, while the actual fixation rate is further regulated by the internal nitrogen and carbon status. Carbon is allocated differently according to fluctuations in the environment. This model has a flexible structure, resulting in a dynamic response of cells to external forcing. Since both photosynthesis and N2 fixation depend on light and on the internal status of cells, it is possible to analyze whether differences in the light patterns can result in different carbon acquisition and growth.
Diurnal dynamics of N2 fixation in Trichodesmium.
Because Trichodesmium fixes N2 during the day, the energy required for diazotrophy is provided mainly by photosynthesis. Such a relation has been clearly pointed out for heterocystous cyanobacteria (46) and for Trichodesmium (26, 48). Trichodesmium showed a rapid decline in the N2 fixation rate when photosynthesis ceased, suggesting that an immediate shortage of energy occurred after darkness (Fig. 2). Hence, our results confirm the dependence of Trichodesmium sp. strain IMS101 on ATP provided by photosynthesis, highlighting the importance of the different substrates required for N2 fixation (N2, ATP, and reducing equivalents). In the model, the lowering of the available Csm reserves in cells at the end of the day resulted in an increased decay of active nitrogenase during the dark (Fig. 4b). The actual N2 fixation rate drops to zero at the end of the day while nitrogenase is still active, indicating that activity is limited by the shortage of carbohydrates in the form of Csm. N2 fixation, driven by the “small-metabolite” pool (Csm) in the model, shows a fast response to light. The role of this compartment is to take into account fast exchanges of carbon to represent, for instance, the steep decline in N2 fixation when the light is turned off. However, this decline is less steep than that measured in the laboratory culture. Furthermore, Csm content originates both from photosynthesis and from storage/catabolism. When cells are exposed to the dark, Csm is fuelled not by photosynthesis but only by catabolism of cellular carbohydrates. Provided that the Csm reserve is available to meet the energy demand, the model also shows that diazotrophy in Trichodesmium can occur during part of the night (Fig. 4b). These results disagree with observations by Mulholland and Bernhardt (37), who did not observe any night N2 fixation in cultures of Trichodesmium sp. strain IMS101. On the other hand, our results are in accordance with the behavior of cultured populations of Trichodesmium sp. strain GBRTRLI101 (20) and with studies of natural Trichodesmium populations from the Pacific (32), supporting the idea that nitrogenase activity is not strictly restricted to the light period in Trichodesmium.
Although photosynthesis and N2 fixation both occur during the day, unbalanced growth leads to a fluctuation of the C/N ratio in cells at a diel scale (Fig. 4c). The maximum hourly N2 fixation rate [expressed in mol of N · (g of Chla)−1 · h−1] and carbon fixation rate [expressed in mol of C · (g of Chla)−1 · h−1] calculated by the model were, respectively, 0.043 and 0.24 in L12 and 0.036 and 0.19 in L16 cultures. These values are in the range reported for different studies on Trichodesmium, listed by Mulholland and Bernhardt (37). The daily averages of the molar C/N2 fixation ratio were 22 in the L12 culture (where the daily average of the dilution rate is 0.28 day−1) and 18.7 in the L16 culture (where the daily average of the dilution rate is 0.29 day−1), consistent with results from acetylene reduction assays reported from continuous-culture experiments with Trichodesmium (37).
Influence of the light rhythm.
Fluctuations of the C/N ratio as a function of light indicate that both the irradiance and the day length affect N2 fixation and cellular elemental composition in Trichodesmium (Fig. 6). L12 and L16 simulations received the same total daily light dose (Ieq × day length) but experienced different light regimes. When the day length increased, nitrogenase was active in cells for a longer period during the day, and although it was progressively down-regulated by the increasing nitrogen reserves, activity proceeded toward the end of the light period because carbon was still available to fuel N2 fixation. Light energy is more efficiently used by photosynthesis at long day lengths: more carbon is fixed per cell and Cs production is higher at the daily scale (and so is pCr), but cells proportionately allocate less nitrogen to their structural biomass. Hence, at shorter day lengths, photosynthesis was better synchronized to nitrogenase requirements and resulted in a lower C/N ratio. In contrast, with longer light periods, an imbalance occurred between photosynthesis and biomass production, and cells redirected the extra carbon fixed into storage. The higher diurnal accumulation of carbon in reserves in L16 also indicates that much less energy was used by the N2 fixation process at the beginning and end of the period of activity. Because nitrogenase activity is not constant during the day, our model simulations emphasize the importance of energy availability when nitrogenase approaches its maximum potential activity, i.e., mainly before midday. The temporal light patterns may thus be part of the factors that influence the proliferation of Trichodesmium populations. The model suggests that cell C/N stoichiometry is tightly regulated, and the range of C/N ratios calculated by the model is in agreement with field observations of natural Trichodesmium populations (3).
Regulation in the modeled system versus laboratory culture.
With the model, we could generate a daily N2 fixation pattern qualitatively similar to the daily patterns measured by us in a laboratory culture of Trichodesmium (Fig. 2) and patterns known from natural populations and other cultures (39).
A special feature of Trichodesmium is that it fixes N2 during the day, concomitantly with photosynthesis. N2 fixation rates are related to photosynthetic activity on a short time scale. Incident irradiance was kept constant during the light period in both the laboratory culture and the model simulations. The observed diel pattern of N2 fixation is not correlated with incident irradiance, suggesting that the process of N2 fixation is not linearly related to light. Rather, the daily pattern had modulations, indicating the occurrence of internal regulatory mechanisms that result from cell history. In the laboratory culture, the increase in the rate of N2 fixation observed in the morning can be due either to (i) an increase in the fixation potential within N2-fixing cells, (ii) an increase in the number of cells that actually fix N2, or (iii) an increase in availability of the substrate (carbon) limiting the N2 fixation rate. The first two possibilities agree with the modeled increase in nitrogenase activity (whether in one cell or in several cells), whereas the third is the modulation of the N2 fixation rate by carbon availability. In the same way, declining rates in the afternoon follow deactivation of the enzyme but could also reflect a decrease in numbers of N2-fixing cells.
Because N2 fixation in the model depends only on physiological factors, enzymatic activities are not confined to the light period. Rather, we assume that diazotrophic activity is determined by nitrogen limitation and cell C/N stoichiometry. This assumption is based on two main ideas: (i) the synthesis and decay of the enzyme are activated and modulated by the internal nutrient and energy states of the cells and (ii) the potential fixation rate is enhanced by nitrogen starvation but limited by electrons originating from the small carbon metabolites or ATP produced by respiration or photosynthesis. As a result, the onset of enzyme activity starts before the onset of the light, and the activity varies along the light period although the applied irradiance is constant. Daily fluctuations calculated by the model proved to be in good agreement with the dynamics of N2 fixation observed in Trichodesmium populations (3), as well as with our own measurements (Fig. 2). Hitherto, models available in the literature have displayed patterns of nitrogenase activity that were most often directly related to light intensity (26, 33, 34). However the synthesis and activity of nitrogenase are probably under the control of a circadian clock (12, 43). In Synechococcus sp. strain Rf-1, the cyclic nitrogenase activity resulted from de novo synthesis of nitrogenase during the dark (13). In Trichodesmium, part of the enzymatic complex is destroyed during the afternoon and synthesized de novo toward the end of the night and the beginning of the day through genetic regulatory mechanisms (5, 53, 54). This transcriptional regulation cannot be described explicitly within our model, because it occurs at different scales than the processes we have focused on. However, regulation is still exerted by the metabolism in the model, which integrates processes at lower levels. In particular, the gene transcriptional level is implicitly involved in the calculation of the potential activity of nitrogenase through the feedback regulation exerted by the internal nitrogen status and a presumed regulatory mechanism based on the Csm and Nr stoichiometry (Fig. 4a and 5a). The model is then able to reproduce the daily patterns of the metabolism that result from these coupled influences of the environment and the internal status of cells.
Finally, the model shows that carbon availability is a simple and efficient mechanism for regulating the balance of carbon and nitrogen fixed (C/N ratio) in cells with a spatial separation of N2 fixation. We conclude that N2 fixation is ultimately determined by substrate availability and that this direct control prevents high fluctuations on a daily basis.
Conclusions.
The dynamics of N2 fixation are complex because they involve the influence of external as well as internal factors. The mathematical model presented here aimed to provide a scientific reflection tool conceptualizing the ecological determinants of the N2 fixation process in Trichodesmium. The combined representation of intracellular reserves of carbon and nitrogen and the description of the activity of nitrogenase are essential parts of the model formulation. The modeling is unconventional in that it precedes observations that could be used to test, validate, or falsify its assumptions. However, where available, the model simulation results agree qualitatively with observations. The model generates a correct timing of the onset of enzyme activity synthesis. Moreover, it also proved to represent the co-occurrence and transition between nitrogen uptake and N2 fixation, and we were able to reproduce a daily pattern of nitrogenase activity as observed in nature. This model is suitable for exploring the influence of environmental factors on the metabolism of this important cyanobacterium and the conditions promoting N2 fixation. In particular, we emphasize the strong relation between N2 fixation and carbon availability. The C/N ratio appeared to be dependent on the light regime. Fluctuations in cell C/N stoichiometry resulted from changes in carbon uptake, assimilation into the different cellular compartments, and energy consumption, according to growth requirements. With the simple mechanism of carbon-limited N2 fixation, the C/N ratio was balanced over days in the model. This modeling exercise thus emphasizes the need for acquisition of data on nitrogenase activity under various environmental conditions, as it may be substrate (carbon) limited. Substrate limitation can directly control C/N ratios and could thus precede the delayed genetic regulatory mechanisms based on the cellular C/N status.
Acknowledgments
We are very grateful to J. Zehr for his contructive comments on the manuscript.
This work was funded by a grant from the European Commission, contract MEIF-CT-2003-500516.
REFERENCES
- 1.Bergman, B., and E. J. Carpenter. 1991. Nitrogenase confined to randomly distributed trichomes in the marine cyanobacterium Trichodesmium thiebautii. J. Phycol. 27:158-165. [Google Scholar]
- 2.Bergman, B., J. R. Gallon, A. N. Rai, and L. J. Stal. 1997. N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol. Rev. 19:139-185. [Google Scholar]
- 3.Berman-Frank, I., J. T. Cullen, Y. Shaked, R. M. Sherrell, and P. G. Falkowski. 2001. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 46:1249-1260. [Google Scholar]
- 4.Berman-Frank, I., P. Lundgren, Y.-B. Chen, H. Kupper, Z. Kolber, B. Bergman, and P. Falkowski. 2001. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294:1534-1537. [DOI] [PubMed] [Google Scholar]
- 5.Capone, D. G., J. M. O'Neil, J. Zehr, and E. J. Carpenter. 1990. Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 56:3532-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capone, D. G., J. P. Zehr, H. W. Paerl, B. Bergman, and E. J. Carpenter. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221-1229. [Google Scholar]
- 7.Carpenter, E. J., B. Bergman, R. Dawson, P. J. A. Siddiqui, E. Soderback, and D. G. Capone. 1992. Glutamine-synthetase and nitrogen cycling in colonies of the marine diazotrophic cyanobacteria Trichodesmium spp. Appl. Environ. Microbiol. 58:3122-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter, E. J., and T. Roenneberg. 1995. The marine planktonic cyanobacteria Trichodesmium spp.—photosynthetic rate measurements in the SW Atlantic Ocean. Mar. Ecol. Prog. Ser. 118:267-273. [Google Scholar]
- 9.Carpenter, E. J., and K. Romans. 1991. Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254:1356-1358. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter, E. J., A. Subramaniam, and D. G. Capone. 2004. Biomass and primary productivity of the cyanobacterium Trichodesmium spp. in the tropical N Atlantic Ocean. Deep-Sea Res. Pt. I 51:173-203. [Google Scholar]
- 11.Cavender-Bares, K. K., D. M. Karl, and S. W. Chisholm. 2001. Nutrient gradients in the western North Atlantic Ocean: relationship to microbial community structure and comparison to patterns in the Pacific Ocean. Deep-Sea Res. Pt. I 48:2373-2395. [Google Scholar]
- 12.Chen, Y. B., J. P. Zehr, and M. Mellon. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. J. Phycol. 32:916-923. [Google Scholar]
- 13.Chow, T. J., and F. R. Tabita. 1994. Reciprocal light-dark transcriptional control of Nif and Rbc expression and light-dependent posttranslational control of nitrogenase activity in Synechococcus sp. strain Rf-1. J. Bacteriol. 176:6281-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coles, V. J., R. R. Hood, M. Pascual, and D. G. Capone. 2004. Modeling the impact of Trichodesmium and nitrogen fixation in the Atlantic Ocean. J. Geophys. Res. Oceans 109:C06007. doi: 10.1029/2002JC001754. [DOI]
- 15.Droop, M. R. 1973. Some thoughts on nutrient limitation in algae. J. Phycol. 9:264-272. [Google Scholar]
- 16.Falkowski, P. G. 1997. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387:272-275. [Google Scholar]
- 17.Faugeras, B., M. Levy, L. Memery, J. Verron, J. Blum, and I. Charpentier. 2003. Can biogeochemical fluxes be recovered from nitrate and chlorophyll data? A case study assimilating data in the Northwestern Mediterranean Sea at the JGOFS-DYFAMED station. J. Mar. Syst. 40:99-125. [Google Scholar]
- 18.Fennel, K., Y. H. Spitz, R. M. Letelier, M. R. Abbott, and D. M. Karl. 2002. A deterministic model for N2 fixation at stn. ALOHA in the subtropical North Pacific Ocean. Deep-Sea Res. Pt. II 49:149-174. [Google Scholar]
- 19.Flynn, K. J., and M. J. R. Fasham. 2003. Operation of light-dark cycles within simple ecosystem models of primary production and the consequences of using phytoplankton models with different abilities to assimilate N in darkness. J. Plankt. Res. 25:83-92. [Google Scholar]
- 20.Fu, F. X., and P. R. F. Bell. 2003. Factors affecting N2 fixation by the cyanobacterium Trichodesmium sp. GBR-TRLI101. FEMS Microbiol. Ecol. 45:203-209. [DOI] [PubMed] [Google Scholar]
- 21.Gallon, J. R. 2001. N2 fixation in phototrophs: adaptation to a specialized way of life. Plant Soil 230:39-48. [Google Scholar]
- 22.Gallon, J. R., J. J. Cheng, L. J. Dougherty, V. A. Gallon, H. Hilz, D. M. Pederson, H. M. Richards, S. Ruggeberg, and C. J. Smith. 2000. A novel covalent modification of nitrogenase in a cyanobacterium. FEBS Lett. 468:231-233. [DOI] [PubMed] [Google Scholar]
- 23.Geider, R. J., H. L. MacIntyre, and T. M. Kana. 1998. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 43:679-694. [Google Scholar]
- 24.Holl, C. M., and J. P. Montoya. 2005. Interactions between nitrate uptake and nitrogen fixation in continuous cultures of the marine diazotroph Trichodesmium (Cyanobacteria). J. Phycol. 41:1178-1183. [Google Scholar]
- 25.Hood, R. R., N. R. Bates, D. G. Capone, and D. B. Olson. 2001. Modeling the effect of nitrogen fixation on carbon and nitrogen fluxes at BATS. Deep-Sea Res. Pt. II 48:1609-1648. [Google Scholar]
- 26.Hood, R. R., A. Subramaniam, L. R. May, E. J. Carpenter, and D. G. Capone. 2002. Remote estimation of nitrogen fixation by Trichodesmium. Deep-Sea Res. Pt. II 49:123-147. [Google Scholar]
- 27.Kana, T. M. 1993. Rapid oxygen cycling in Trichodesmium thiebautii. Limnol. Oceanogr. 38:18-24. [Google Scholar]
- 28.Karl, D., A. Michaels, B. Bergman, D. Capone, E. Carpenter, R. Letelier, F. Lipschultz, H. Paerl, D. Sigman, and L. Stal. 2002. Dinitrogen fixation in the world's oceans. Biogeochemistry 57/58:47-98. [Google Scholar]
- 29.Lancelot, C., and G. Billen. 1985. Carbon-nitrogen relationships in nutrient metabolism of coastal marine ecosystems. Adv. Aquat. Microbiol. 3:263-321. [Google Scholar]
- 30.Lancelot, C., G. Billen, C. Veth, S. Becquevort, and S. Mathot. 1991. Modeling carbon cycling through phytoplankton and microbes in the Scotia-Weddell Sea area during sea ice retreat. Mar. Chem. 35:305-324. [Google Scholar]
- 31.Lee, K., D. M. Karl, R. Wanninkhof, and J.-Z. Zhang. 2002. Global estimates of net carbon production in the nitrate-depleted tropical and subtropical oceans. Geophys. Res. Lett. 29:1907. doi: 10.1029/2001GL014198. [DOI] [Google Scholar]
- 32.Letelier, R. M., and D. M. Karl. 1996. Role of Trichodesmium spp. in the productivity of the subtropical North Pacific Ocean. Mar. Ecol. Prog. Ser. 133:263-273. [Google Scholar]
- 33.Levine, S. N., and W. M. Lewis, Jr. 1984. Diel variation of nitrogen fixation in Lake Valencia, Venezuela. Limnol. Oceanogr. 29:887-893. [Google Scholar]
- 34.Levine, S. N., and W. M. Lewis, Jr. 1987. A numerical model of nitrogen fixation and its application to Lake Valencia. Freshwat. Biol. 17:265-274. [Google Scholar]
- 35.Moore, J. K., S. C. Doney, D. M. Glover, and I. Y. Fung. 2002. Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep-Sea Res. Pt. II 49:463-507. [Google Scholar]
- 36.Moore, J. K., S. C. Doney, J. A. Kleypas, D. M. Glover, and I. Y. Fung. 2002. An intermediate complexity marine ecosystem model for the global domain. Deep-Sea Res. Pt. II 49:403-462. [Google Scholar]
- 37.Mulholland, M. R., and P. W. Bernhardt. 2005. The effect of growth rate, phosphorus concentration, and temperature on N2 fixation, carbon fixation, and nitrogen release in continuous cultures of Trichodesmium IMS101. Limnol. Oceanogr. 50:839-849. [Google Scholar]
- 38.Mulholland, M. R., and D. G. Capone. 1999. Nitrogen fixation, uptake and metabolism in natural and cultured populations of Trichodesmium spp. Mar. Ecol. Prog. Ser. 188:33-49. [Google Scholar]
- 39.Mulholland, M. R., and D. G. Capone. 2000. The nitrogen physiology of the marine N2-fixing cyanobacteria Trichodesmium spp. Trends Plant Sci. 5:148-153. [DOI] [PubMed] [Google Scholar]
- 40.Mulholland, M. R., K. Ohki, and D. G. Capone. 2001. Nutrient controls on nitrogen uptake and metabolism by natural populations and cultures of Trichodesmium (Cyanobacteria). J. Phycol. 37:1001-1009. [Google Scholar]
- 41.Platt, T., C. L. Gallegos, and W. G. Harrison. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38:687-701. [Google Scholar]
- 42.Roenneberg, T., and E. J. Carpenter. 1993. Daily rhythm of O2-evolution in the cyanobacterium Trichodesmium thiebautii under natural and constant conditions. Mar. Biol. 117:693-697. [Google Scholar]
- 43.Schneegurt, M. A., D. L. Tucker, J. K. Ondr, D. M. Sherman, and L. A. Sherman. 2000. Metabolic rhythms of a diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, heterotrophically grown in continuous dark. J. Phycol. 36:107-117. [Google Scholar]
- 44.Soetaert, K., V. deClippele, and P. Herman. 2002. FEMME, a flexible environment for mathematically modelling the environment. Ecol. Model. 151:177-193. [Google Scholar]
- 45.Soetaert, K., P. M. J. Herman, J. J. Middelburg, C. Heip, C. L. Smith, P. Tett, and K. Wild-Allen. 2001. Numerical modelling of the shelf break ecosystem: reproducing benthic and pelagic measurements. Deep-Sea Res. Pt. II 48:3141-3177. [Google Scholar]
- 46.Staal, M., S. T. L. Hekkert, P. Herman, and L. J. Stal. 2002. Comparison of models describing light dependence of N2 fixation in heterocystous cyanobacteria. Appl. Environ. Microbiol. 68:4679-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staal, M., S. T. Lintel-Hekkert, F. Harren, and L. Stal. 2001. Nitrogenase activity in cyanobacteria measured by the acetylene reduction assay: a comparison between batch incubation and on-line monitoring. Environ. Microbiol. 3:343-351. [DOI] [PubMed] [Google Scholar]
- 48.Staal, M., F. J. R. Meysman, and L. J. Stal. 2003. Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans. Nature 425:504-507. [DOI] [PubMed] [Google Scholar]
- 49.Stephens, N., K. J. Flynn, and J. R. Gallon. 2003. Interrelationships between the pathways of inorganic nitrogen assimilation in the cyanobacterium Gloeothece can be described using a mechanistic mathematical model. New Phytol. 160:545-555. [DOI] [PubMed] [Google Scholar]
- 50.Tett, P. 1998. Parameterising a microplankton model. Napier University, Edinburgh, United Kingdom.
- 51.Tyrrell, T. 1999. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400:525-531. [Google Scholar]
- 52.Van den Meersche, K., J. J. Middelburg, K. Soetaert, P. van Rijswijk, H. T. S. Boschker, and C. H. R. Heip. 2004. Carbon-nitrogen coupling and algal-bacterial interactions during an experimental bloom: modeling a C-13 tracer experiment. Limnol. Oceanogr. 49:862-878. [Google Scholar]
- 53.Wyman, M., J. P. Zehr, and D. G. Capone. 1996. Temporal variability in nitrogenase gene expression in natural populations of the marine cyanobacterium Trichodesmium thiebautii. Appl. Environ. Microbiol. 62:1073-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zehr, J. P., M. Wyman, V. Miller, L. Duguay, and D. G. Capone. 1993. Modification of the Fe protein of nitrogenase in natural populations of Trichodesmium thiebautii. Appl. Environ. Microbiol. 59:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]








