Abstract
The microbial community of a pig slurry on a farm was monitored for 6 months using both molecular and cultural approaches. Sampling was carried out at all the different stages of effluent handling, from the rearing build-up to slurry spreading. Total DNA of each sample was extracted and analyzed by PCR-single-strand conformation polymorphism (SSCP) analysis using primers targeting the 16S rRNA genes from the archaeal and bacterial domains and also the Eubacterium-Clostridium, Bacillus-Streptococcus-Lactobacillus, and Bacteroides-Prevotella groups. A comparison of the SSCP profiles showed that there were rapid changes in the dominant bacterial community during the first 2 weeks of anaerobic storage and that the community was relatively stable thereafter. Several bacterial populations, identified as populations closely related to uncultured Clostridium and Porphyromonas and to Lactobacillus and Streptococcus cultured species commonly isolated from pig feces, remained present and dominant from the rearing build-up to the time of spreading. Enumeration of fecal indicators (enterococci and Escherichia coli) performed in parallel using cultural methods revealed the same trends. On the other hand, the archaeal community adapted slowly during pig slurry storage, and its diversity increased. A shift between two hydrogenotrophic methanogenic Methanobrevibacter populations from the storage pit to the pond was observed. Microorganisms present in pig slurry at the time of spreading could not be detected in soil after spreading by either molecular or cultural techniques, probably because of the detection limit inherent in the two techniques.
The majority of pigs produced in industrialized countries are raised in buildings that are especially designed for this type of husbandry. The livestock buildings are entirely covered and insulated, and the pigs are housed in stalls with concrete floors for easy evacuation of feces and urine. Pits located below the animals receive the excrement for temporary indoor storage. After a few weeks, the effluent is generally evacuated to a large outdoor storage tank, where it stays for 4 to 6 months until it can be spread onto arable land (5). Although this type of husbandry is a success for pig production, the different stages of waste management generate severe air, soil, and water pollution (5, 14, 24, 25, 46, 47). Moreover, spreading large amounts of manure onto fields raises questions about the sanitary aspects of such practices (5, 7, 26, 27, 30).
The pig slurry microbial community, which was previously characterized by cultural methods (15, 16, 38), has recently been characterized by molecular techniques (37, 43). Of the 109 to 1010 cells per ml present in pig slurry, only 10 to 20% could be cultured on various media based on pig slurry (8, 33). Moreover, less than 50% of the phylotypes observed in pig slurry by 16S rRNA gene analysis were closely related to known microorganisms (37, 43). Nevertheless, based on both cultural and molecular approaches, the pig slurry microbial community appears to be an anaerobic digestion ecosystem that is dominated by groups of fermentative bacteria, such as the Eubacterium-Clostridium (EC) and Lactobacillus-Streptococcus groups of low-G+C-content gram-positive bacteria and the Bacteroides group of gram-negative bacteria. The members of the domain Archaea are dominated by hydrogenotrophic methanogens, as well as by a group of uncultured Archaea whose function is unknown.
Despite the increasing interest now being paid to characterization of pig slurry microbiology (48), few workers have tried to evaluate the impact of pig slurry storage conditions on the composition of the slurry microbial community (2, 26, 27, 33, 49). In most of the studies the researchers used cultural methods and focused on the fate of pathogenic or fecal indicators (26, 27). These studies have shown that the numbers of fecal indicators and pathogenic bacteria decrease rapidly and exponentially with time during pig slurry storage. In contrast, analyses of the whole microbial community by cultural (2, 33) and molecular techniques (20) have suggested that a more static view is possible and that the dominant microbial groups are relatively stable. A more systematic analysis of the pig slurry microbial community dynamics in pig-rearing structures should contribute to our understanding of metabolic transformations which occur during slurry storage. The aim of this work was to monitor the dynamics of a pig slurry microbial community throughout an intensive production installation in order to evaluate the impact of each waste management unit on the pig slurry microbial community. Dominant bacterial and archaeal communities were monitored by 16S rRNA gene-targeted PCR amplification and single-strand conformation polymorphism (SSCP) analysis (18, 28, 36, 50). Fecal indicators (enterococci and Escherichia coli) were monitored by using cultural methods. The most prominent microbial populations observed by PCR-SSCP analysis were identified by DNA sequence analysis.
MATERIALS AND METHODS
Farm description and sampling.
Sampling was carried out between November 2002 and June 2003 on an intensive pig-fattening farm located in southwest France near Albi. This farm breeds 220 Broad-White sows plus finishers that weigh up to 100 kg (live weight) and produces about 4,500 m3 of pig slurry per year. On this farm, pig slurry is stored for about 2 weeks in pits located under stalls before it is evacuated by a flushing procedure to a large, outside, covered, homogenized tank with a total capacity of 800 m3. The flushing procedure is operated by pumping the pig slurry from the outside storage tank back into the rearing house pits in order to avoid accumulation of solids in the indoor pits. After a few weeks of storage in the outdoor tank, the pig slurry is treated in a second stage with an auger press to separate the liquid and solid parts. The liquid part is evacuated to a pond with a capacity of 2,500 m3, while the solid part is stored in an open building. After storage for 4 to 6 months, the liquid from the pond is spread on farmland at a level of about 170 kg hectare−1 (about 69 kg acre−1) of organic nitrogen from livestock waste.
A total of 28 samples were taken at the different stages of the pig slurry accumulation and handling process. Slurry samples were collected at different locations within the indoor pits with a sampling cane and then mixed together. Samples from the outdoor storage tank were taken every 2 or 3 weeks after 1 h of homogenization, starting with the initial filling in December 2002. The pond was sampled four times during refilling in March 2003. Soil sampling was carried out 15 days before and immediately before pig slurry spreading. Samples were also taken just after spreading and during a subsequent 2-month period. At each sampling time, nine core samples from a depth of 0 to 15 cm were obtained with a drill and thoroughly mixed. After mixing, a subsample consisting of about 1 kg of soil was collected. All samples were brought to the laboratory within 12 h.
Physicochemical characterization of pig slurry samples.
pH was determined by direct measurement with a glass electrode pH meter. Total ammonia nitrogen and total Kjeldahl nitrogen were analyzed by steam distillation coupled with a titration unit. For total nitrogen, prior mineralization at 350°C was required to reduce all nitrogen forms to ammonium. The dry matter was analyzed by drying samples at 105°C until the weight was constant. The volatile fatty acid content was analyzed by high-performance liquid chromatography (29).
Enumeration of classical fecal indicators by cultural techniques.
Enumeration was performed by using 25 g of solid samples or, for liquid samples, a 25-ml pellet obtained by centrifugation at 8,000 rpm for 12 min of 500 ml of pig slurry. Samples were homogenized in a stomacher bag with 225 ml of tryptone-salt solution for 10 min. Plate counts of enterococci were determined by using kanamycin esculin azide agar base (Oxoid) that was incubated at 37°C for 24 h. E. coli counts were determined using Select E. coli Count Plate Petrifilm (3 M) that was incubated at 44°C for 24 h. The data were expressed as numbers of CFU per gram (dry weight). Means of unadjusted variables were compared using Student's t test.
DNA extraction.
Liquid pig slurry samples (10 ml) were centrifuged for 10 min at 17,500 × g. Each pellet was homogenized in a mortar containing 4 ml of 4 M guanidine thiocyanate-0.1 M Tris-HCl (pH 7.5) and 0.6 ml of 10% N-lauroyl sarcosine. Fresh soil samples were sieved using a 0.04-cm2 mesh. Ten-gram aliquots were crushed in a mortar in the same way with 8 ml of guanidine thiocyanate and 1.2 ml of N-lauroyl sarcosine. Four 0.5-ml aliquots were transferred into microtubes and immediately stored at −20°C. Nucleic acids were extracted as described previously (13) except for the soil samples, to which 0.2 ml of aluminum ammonium bisulfate [AlNH4(SO4)2] was added at the beginning of the extraction procedure in order to eliminate most PCR inhibitors (3).
Direct and nested PCR-SSCP analysis of microbial 16S rRNA genes.
The pig slurry bacterial and archaeal communities were analyzed by PCR amplification of the 16S rRNA gene V3 region using the W49-W104 and W96-W104 primer pairs, respectively (Table 1), and Pfu Turbo DNA polymerase (Stratagene) as described by Chachkhiani et al. (6). Twenty-five cycles of amplification were performed with a GeneAmp 9700 thermocycler (PE Applied Biosystems). The resulting PCR products were then separated by SSCP capillary electrophoresis with an ABI 310 genetic analyzer (Applied Biosystems) as described by Delbes et al. (10).
TABLE 1.
Sequences and target positions of the primers used in this study
| Primer | Directiona | Primer sequence (5′-3′) | E. coli positionc | Targeted 16S rRNA | Reference |
|---|---|---|---|---|---|
| W18 | F | GAGTTTGATCMTGGCTCAG | 9 | Bacteria | 13 |
| W17 | F | ATTCYGGTTGATCCYGSCRG | 6 | Archaea | 13 |
| W02 | R | GNTACCTTGTTACGACTT | 1509 | Universal | 42 |
| W49 | F | AGGTCCAGACTCCTACGGG | 330 | Bacteria | 9 |
| W96 | F | TCCAGGCCCACGGGG | 333 | Archaea | 9 |
| W104 | R | TTACCGCGGCTGCTGGCACb | 500 | Universal | 9 |
| W31 | R | TTACCGCGGCTGCTGGCAC | 500 | Universal | 9 |
| W108 | R | ATTYCACCGCTACACATG | 679 | Bacillus, Lactobacillus, Lactococcus, Pediococcus, Leuconostoc, Weissella, Streptococcus, Enterococcus, Staphylococcus | 41 |
| W109 | R | CCCTTTACACCCAGTAA | 561 | Eubacterium, Clostridiaceae (clusters I, III, IV, XIVa, XIVb, Rumen) | 40 |
| W112 | R | TCACCGTTGCCGGCGTACTC | 887 | Prevotella, Bacteroides, Porphyromonas | 45 |
F, forward; R, reverse.
Labeled with the fluorophore 6-carboxyfluorescein.
Data from reference 4.
A more precise analysis of dominant phylogenetic groups was carried out by nested PCR. In the first step, the 16S rRNA genes of the targeted bacterial group were amplified using AccuPrime Taq DNA polymerase (Invitrogen, The Netherlands) and a group-specific primer (W108, W109, or W112) along with a bacterial domain primer (W18). On the basis of the June 2002 ARB database release (updated with deposited sequences related to pig fecal flora and pig slurry flora [19, 31, 37, 43]), primer W108 targets 60% of the sequences belonging to the Bacillus-Streptococcus-Lactobacillus (BSL) group (41), primer W109 targets 80% of the sequences belonging to the Eubacterium-Clostridium (EC) group (40), and W112 targets 85% of the sequences belonging to the Bacteroides-Prevotella (BP) group (45). PCRs were performed according to recommendations of the supplier (Invitrogen). The reaction mixtures contained about 100 ng of sample genomic DNA, 1× AccuPrime Taq polymerase buffer, 50 ng of each primer, 2.5 U of AccuPrime Taq polymerase (Invitrogen), and enough H2O to bring the volume to 25 μl. The PCR conditions were as follows: denaturation for 2 min at 94°C and then 30 cycles of 30 s at 94°C, 30 s at the annealing temperature, and 90 s at 68°C. No final elongation was performed, as recommended by the supplier (Invitrogen). The reaction was stopped by cooling the mixture to 4°C. The annealing temperatures of the primers were 52°C for W108, 50°C for W109, and 61°C for W112. Amplification product sizes were confirmed by electrophoresis on a 0.7% (wt/vol) agarose gel. Products obtained from the PCR were diluted 100-fold, and 1 μl of each product was used as a template for a PCR-SSCP analysis carried out using the primers and conditions described above.
Clone library, sequencing, and identification of dominant 16S rRNA gene fragments.
The dominant peaks in the SSCP profiles for pig slurry samples collected from the storage tank on day 21 were identified. For the Archaea, the 16S rRNA gene V3 region was amplified from total DNA using the redTaq DNA polymerase (Sigma, France) and the archaeal W96-W31 primer pair (Table 1). For each targeted microbial group (BSL, BP, and EC groups), the AccuPrime Taq polymerase PCR products obtained as described above were amplified again using bacterial primers W49 and W31 (Table 1). These amplifications generated the same DNA fragments that the amplifications performed for the SSCP analysis generated, except that, since the W31 primer was not labeled, the DNA fragments could be cloned into E. coli using a TOPO-TA vector cloning kit (Invitrogen) to generate 16S rRNA gene V3 libraries. Then about 40 clones from each library (45 clones for the BSL library, 42 clones for the BP library, 52 clones for the EC library, and 40 clones for the Archaea library) were randomly picked, and their inserts were screened by PCR-SSCP analysis as described previously (6). Inserts that comigrated with distinguishable peaks from the total DNA SSCP profile were sequenced to finalize the identification of peaks. DNA sequences were obtained using a dye terminator cycle sequencing Ready Reaction kit (Big Dye Terminator; Applied Biosystems) and a 373A Genetic Analyzer from Applied Biosystems. DNA sequences were identified by comparison with their closest relatives available in databases using BLAST from the National Center for Biotechnology Information and the Ribosomal Database Project (1, 23) and by fitting the sequences into preexisting trees using the parsimony interactive tool from the ARB software package (22).
Nucleotide sequence accession numbers.
Sequences have been deposited in the EMBL database under accession numbers AM229418 to AM229448.
RESULTS
Physical and chemical characteristics of the effluent.
Pig slurry collected from the storage tank and pond had chemical characteristics similar to those usually observed in European countries (29). The pH was around 7; the dry matter contents were 3.6% and 2.1% (wt/wt), respectively; the total Kjeldahl nitrogen contents were 3.1 and 3.6 g · liter−1, respectively; and the ammonium contents were 80% and 45% of the total nitrogen, respectively. The concentrations of total volatile fatty acids were 6,888 mg · liter−1 in the storage tank and 331 g · liter−1 in the pond. As expected, the dry matter, total nitrogen, ammonia nitrogen, and volatile fatty acid contents were lower in the pond than in the storage tank. The differences resulted from the action of the press auger located between the outlet of the storage tank and the pond that received only the liquid phase of the manure. The distributions of the volatile fatty acids were the same in the two storage units: 60% acetic acid, 25% propionic acid, 5% butyric acid, and 10% isobutyric and isovaleric acids.
Bacterial 16S rRNA gene dynamics in pig slurry.
The population dynamics of the pig slurry microbial community throughout the pig-rearing system were determined by 16S rRNA gene-targeted PCR-SSCP analysis.
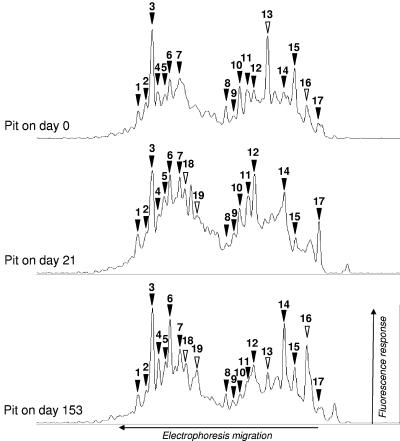
The SSCP profiles obtained for fresh slurry samples (less than 2 weeks of storage) collected from the indoor pit at three different times had 15 to 20 distinguishable peaks that clearly emerged from the background of subdominant bacterial diversity (Fig. 1) (21). The presence of several comigrating peaks in the profiles suggests that there were microbial populations that were present in all samples.
FIG. 1.
Comparison of bacterial 16S rRNA gene-targeted PCR-SSCP profiles for fresh pig slurry (less than 2 weeks old) collected from the fattening building pit at three different times. SSCP electrophoresis was performed from right to left. The horizontal and vertical axes indicate time (number of scans) and detection of fluorescently labeled PCR products, respectively. The SSCP profiles for each sample are aligned for comparison. Peaks that comigrated in all profiles are indicated by solid arrowheads, while peaks 13, 16, 18, and 19, which comigrated in only two profiles, are indicated by open arrowheads. Peaks that were present in only one profile are not labeled.
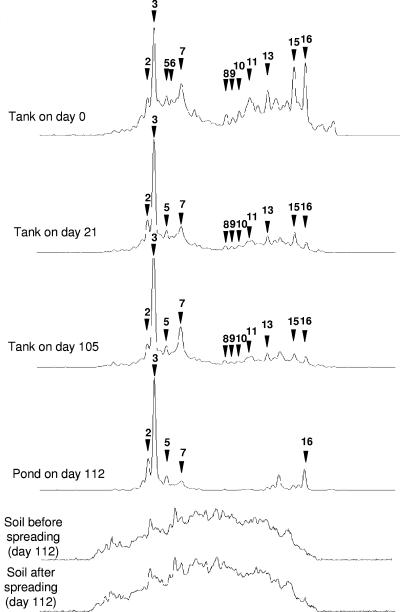
The bacterial community SSCP profiles obtained during several stages of the slurry management process, including pig slurry storage in the outdoor tank and pond and subsequent spreading on the field, are aligned in Fig. 2. The profile for the storage tank at zero time, when the tank was almost empty, shares several comigrating peaks with profiles obtained for pit fresh pig slurry, as shown in Fig. 1. However, the relative proportions of these peaks in the profile are different. After about 2 weeks of pig rearing and the addition of fresh pig slurry to the storage tank, the SSCP profile changed, and peak 3 became dominant in the community profile (day 21) (Fig. 2). Nevertheless, numerous small peaks remained in the profile and continued to comigrate with distinguishable peaks in the pit pig slurry SSCP profiles, suggesting that high microbial diversity persisted in the slurry during storage. After this, for the next 6 months of sampling, the SSCP profiles obtained for the storage tank samples remained very similar (Fig. 2) except for peak 7, whose relative proportion in the profiles seemed to increase.
FIG. 2.
Modification of SSCP profiles for the bacterial pig slurry community with time in the storage tank and at the time of spreading onto the field. SSCP profiles obtained for pig slurry collected at different times in the storage tank and at the time of spreading in the pond and onto the soil are aligned for comparison. Peaks present in Fig. 1 are indicated by arrowheads, and the same numbers are used.
The bacterial community SSCP profile for the pond sampled just before spreading indicates that there was a further apparent reduction in the bacterial diversity compared to the pig slurry profiles for the storage tank (Fig. 2). However, the majority of the peaks that were distinguishable in the pond SSCP profile comigrated with peaks present in the storage tank pig slurry profile. The analysis of four other pond samples obtained from April to June 2003 revealed that the bacterial community SSCP profiles for this structure remained the same (data not shown).
Finally, alignment of the pond SSCP profile with soil SSCP profiles obtained both just before and after spreading of the liquid from the pond showed that none of the dominant peaks observed in the pond profile could be detected in soil after spreading. This remained true for soil samples collected 3, 7, 15, 28, 49, and 77 days after spreading (data not shown).
Monitoring of bacterial subgroups.
The data presented above suggest that the bacterial community was stable throughout the pig slurry storage system. However, the use of general bacterial primers, the great diversity observed, and the presence of a very dominant peak (peak 3 in this study) may hide the variation of less dominant microbial groups (11). In order to better visualize the data, a nested PCR approach was used to artificially divide the bacterial SSCP profile. A set of primers targeting the Eubacterium-Clostridium, Bacillus-Streptococcus-Lactobacillus, and Bacteroides-Prevotella groups was used in combination with a general bacterial primer. According to data published previously (19, 31, 37, 43), these primers target more than 80% of the phylotypes observed in pig feces and pig slurry.
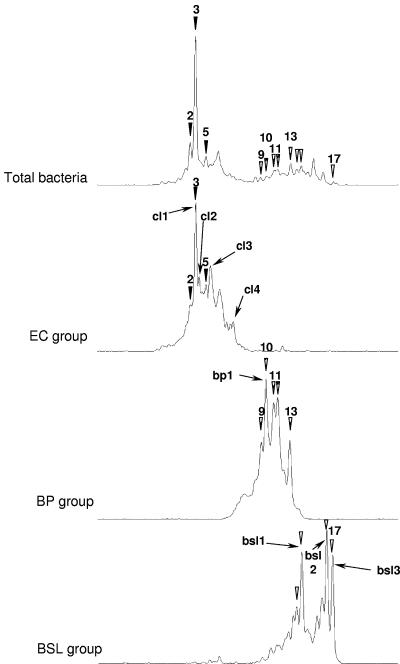
Figure 3 shows an alignment of the SSCP profiles obtained for the same pig slurry sample for the bacterial community and for each targeted group. Each specific microbial group profile presents an individualized window of electrophoretic mobility in agreement with the expected size of its 16S rRNA V3 region that is centered around 179, 199, and 204 nucleotides for the EC, BP, and BSL groups, respectively. Use of this group-specific approach revealed diversity that was not detectable in the bacterial profiles. Nevertheless, several dominant peaks for each group comigrated with dominant or distinguishable peaks in the total Bacteria profile (Fig. 3). Thus, the very dominant peak 3 of the bacterial profile comigrated perfectly with the dominant peak of the EC profile.
FIG. 3.
Comparison of the bacterial SSCP profile with profiles obtained after nested PCR-SSCP targeting the Clostridiaceae (EC group), the BP group, and the BSL group. Amplification was performed with storage tank pig slurry samples obtained on day 21. The peak numbers are the same as the numbers in Fig. 1 and 2. Peaks that comigrated in the bacterial profile and group profiles are indicated by solid arrowheads for the EC group, by striped arrowheads for the BP group, and by open arrowheads for the BSL group. Peaks that could be identified are designated cl1 to cl4, bp1, and bsl1 to bsl3, as shown in Table 3.
This set of primers was used to monitor the population dynamics of each microbial group individually in the pig-rearing system and at the time of pig slurry spreading. As we observed for the bacterial community, the SSCP profiles obtained for the EC, BSL, and BP groups remained very similar over time for the pig slurry storage tank and to some degree for the pond. However, none of them was detected in soil after spreading (data not shown).
Identification of the persistent bacterial populations.
We attempted to identify the dominant peaks observed for each microbial group by cloning and sequencing the corresponding 16S rRNA gene fragments, as described in Materials and Methods. Most of the dominant peaks in the EC and BSL profiles could be identified, while only the dominant peak in the BP profile was assigned (Fig. 3). The phylogenetic affiliations are shown in Table 2. The closest relatives of five of the eight sequences identified were sequences from uncultured bacteria that were obtained either from pig gastrointestinal tracts (peaks cl3 and bp1) (19), from a bioreactor treating piggery waste (peaks cl1 and cl2) (unpublished data), or from a pig slurry storage pit (peak cl4) (43).
TABLE 2.
Phylogenetic affiliations of 16S rRNA gene sequences
| Peak designation | Sequence length (bp) | Closest relative
|
|||
|---|---|---|---|---|---|
| Name (accession no.) | Affiliation group | % Similarity | Source | ||
| cl1 | 141 | Clone THM-10 (AY147280) | Clostridium botulinum, cluster I | 100 | Thermophilic bioreactor treating piggery waste |
| cl2 | 141 | Clone THM-8 (AY147278) | Clostridium botulinum, cluster Rumen | 98 | Thermophilic bioreactor treating piggery waste |
| cl3 | 144 | Clone p-249-o5 (AF371793) | Clostridium leptum, cluster IV | 95 | Pig gastrointestinal tract |
| cl4 | 144 | Clone P316 (AF261803) | Clostridium leptum, cluster IV | 91 | Swine manure storage pit |
| bp1 | 162 | Clone p-987-s962-5 (AF371910) | Porphyromonas | 97 | Pig gastrointestinal tract |
| bsl1a | 166 | Clone SM8-19 (AY773140) | Lactobacillus | 100 | Swine manure |
| bsl2a | 167 | Streptococcus alactolyticus (AF201899) | Streptococcus | 99 | Swine feces |
| bsl3a | 167 | Lactobacillus crispatus (AF257097) | Lactobacillus | 95 | Human gut |
| c | 119 | Methanogenium organophilum (M59131) | Methanomicrobiales | 97 | Sediment |
| d | 124 | Methanobrevibacter ruminantium (AY196666) | Methanobacteriales | 99 | Bovine rumen |
| e | 123 | Methanobrevibacter smithii (AY196669) | Methanobacteriales | 100 | Intestinal tract of animal and sewage sludge |
Because of the short length of the 16S rRNA V3 region sequence, several other Lactobacillus (for peaks bsl1 and bsl3) or Streptococcus (for peak bsl2) sequences exhibited the same level of similarity as the closest relative shown.
Archaeal 16S rRNA gene dynamics.
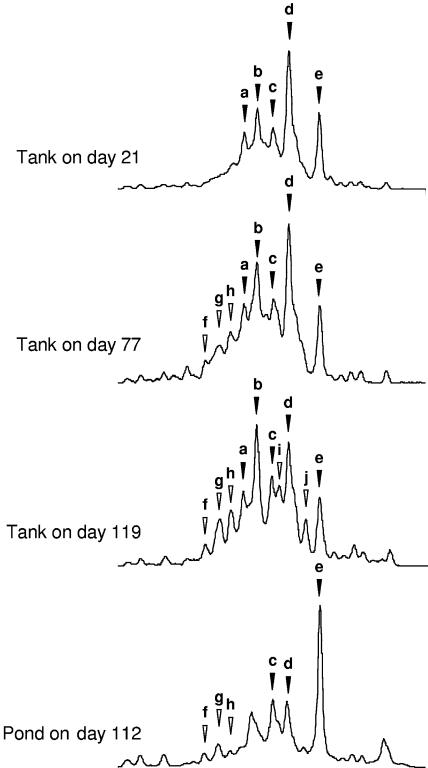
Monitoring of the archaeal community did not require a nested PCR and was performed by direct amplification of the archaeal 16S rRNA gene V3 region with domain-specific primers. In contrast to the results obtained for the bacterial groups, the diversity of the pig slurry archaeal community appeared to increase slowly during storage (Fig. 4). While the profiles at the beginning of the storage period contained only five dominant peaks (peaks a, b, c, d, and e), the profile obtained after 3 months of storage contained at least five other distinguishable peaks (peaks f, g, h, i, and j). At the same time, the dominance in the profile of peak d, identified as belonging to Methanobrevibacter ruminantium group I (Table 2), was slowly replaced by a dominant peak, peak b, that could not be identified.
FIG. 4.
Archaea pig slurry community dynamics during the pig slurry management process. The SSCP profiles for pig slurry samples collected at different times in the storage tank and in the pond are aligned for comparison. Peaks that dominated the profile at the beginning of pig slurry storage are indicated by solid arrowheads to facilitate comparison of the profiles. Peaks that appeared during storage are indicated by open arrowheads.
Samples collected from the pond produced a simplified profile with reduced apparent diversity and the remarkable dominance of peak e, which was identified as Methanobrevibacter smithii. Judging from the peak alignment, this peak, as well as peak d (M. ruminantium) and peak c (Methanogenium organophilum), persisted from the storage tank to the pond. Detection of the archaeal community in soil before and after pig slurry spreading was attempted, but the 16S rRNA gene could not be amplified.
Fecal indicator dynamics in pig slurry.
In order to obtain more insight into the behavior of the pig fecal microbial community, enterococci and E. coli were enumerated by cultural techniques during the pig slurry management process (Table 3). The concentrations of these microorganisms during the 6 months of storage were not significantly different for the building pit and the anaerobic storage tank, whereas the numbers of both fecal indicators in the pond decreased significantly (P < 0.05). In the soil there was no difference between samples collected before spreading and samples collected after spreading.
TABLE 3.
Average concentrations and ranges of concentrations of fecal indicators in the pig slurry management process
| Parameter | Concn of fecal indicators (CFU g−1 [dry wt])
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Building pit (day 0-day 189) (n = 4)a
|
Storage tank (day 21-day 189) (n = 8)
|
Pond (day 112-day 189) (n = 4)
|
Soil before spreading (day 84-day 112) (n = 2)
|
Soil after spreading (day 112-day 119) (n = 3)
|
||||||
| Enterococci | E. coli | Enterococci | E. coli | Enterococci | E. coli | Enterococci | E. coli | Enterococci | E. coli | |
| Mean | 8.6 × 106 | 7.9 × 106 | 1.4 × 107 | 4.5 × 106 | 1.8 × 106 | 1.1 × 105 | ||||
| Minimum | 2.0 × 105 | 1.3 × 106 | 6.8 × 104 | 5.3 × 105 | 9.5 × 104 | 8.2 × 102 | ||||
| Maximum | 1.6 × 107 | 2.0 × 107 | 2.5 × 107 | 6.3 × 106 | 4.2 × 106 | 2.7 × 105 | ≤2 × 102 | ≤12 | ≤9 × 102 | ≤12 |
n, number of samples.
DISCUSSION
In this study, we used 16S rRNA gene-targeted PCR-SSCP analysis coupled with capillary electrophoresis and classical cultural techniques to monitor a pig slurry microbial community from an indoor pig-rearing pit to spreading on fields.
When molecular monitoring of the microbial community was used, very good reproducibility was observed for the SSCP profiles obtained from samples collected from the different matrices (pig slurry, pond, and soil) and at different times during the experiment over several months (Fig. 2). This can be explained by technical factors such as (i) the homogenization of the samples, (ii) the reproducibility of the sampling, (iii) the DNA extraction method used, and (iv) the use of capillary electrophoresis and automated DNA band detection, but also by the fact that the PCR-SSCP profiles revealed only the most dominant populations in the microbial community (the populations that were least prone to sampling bias). However, two limitations were clear when general bacterial primers were used (Fig. 2). The first limitation was the reduction in the apparent diversity of the profile in the presence of a very dominant population. The second limitation was the very peculiar shape of the SSCP soil profiles, which may be explained both by the high bacterial diversity present in the soil and by the absence of any very dominant microbial group (21). These two limitations could be partially overcome by the use of a nested PCR-SSCP approach. Focusing on specific microbial groups permitted visualization of subdominant populations that were not clearly visible in the total bacterial profile and allowed easier peak identification (Fig. 3). Each microbial group had a specific window of electrophoretic migration, confirming that SSCP electrophoresis separates single-stranded DNA fragments first according to size and second according to the secondary structure (18). The absence of detection of the spread microorganisms in soil, by both molecular and cultural techniques, was in all likelihood due to the high dilution rate for manure in soil because of the spreading itself and the concomitant dilution of the manure microorganisms in the soil microbial community. With slurry spread at a level of 170 kg hectare−1 of organic N and a density of 109 bacteria ml−1 of slurry, the number of microorganisms spread on the soil was about 1012 cells m−2. If we assume that the density of microorganisms in soil is about 1010 bacteria g−1 (39) and the apparent density of soil is 1.5 kg liter−1, the bacteria added with the slurry represented about 0.1% of the soil microflora. This value is too low to be detected by the techniques used in this study.
From a manure management perspective, SSCP monitoring of the dominant microbial populations showed that the pig slurry microbial community changed primarily during the first 2 to 3 weeks of anaerobic storage and then after the slurry was transferred to the pond. In contrast, the community remained very stable during passive anaerobic storage in both units. Cultural enumeration of fecal indicators revealed the same trend. The only significant decrease occurred between the storage tank and the pond. This stability of fecal indicator concentrations observed during pig slurry anaerobic storage contrasts with the decrease previously reported in batch studies (26, 27, 33). The difference may have been due to the constant addition of fresh feces to the slurry in real systems, which may logically lead to stabilization of the number of fecal indicators. Indeed, passive conditions that prevail in anaerobic storage tanks may not foster rapid change in the microbial community. According to this hypothesis, important microbial changes were observed in this study when the community was moved from the feces to the manure and then from the storage tank to the pond. Previous studies have demonstrated the stronger effects of drastic conditions like aeration or thermophilic treatment for modifying the pig slurry microbial community (17, 20, 26, 27).
Both methods showed that several microbial populations remained present and dominant from rearing until the time of spreading. The fecal origin of the persistent microorganisms visualized by the SSCP profiles was confirmed by identification of the organisms. The eight bacterial SSCP peaks identified were closely related to microorganisms present in manure or feces (Table 3). Strongly dominant peak cl1 observed in stored pig slurry was identified as Clostridium disporicum (Y18176) and exhibited 99% sequence similarity for a 141-bp fragment. Although identification of microorganisms by using such a short sequence must be used carefully (35), it is interesting that this microorganism has been observed previously in a thermophilic aerobic digestor treating pig slurry (J. W. Lee, S. Y. Lee, and Y. K. Park, unpublished data) and as a persistent population in pig slurry after 7 weeks of aerated or unaerated storage (20). This group of Clostridium may be especially resistant to environmental changes.
The apparent stability of the pig slurry microbial community was tempered by the slow dynamics and increasing diversity observed for the archaeal community. Archaeal SSCP profiles showed that subdominant populations slowly adapted to their new environments in the storage tank and pond. All the peaks identified correspond to cultured H2/CO2− and/or formate-utilizing methanogens, but several subdominant peaks were not identified (Table 3). M. ruminantium, which was dominant in the community at the beginning of the storage stage, was replaced by M. smithii in the pond. Both of these species are commonly isolated from human or animal feces; however, the former requires more growth factors, including acetate (12). Differences between the concentration of organic matter (especially acetate) in the pig slurry in the storage tank and the concentration in the pond may explain this microbial shift. Peak c, which persisted until the end of storage, belongs to a group of microorganisms comprising M. organophilum, Methanogenium frigidum, and uncultured microorganisms observed in Antarctic sediment (32). The persistence of these archaeal groups is consistent with the methane production observed during pig slurry anaerobic storage (5 to 70 g C-CH4 · m−3 · day−1) (24). No acetotrophic methanogen was identified, despite the high acetate concentration in pig slurry. This absence has been noticed in two previous analyses of other pig slurry ecosystems (37, 44). However, we cannot rule out the possibility that such organisms were represented here by one of the subdominant unidentified peaks that appeared during the experiment.
The persistence of fecal microorganisms in human and animal organic wastes raises questions for all intensive manure management processes. This study showed that 16S rRNA gene-targeted molecular techniques can be used to detect microorganisms discharged into the environment by such processes. Further work should be done to identify these microorganisms and to determine their activity and quantity in effluent. For microorganisms that have not been cultured yet, this requires the use of quantitative molecular tools, such as real-time PCR (34).
Acknowledgments
This work received financial support from the GIS Porcherie Verte and from the Epiemerge program of INRA.
We thank G. Meynaud, ASAMIP (Castanet Tolosan Cedex, France), who carried out the sampling, and Théodore Bouchez (CEMAGREF, Antony, France) for helpful reading of the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisaillon, J. G., R. Beaudet, M. Sylvestre, M. Ishaque, A. Morin, E. Di Franco, and A. M. Guérin. 1984. Aspects microbiologiques du lisier de porc. Sci. Tech. Eau 17:397-400. [Google Scholar]
- 3.Braid, M. D., L. M. Daniels, and C. L. Kitts. 2003. Removal of PCR inhibitors from soil DNA by chemical flocculation. J. Microbiol. Methods 52:389-393. [DOI] [PubMed] [Google Scholar]
- 4.Brosius, J., T. J. Dull, D. Sleeter, and H. F. Noller. 1981. Genes organisation and primary structure of a ribosomal DNA operon from Escherichia coli. J. Mol. Biol. 148:107-127. [DOI] [PubMed] [Google Scholar]
- 5.Burton, C. H., and C. Turner. 2003. Manure management. Treatment strategies for sustainable agriculture, 2nd ed. Silsoe Research Institute, Bedford, United Kingdom.
- 6.Chackhiani, M., P. Dabert, T. Abzianidze, G. Partskhaladze, L. Tsiklauri, T. Dudauri, and J. J. Godon. 2004. 16S rDNA characterisation of bacterial and archaeal communities during start-up of anaerobic thermophilic digestion of cattle manure. Bioresour. Technol. 93:227-232. [DOI] [PubMed] [Google Scholar]
- 7.Chinivasagam, H. N., R. J. Thomas, K. Casey, E. McGahan, E. A. Gardner, M. Rafiee, and P. J. Blackall. 2004. Microbiological status of piggery effluent from 13 piggeries in the southeast Queensland region of Australia. J. Appl. Microbiol. 97:883-891. [DOI] [PubMed] [Google Scholar]
- 8.Cotta, M. A., T. R. Whitehead, and R. L. Zeltwanger. 2003. Isolation, characterization and comparison of bacteria from swine faeces and manure storage pits. Environ. Microbiol. 5:737-745. [DOI] [PubMed] [Google Scholar]
- 9.Delbes, C., R. Moletta, and J. J. Godon. 2001. Bacterial and archaeal 16S rDNA and 16S rRNA dynamics during an acetate crisis in an anaerobic digestor ecosystem. FEMS Microbiol. Ecol. 35:19-26. [DOI] [PubMed] [Google Scholar]
- 10.Delbes, C., R. Moletta, and J. J. Godon. 2000. Monitoring of activity dynamics of an anaerobic digester bacterial community using 16S rRNA polymerase chain reaction-single-strand conformation polymorphism analysis. Environ. Microbiol. 2:506-515. [DOI] [PubMed] [Google Scholar]
- 11.Duthoit, F., J. J. Godon, and M. C. Montel. 2003. Bacterial community dynamics during production of registered designation of origin Salers cheese as evaluated by 16S rRNA gene single-strand conformation polymorphism analysis. Appl. Environ. Microbiol. 69:3840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia, J. L., B. K. C. Patel, and B. Ollivier. 2000. Taxonomic phylogenetic and ecological diversity of methanogenic Archaea. Anaerobe 6:205-226. [DOI] [PubMed] [Google Scholar]
- 13.Godon, J. J., E. Zumstein, P. Dabert, F. Habouzit, and R. Moletta. 1997. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 63:2802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartung, J., and V. R. Phillips. 1994. Control of gaseous emissions from livestock buildings and manure stores. J. Agric. Eng. Res. 57:173-189. [Google Scholar]
- 15.Hobson, P. N., and B. G. Shaw. 1974. The bacterial population of piggery waste anaerobic digesters. Water Res. 8:507-516. [Google Scholar]
- 16.Iannoti, E. L., J. R. Fischer, and D. M. Sievers. 1982. Characterization of bacteria from swine manure digester. Appl. Environ. Microbiol. 43:136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, R., M. K. Gupta, and S. S. Kanwar. 1999. Fate of bacterial pathogens in cattle dung slurry subjected to anaerobic digestion. World J. Microbiol. Biotechnol. 15:335-338. [Google Scholar]
- 18.Lee, D. H., Y. G. Zo, and S. J. Kim. 1996. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 62:3112-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Moller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung, K., and E. Topp. 2001. Bacterial community dynamics in liquid swine manure during storage: molecular analysis using DGGE/PCR of 16S rDNA. FEMS Microbiol. Ecol. 38:169-177. [Google Scholar]
- 21.Loisel, P., J. Harmand, O. Zemb, E. Latrille, C. Lobry, J. P. Delgenès, and J. J. Godon. 2006. Denaturing gradient electrophoresis (DGE) and single-strand conformation polymorphism (SSCP) molecular fingerprintings revisited by simulation and used as a tool to measure microbial diversity. Environ. Microbiol. 8:720-731. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maidak, B. L., G. J. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey, and C. R. Woese. 1996. The Ribosomal Database Project (RDP). Nucleic Acids Res. 24:82-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez, J., F. Guiziou, P. Peu, and V. Gueutier. 2003. Influence of treatment techniques for pig slurry on methane emissions during subsequent storage. Biosyst. Eng. 85:347-354. [Google Scholar]
- 25.Martinez, J., and G. Le Bozec. 2000. Pig manure and environmental concerns in Europe. Cah. Agric. 9:181-190. [Google Scholar]
- 26.Munch, B., H. Errebo-Larsen, and B. Aalbaek. 1987. Experimental studies on the survival of pathogenic and indicator bacteria in aerated and non-aerated cattle and pig slurry. Biol. Wastes 22:49-65. [Google Scholar]
- 27.Olsen, J. E. 1988. Studies on the reduction of pathogenic and indicator bacteria in liquid pig manure treated by sedimentation and anaerobic filter digestion for methane generation. Biol. Wastes 24:17-26. [Google Scholar]
- 28.Peters, S., S. Koschinsky, F. Schwieger, and C. C. Tebbe. 2000. Succession of microbial communities during hot composting as detected by PCR-single-strand-conformation polymorphism-based genetic profiles of small-subunit rRNA genes. Appl. Environ. Microbiol. 66:930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peu, P., F. Beline, and J. Martinez. 2004. Volatile fatty acids analysis from pig slurry using high-performance liquid chromatography. Int. J. Environ. Anal. Chem. 84:1017-1022. [Google Scholar]
- 30.Placha, I., J. Venglovsky, N. Sasakova, and I. F. Svoboda. 2001. The effect of summer and winter seasons on the survival of Salmonella typhimurium and indicator micro-organisms during the storage of solid fraction of pig slurry. J. Appl. Microbiol. 91:1036-1043. [DOI] [PubMed] [Google Scholar]
- 31.Pryde, S. E., A. J. Richardson, C. S. Stewart, and H. J. Flint. 1999. Molecular analysis of the microbial diversity present in the colonic wall, colonic lumen, and cecal lumen of a pig. Appl. Environ. Microbiol. 65:5372-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purdy, K. J., D. B. Nedwell, and T. M. Embley. 2003. Analysis of the sulfate-reducing bacterial and methanogenic archaeal populations in contrasting Antarctic sediments. Appl. Environ. Microbiol. 69:3181-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivière, J., J. C. Subtil, and G. Catroux. 1974. Etude de l'évolution physico-chimique et microbiologique du lisier de porcs pendant le stockage anaérobie. Ann. Agron. 25:383-401. [Google Scholar]
- 34.Rousselon, N., J. P. Delgenes, and J. J. Godon. 2004. A new real time PCR (TaqMan PCR) system for detection of the 16S rDNA gene associated with fecal bacteria. J. Microbiol. Methods 59:15-22. [DOI] [PubMed] [Google Scholar]
- 35.Schmalenberger, A., F. Schwieger, and C. C. Tebbe. 2001. Effect of primers hybridizing to different evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl. Environ. Microbiol. 67:3557-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwieger, F., and C. C. Tebbe. 1998. A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl. Environ. Microbiol. 64:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snell-Castro, R., J. J. Godon, J. P. Delgenes, and P. Dabert. 2005. Characterisation of the microbial diversity in a pig manure storage pit using small subunit rDNA sequence analysis. FEMS Microbiol. Ecol. 52:229-242. [DOI] [PubMed] [Google Scholar]
- 38.Spoelstra, S. F. 1978. Enumeration and isolation of anaerobic microbiota of piggery waste. Appl. Environ. Microbiol. 35:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torsvik, V., F. L. Daae, R. A. Sandaa, and L. Ovreas. 1998. Novel techniques for analysing microbial diversity in natural and perturbed environments. J. Biotechnol. 64:53-62. [DOI] [PubMed] [Google Scholar]
- 40.Van Dyke, M. I., and A. J. McCarthy. 2002. Molecular biological detection and characterization of Clostridium populations in municipal landfill sites. Appl. Environ. Microbiol. 68:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead, T. R., and M. A. Cotta. 2001. Characterisation and comparison of microbial populations in swine faeces and manure storage pits by 16S rDNA gene sequence analyses. Anaerobe 7:181-187. [Google Scholar]
- 44.Whitehead, T. R., and M. A. Cotta. 1999. Phylogenetic diversity of methanogenic archaea in swine waste storage pits. FEMS Microbiol. Lett. 179:223-226. [DOI] [PubMed] [Google Scholar]
- 45.Wood, J., K. P. Scott, G. Avgustin, C. J. Newbold, and H. J. Flint. 1998. Estimation of the relative abundance of different Bacteroides and Prevotella ribotypes in gut samples by restriction enzyme profiling of PCR-amplified 16S rRNA gene sequences. Appl. Environ. Microbiol. 64:3683-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, R. H., and D. L. Day. 1996. Anaerobic decomposition of swine manure and ammonia generation in a deep pit. Trans. ASAE 39:1811-1815. [Google Scholar]
- 47.Zhu, J. 2000. A review of microbiology in swine manure odor control. Agric. Ecosyst. Environ. 78:93-106. [Google Scholar]
- 48.Zhu, J., and L. D. Jacobson. 1999. Correlating microbes to major odorous compounds in swine manure. J. Environ. Qual. 28:737-744. [Google Scholar]
- 49.Ziemer, C. J., M. A. Cotta, and T. R. Whitehead. 2004. Application of group specific amplified rDNA restriction analysis to characterize swine fecal and manure storage pit samples. Anaerobe 10:217-227. [DOI] [PubMed] [Google Scholar]
- 50.Zumstein, E., R. Moletta, and J. J. Godon. 2000. Examination of two years of community dynamics in an anaerobic bioreactor using fluorescence polymerase chain reaction (PCR) single-strand conformation polymorphism analysis. Environ. Microbiol. 2:69-78. [DOI] [PubMed] [Google Scholar]