Abstract
The genome of Sinorhizobium meliloti type strain Rm1021 consists of three replicons: the chromosome and two megaplasmids, pSymA and pSymB. Additionally, many indigenous S. meliloti strains possess one or more smaller plasmids, which represent the accessory genome of this species. Here we describe the complete nucleotide sequence of an accessory plasmid, designated pSmeSM11a, that was isolated from a dominant indigenous S. meliloti subpopulation in the context of a long-term field release experiment with genetically modified S. meliloti strains. Sequence analysis of plasmid pSmeSM11a revealed that it is 144,170 bp long and has a mean G+C content of 59.5 mol%. Annotation of the sequence resulted in a total of 160 coding sequences. Functional predictions could be made for 43% of the genes, whereas 57% of the genes encode hypothetical or unknown gene products. Two plasmid replication modules, one belonging to the repABC replicon family and the other belonging to the plasmid type A replicator region family, were identified. Plasmid pSmeSM11a contains a mobilization (mob) module composed of the type IV secretion system-related genes traG and traA and a putative mobC gene. A large continuous region that is about 42 kb long is very similar to a corresponding region located on S. meliloti Rm1021 megaplasmid pSymA. Single-base-pair deletions in the homologous regions are responsible for frameshifts that result in nonparalogous coding sequences. Plasmid pSmeSM11a carries additional copies of the nodulation genes nodP and nodQ that are responsible for Nod factor sulfation. Furthermore, a tauD gene encoding a putative taurine dioxygenase was identified on pSmeSM11a. An acdS gene located on pSmeSM11a is the first example of such a gene in S. meliloti. The deduced acdS gene product is able to deaminate 1-aminocyclopropane-1-carboxylate and is proposed to be involved in reducing the phytohormone ethylene, thus influencing nodulation events. The presence of numerous insertion sequences suggests that these elements mediated acquisition of accessory plasmid modules.
Rhizobia are gram-negative soil bacteria that are able to live in symbiosis with leguminous plants by induction of nitrogen-fixing nodules on host plant roots. The development and maintenance of these complex organs are determined by the regulated expression of plant and bacterial genes (14, 25, 41, 74).
Sinorhizobium meliloti is the symbiont of alfalfa (Medicago sativa), as well as its close relatives Medicago truncatula, Melilotus, and Trigonella. The genome of S. meliloti type strain Rm1021 consists of three replicons, the chromosome (3,654 kb) and two megaplasmids that are approximately 1,354 kb and 1,683 kb long, designated pSymA and pSymB, respectively (26). These three replicons seem to accomplish different tasks. Most of the essential housekeeping genes are chromosomally encoded (11). Many of the genes involved in root nodule formation (nod genes) and nitrogen fixation (nif and fix genes) are located on pSymA (2, 3, 8, 24, 55). Genes for the production of extracellular polysaccharides (exo and exp genes), lipopolysaccharide synthesis, carboxylic acid transport (dct), utilization of lactose (lac), and thiamine synthesis (thi) have been found on pSymB (17, 22, 23, 33, 76).
Besides the two megaplasmids, several rhizobial strains carry one or more smaller accessory plasmids, which vary in number and size. Prior to the S. meliloti Rm1021 sequencing project, there was already interest in genes located on accessory plasmids. Population analysis revealed that some plasmids are widespread in indigenous rhizobial populations and occur at frequencies of at least 50% (1, 4, 56). It is assumed that rhizobial accessory plasmids are interchangeable among indigenous rhizobial populations. Mercado-Blanco and Toro (48) reviewed different functions of accessory plasmids in rhizobia. Besides traits that affect symbiosis, some of these plasmids also encode functions that enhance the growth and survival of their hosts (48). S. meliloti strain GR4 carries two accessory plasmids, designated pRmeGR4a and pRmeGR4b. Increased efficiency of nodule formation by S. meliloti strain GR4 was correlated with the presence of nfe genes located on plasmid pRmeGR4b (58, 68). To our knowledge, apart from several replication genes, genetic information about accessory S. meliloti plasmids is rare. Thus, sequence analysis of some widespread accessory plasmids would broaden our understanding of genetic variation and evolution of these accompanying DNA elements (73).
In the context of a joint project, the first deliberate release of genetically engineered microorganisms was performed in Germany (61). The genetically engineered microorganisms released were derivatives of S. meliloti strain 2011 genetically tagged with the firefly luciferase gene (luc) mediating bioluminescence (62, 63). These bacteria were released in field plots of the Federal Research Center of Agriculture (FAL, Braunschweig, Germany) in 1995 and in field plots in Strassmoos (Bavaria, Germany) in 1997. The impact of the genetically modified S. meliloti strains on the indigenous rhizobial populations was analyzed during the release experiment. Fingerprint analysis revealed that indigenous nodulating S. meliloti strains could be subdivided into several dominant fingerprint groups (64). In this paper we first describe isolation and characterization of accessory plasmids from selected members of dominant indigenous S. meliloti subpopulations. The main objective of this work was to select an accessory plasmid for complete nucleotide sequence analysis. For this purpose the accessory plasmid pSmeSM11a residing in S. meliloti strain SM11 was completely sequenced and analyzed to identify possible advantageous traits.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani medium at 37°C. S. meliloti and Agrobacterium tumefaciens strains were grown at 30°C in tryptone-yeast (TY) medium. The final concentrations of antibiotics for selective growth were 300 μg streptomycin ml−1, 120 μg neomycin ml−1, 50 μg kanamycin ml−1, 50 μg rifampin ml−1, 10 μg nalidixic acid ml−1, and 6 μg tetracycline ml−1. Bacterial strains were stored at −20°C in 50% glycerol.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| Sinorhizobium meliloti strains | ||
| Rm1021 | SU47, Smr | 46 |
| SM11 | Indigenous S. meliloti strain carrying two accessory plasmids | Strain collection, Bielefeld |
| SM15 | Indigenous S. meliloti strain carrying three accessory plasmids | Strain collection, Bielefeld |
| SM19 | Indigenous S. meliloti strain carrying two accessory plasmids | Strain collection, Bielefeld |
| SM31 | Indigenous S. meliloti strain carrying four accessory plasmids | Strain collection, Bielefeld |
| BS9 | Indigenous S. meliloti strain carrying three accessory plasmids | Strain collection, Bielefeld |
| BS13 | Indigenous S. meliloti strain carrying two accessory plasmids | Strain collection, Bielefeld |
| Escherichia coli S17-1 | Chromosomally integrated RP4 derivative, Tpr | 66 |
| Other bacterial strains | ||
| Pseudomonas sp. strain B13 GFP1 | Kmr Gmr, green fluorescent derivative of Pseudomonas sp. strain B13, γ-proteobacterium | 15 |
| Ralstonia eutropha GFP3 | Kmr Gmr, gfp, β-proteobacterium | 70 |
| Agrobacterium tumefaciens UBAPF2 | Plasmid-free derivative of A. tumefaciens strain C58, Rifr | 34 |
| Sinorhizobium meliloti plasmids | ||
| pSmeBS9aT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeBS9bT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeBS9cT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeBS13aT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeBS13bT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeSM11aT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeSM11bT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeSM15bT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| pSmeSM31aT | Tn5-B10-tagged S. meliloti accessory plasmid, Nmr | This study |
| Escherichia coli plasmids | ||
| pRK2013 | Helper plasmid for mobilization, Kmr | 21 |
| pSUP5011 | Derivative of plasmid pBR325, Bam− (Tcs), carrying Tn5-B10, Apr Cmr Kmr | 65 |
| pJP2 | Mini-RK2 derivative, Apr Tcr | 53 |
| pACC1 | pJP2 containing the acdS and lrpL genes of pSmeSM11a | This study |
Abbreviations: Ap, ampicillin; Cm, chloramphenicol; Km, kanamycin; Nm, neomycin; Rif, rifampin; Sm, streptomycin; Tc, tetracycline; Tp, trimethoprim.
Tn5-B10 tagging and mobilization of accessory S. meliloti plasmids into plasmid-free A. tumefaciens strain UBAPF2.
To separate individual accessory plasmids from other plasmids residing in the same S. meliloti cells, the RP4 mobilization (mob) region was introduced by using suicide plasmid pSUP5011 containing Tn5-B10 (65, 67). For this reason pSUP5011 was mobilized from mobilizator strain E. coli S17-1 into S. meliloti strains by filter mating. Neomycin-resistant transconjugants were selected on TY agar containing 120 μg neomycin ml−1 and 10 μg nalidixic acid ml−1. Transconjugants carrying Tn5-B10 in the genome were pooled and subsequently used as donors in triparental matings with E. coli carrying helper plasmid pRK2013 (21) and A. tumefaciens plasmid-free strain UBAPF2 (34) as the recipient. Transconjugants were selected on TY agar containing 120 μg neomycin ml−1 and 50 μg rifampin ml−1. Subsequently, the plasmid profiles of all A. tumefaciens transconjugants were determined by Eckhardt analyses (34).
Plasmid transfer test by filter mating.
A 0.5-ml aliquot of the donor culture (optical density at 560 nm, 0.6) was mixed with 0.5 ml of the recipient culture (optical density at 560 nm, 0.9 to 1.0) and concentrated by centrifugation for 30 s at 14,000 × g. Mating was carried out by using nitrocellulose filters placed on TY agar. Serial dilutions of mating mixtures were plated onto TY agar plates supplemented with appropriate antibiotics.
Plasmid isolation, shotgun library construction, and sequencing.
DNA of plasmid pSmeSM11a was isolated from A. tumefaciens by alkaline sodium dodecyl sulfate lysis and column purification with a Nucleobond PC100 kit, using the NucleoBond plasmid purification protocol (Macherey-Nagel, Düren, Germany).
A pSmeSM11a shotgun library was constructed by MWG-Biotech AG (Ebersberg, Germany) using the hydroshearing approach. Fragments that were 1.3 to 2.0 kb long were cloned into the vector pGEM-T Easy. Templates for nucleotide sequencing were prepared from E. coli shotgun clones by automated lysis with a RoboPrep 2500 (MWG) and a BioRobot 9600 (QIAGEN). Cycle sequencing reaction mixtures obtained using dye terminator chemistry were separated with a MegaBACE 1000 capillary sequencer (Amersham Bioscience) and an ABI377 (Applied Biosystems) DNA sequencer (IIT Biotech GmbH, Bielefeld, Germany). Quality control of the sequence data was performed by using the in-house software tool BioMake (Bielefeld University, unpublished data), in which a normalization step applying PHRED (18, 19) was implemented. Computer-assisted assembly of random shotgun sequencing results was carried out with the CONSED/AUTOFINISH software tool (29, 30). Gap closure and polishing of the sequence were performed by primer walking using walking primers designed based on contig nucleotide sequences. We relied on Phred 40 quality in the consensus sequence.
DNA sequence analysis and annotation.
Annotation of the complete pSmeSM11a nucleotide sequence was performed by using the GenDB (version 2.0) annotation tool (49). Repeat regions within the pSmeSM11a sequence were identified and analyzed by using the REPuter software (38). Insertion elements were annotated by using the IS database homepage (http://www-is.biotoul.fr/is.html).
Construction of vector pACC1 carrying acdS and lrpL.
A 2-kb region carrying the acdS and lrpL genes of plasmid pSmeSM11a was amplified by PCR and inserted into the EcoRI site of vector pJP2 (53) to construct pACC1. Vector pACC1 was introduced into S. meliloti Rm1021 by conjugation with donor strain E. coli S17-1(pACC1). Transconjugants were selected on TY medium supplemented with streptomycin and tetracycline. As a negative control, vector pJP2 was also introduced into S. meliloti Rm1021.
ACC deaminase activity assay.
S. meliloti cells were grown in 5 ml TY medium supplemented with appropriate antibiotics at 30°C to the stationary phase. After centrifugation, cell pellets were washed twice with 0.1 M Tris-HCl (pH 7.5). 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase activity was induced by resuspending cells in 2 ml of M9 minimal medium supplemented with appropriate antibiotics and 5 mM ACC as the sole source of nitrogen and incubating the preparation for 24 h at 30°C with shaking. ACC deaminase activity was determined by spectrophotometrically measuring the production of α-ketobutyrate (32).
Growth assay with taurine.
S. meliloti cells were grown in 5 ml TY medium supplemented with appropriate antibiotics at 30°C to the early stationary phase. One milliliter of each culture was washed twice in sulfur-free Vincent minimal medium, resuspended in 10 ml of the same medium, and incubated for 48 h at 30°C in Greiner tubes with continuous shaking to starve the cells. To determine the growth behavior with taurine as the sole sulfur source, 0.1-ml portions of the culture were diluted in 10 ml of sulfur-free Vincent minimal medium and 10 ml of Vincent minimal medium supplemented with 20 mM taurine as the sole source of sulfur and incubated for an additional 48 h under the conditions described above.
Southern hybridization.
Rhizobial genomic DNA was isolated with a Gene Elute bacterial genomic DNA kit (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) used according to the manufacturer's instructions, digested with EcoRI, and used for hybridization. DNA fragments were amplified from plasmid pSmeSM11a DNA by PCR, using a PCR digoxigenin probe synthesis kit (Roche-Diagnostics GmbH, Mannheim, Germany). The labeled PCR products were used as probes with EcoRI-restricted genomic DNA. Plasmid DNA was labeled by using a digoxigenin DNA labeling kit (Roche-Diagnostics GmbH, Mannheim, Germany). Labeled plasmid DNA was hybridized with EcoRI-restricted plasmid DNA and pSmeSM11a-specific PCR amplicons. Hybridization was carried out at 68°C.
Nucleotide sequence accession number.
The annotated sequence of pSmeSM11a has been deposited in the EMBL database under accession number DQ145546.
RESULTS AND DISCUSSION
Isolation and characterization of accessory plasmids from dominant indigenous S. meliloti strains nodulating alfalfa.
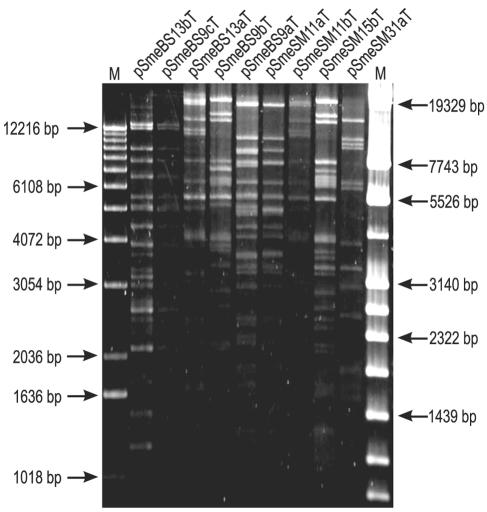
The main objective of this work was analysis of accessory plasmids residing in dominant indigenous S. meliloti strains isolated from two different field plots in Germany where S. meliloti genetically engineered microorganisms tagged with the firefly luc gene had been released. It could be demonstrated that during the field release experiment the released S. meliloti strains were outcompeted by an indigenous S. meliloti subpopulation. Characterization of this population by enterobacterial repetitive intergenic consensus-PCR fingerprinting showed that most of the indigenous S. meliloti strains could be classified into dominant fingerprint groups, which indicated that a few dominant strain types had outcompeted the released strains (69). Six competitive S. meliloti strains belonging to dominant fingerprint groups were chosen from a collection of indigenous S. meliloti strains for plasmid analysis. Two indigenous S. meliloti strains were selected from the release site in Braunschweig (Lower Saxony), whereas four strains originated from the release site in Strassmoos (Bavaria). Determination of the plasmid contents of these strains by Eckhardt lysis revealed that two strains carried three accessory plasmids, three strains carried two accessory plasmids, and one strain carried four accessory plasmids. These accessory S. meliloti plasmids were separated from each other by employing the Tn5-mob (Tn5-B10) tagging method and subsequently mobilizing Tn5-B10-tagged plasmids with the help of plasmid pRK2013 into plasmid-free, rifampin-resistant A. tumefaciens strain UBAPF2 as the recipient (34). Rifampin- and neomycin-resistant A. tumefaciens transconjugants were recovered and tested by Eckhardt lysis to determine their plasmid contents. A total of nine accessory S. meliloti plasmids that ranged in size from 75 to 300 kb and were derived from different indigenous S. meliloti strains were isolated (Table 1). The plasmids were designated in accordance with the system proposed by Casse et al. (12). An additional T in a designation indicates that a plasmid carries the Tn5-B10 transposon. A restriction profile analysis was performed with all nine separated accessory plasmids. To do this, plasmid DNA was isolated from plasmid-carrying A. tumefaciens UBAPF2 strains and restricted with endonuclease BamHI. It appeared that accessory plasmids pSmeSM11aT and pSmeBS9aT had nearly identical BamHI restriction patterns, which showed that the plasmid sizes were similar (approximately 150 kb) (Fig. 1). Interestingly, these two accessory plasmids originated from two indigenous S. meliloti strains found at different release sites. Plasmid pSmeSM11aT was isolated from indigenous S. meliloti strain SM11 found at the release site in Strassmoos (Bavaria, Germany), whereas plasmid pSmeBS9aT was isolated from strain BS9, which was obtained from field plots at the Federal Research Center of Agriculture (Braunschweig, Lower Saxony). One of these two plasmids, pSmeSM11aT, was chosen for complete nucleotide sequence analysis.
FIG. 1.
Characterization of Tn5-B10-tagged accessory S. meliloti plasmids by restriction profile analysis. Fragments of BamHI-digested accessory plasmid DNA isolated from A. tumefaciens UBAPF2 were separated on a 1% agarose gel. Lanes M contained standard length markers X (left) and IV (right).
General features of the annotated pSmeSM11a nucleotide sequence.
The complete nucleotide sequence of plasmid pSmeSM11a was determined by using a shotgun sequencing approach with the Tn5-mob-tagged derivative pSmeSM11aT. Assembly of 2,150 shotgun sequencing reads resulted in 10 contigs with 6.5-fold coverage of the sequence. Gap closure and polishing by primer walking on linking clones or pSmeSM11aT plasmid DNA yielded a circular contiguous DNA sequence consisting of 151,723 bp. Since the Tn5-mob used for plasmid tagging is 7,553 bp long, the original pSmeSM11a plasmid is 144,170 bp long. The mean G+C content is 59.5 mol%. Subsequent annotation of the finished pSmeSM11a nucleotide sequence by using the GenDB 2.0 tool (49) revealed 160 coding open reading frames (ORFs). Functional predictions for putative pSmeSM11a genes are summarized in Table S1 in the supplemental material. A circular map and the genetic organization of plasmid pSmeSM11a are shown in Fig. 2. The majority of gene products have predicted functions in DNA replication, recombination, and repair (17 ORFs). This class includes integrases, recombinases, resolvases, and transposases encoded by mobile genetic elements. Interestingly, these elements are not evenly distributed over the entire plasmid sequence. All mobile genetic elements present on pSmeSM11a are located in a region between orf6 and orf87. We assume that the remaining part of plasmid pSmeSM11a was acquired very recently, since it is not interrupted by transposable elements. Insertion elements and transposons identified on pSmeSM11a are listed in Table S2 in the supplemental material (see also references 39 and 77). The second largest category comprises transport- and metabolism-related gene products (13 ORFs), including proteins with predicted functions in carbohydrate (3 ORFs), inorganic ion (6 ORFs), and amino acid (4 ORFs) transport and metabolism. Additionally, the annotation revealed that seven gene products have possible regulatory functions. The class of miscellaneous gene products (24 ORFs) includes, among other categories, products involved in intracellular trafficking, energy production and conversion, and coenzyme metabolism. Functional predictions can be made for only 43% of the genes, whereas 57% of the genes encode products with hypothetical or unknown functions.
FIG. 2.
Genetic map and organization of plasmid pSmeSM11a. The modular structure of plasmid pSmeSM11a is indicated by gray regions in the outer circle, as follows: a repABC replication region (region I), a region probably involved in sulfur metabolism (region II), a putative ethylene level modulation region (region III), a region carrying a mobilization module (region VI), a region homologous to pSymA (region V), and a plasmid type A replication region (region VI). The arrows in the next circle indicate the localization and orientation of coding regions that are numbered clockwise from 1 to 160. Different colors of coding sequences indicate different COG classes, as follows: dark blue, plasmid replication and partitioning; green, DNA replication, recombination, and repair; light magenta, signal transduction and metabolism; red, transcription; light blue, inorganic ion transport and metabolism; yellow, carbohydrate transport and metabolism; dark magenta, posttranslational modification, protein turnover, and chaperones; orange, miscellaneous; dark gray, function unknown; and light gray, orphan. The relative G+C content and the GC skew [(G − C)/(G + C)] are shown in the two inner circles, respectively, in which a G+C content of >50% is indicated by black and a G+C content of <50% is indicated by red. G+C plots were generated using GenDB (version 2.0) (48) with a 300-nucleotide window in 50-nucleotide steps. The innermost circle indicates the scale (in base pairs). The first nucleotide of the repA coding region was chosen as the starting point for the nucleotide sequence.
Six remarkable DNA regions were identified on plasmid pSmeSM11a and are indicated in Fig. 2. Region I (comprising nucleotides 1 to 3622) consists of three rep genes (repABC) arranged in an operon. Genes that are probably involved in sulfur metabolism were identified in region II (nucleotides 13548 to 17520). The third region (nucleotides 69291 to 70931) is composed of two genes, lrpL and acdS, which are predicted to be involved in modulation of the level of the phytohormone ethylene. A putative mobilization module that is composed of the type IV secretion system-related genes traG and traA and a mobC gene is present in region IV (nucleotides 79307 to 85943). Region V (nucleotides 91096 to 133462) is homologous to a segment on symbiotic plasmid pSymA (SMa1076 to SMa1169) and constitutes about one-third of the whole plasmid genome. Region VI (nucleotides 137401 to 141400) carries a plasmid type A replication region that is homologous to the replication regions of plasmids pRmeGR4a and pRmeGR4b of Sinorhizobium meliloti strain GR4. Detailed descriptions of the different pSmeSM11a modules are given below.
Plasmid pSmeSM11a possesses a repABC replicon and a putative type A replication module.
A 3,622-bp region of plasmid pSmeSM11a, designated region I (Fig. 2), consists of three genes belonging to the repABC replicon family that includes several plasmids of α-proteobacteria (5-7, 13, 26, 36, 40, 50, 69, 78).
The repABC module encodes the partitioning proteins RepA (ParA family ATPase) and RepB (ParB-like nuclease domain) and the replication protein RepC. The repA gene product is 70% identical to RepA of Rhizobium etli plasmid p42d (accession no. AAM88941). The repB gene product is 69% identical to the corresponding gene product of Mesorhizobium sp. strain BNC1 (accession no. ZP_00614184), and RepC exhibits 68% identity to possible replication protein C of Agrobacterium rhizogenes plasmid pRiA4b (accession no. P05684).
A large intergenic sequence between repB and repC, which is typical for repABC replicons, was also identified on pSmeSM11a (Fig. 3A). This intergenic sequence contains A+T-rich segments and is thought to be a cis-acting incompatibility site, termed incα, of repABC family replicons (7, 54, 69). Very recently, two novel regulatory elements, a small antisense RNA and a stem-loop structure in the repABC mRNA, were identified in the incα regions of different rhizobial repABC plasmids (45, 75). Our analysis revealed that the intergenic sequence of pSmeSM11a also contains the characteristic sequences needed to encode a putative antisense RNA (Fig. 3B).
FIG. 3.
repABC replicon module present on plasmid pSmeSM11a. The repABC replicon module of plasmid pSmeSM11a constitutes region I, which is shown in Fig. 2. (A) Schematic representation of the repABC replicon module. The arrows indicate the positions of the repA, repB, and repC genes. The shaded box shows the position of the conserved intergenic region (igs). (B) Nucleotide sequence of the intergenic region between repB and repC. The RepB C-terminal segment and the RepC N-terminal segment are shown below the DNA sequence. The −35 and −10 elements of the promoter and the putative transcription start site (+1) of the putative small antisense RNA are indicated and underlined. The shaded sequence indicates a motif able to form a stem-loop structure in the small antisense RNA. The T-tract, which probably constitutes the end of the transcript, is underlined. A putative ribosome binding site for repC is indicated by boldface type.
In addition to the repABC module, pSmeSM11a harbors a second putative replication region (designated region VI [Fig. 2]) that is located upstream of repABC. A 4,000-bp region comprising orf154 to orf157 encodes a putative ParB-like partitioning protein (RepB2), replication protein C (RepC2), a predicted nuclease (Orf156), and a protein whose function is unknown (Orf157). RepB2 is 91% identical to OrfB encoded by S. meliloti strain GR4 plasmid pRmeGR4a (accession no. ABA55660), whereas RepC2 exhibits 94% identity to the corresponding gene product of plasmid pRmeGR4b. The pSmeSM11a type A replicator module was tentatively classified as a member of the A(II) subgroup since it exhibits the highest degree of similarity to members of this replicator group (10, 47). Recently, a small antisense RNA, nested within the repC mRNA leader, was found to be involved in replication control of plasmid pRmeGR4a (35). Our analysis revealed that the corresponding pSmeSM11a sequence is 100% identical to the small antisense RNA encoded on plasmid pRmeGR4b.
The presence of two replication modules on pSmeSM11a might broaden the host range of the plasmid and could indicate that the plasmid resulted from fusion of two formerly autonomous replicons.
A putative mobilization module similar to that of Agrobacterium radiobacter plasmid pAgK84 is located on pSmeSM11a.
Region IV of plasmid pSmeSM11a (Fig. 2) represents a putative mobilization (mob) module that encodes a predicted coupling protein belonging to the TraG/VirD4 family of bacterial conjugation proteins (59), probable mobilization protein C, TraA (a putative helicase belonging to the MobA/MobL family [Pfam03389]), and two presumptive ORF products whose functions are unknown. The pSmeSM11a mob module exhibits the highest level of similarity to corresponding regions located on the linear chromosome of A. tumefaciens strain C58 (accession no. NC_003063) and on A. radiobacter plasmid pAgK84 (accession no. AY442931) (Fig. 4).
FIG. 4.
Alignment of the mob region of plasmid pSmeSM11a with the corresponding region of A. radiobacter plasmid pAgK84 and the linear chromosome of A. tumefaciens strain C58. Coding regions are indicated by arrows. Homologous genes are indicated by the same shade of gray. ORFs whose functions are unknown or ORFs without counterparts in the pSmeSM11a mob region are indicated by open arrows. The mob region of plasmid pSmeSM11a is designated region IV in Fig. 2. The GenBank accession numbers for the nucleotide sequences are as follows: A. radiobacter plasmid pAgK84, AY442931; and A. tumefaciens linear chromosome, NC_003063.
It could be shown that plasmid pAgK84 can be mobilized to several Agrobacterium strains and, interestingly, also to S. meliloti (20). Plasmid pSmeSM11a was marked with transposon Tn5-B10 and could therefore be mobilized by helper plasmid pRK2013 to A. tumefaciens or other S. meliloti strains. To further test the mobilization of plasmid pSmeSM11a, we carried out several mating experiments with pSmeSM11a::Tn5-B10-carrying S. meliloti strain Rm1021 without the help of plasmid pRK2013 and with A. tumefaciens UBAPF2 as the recipient. Since no transconjugants were obtained (frequency, <10−9 transconjugant per recipient strain), we concluded that the pSymA type IV secretion system does not function in mobilization of pSmeSM11a. Furthermore, the host range of pSmeSM11a seems to be restricted to the α-proteobacteria since pSmeSM11a::Tn5-B10 could be mobilized by helper plasmid pRK2013 (IncP-1α) only to A. tumefaciens UBAPF2 and S. meliloti strains (α-proteobacteria) and not to Pseudomonas sp. strain B13 GFP1 (a γ-proteobacterium) or Ralstonia eutropha GFP3 (a β-proteobacterium).
A DNA region of plasmid pSmeSM11a that is more than 42 kb long is homologous to S. meliloti megaplasmid pSymA.
A 42,367-bp continuous region on pSmeSM11a designated region V (Fig. 2) is homologous to a region located on megaplasmid pSymA of S. meliloti strain Rm1021 and consists of orf107 to orf149. A comparative analysis of the homologous regions is shown in Fig. 5. Interestingly, the synteny of the two homologous regions is not continuous since a 10-kb pSymA region composed of open reading frames SMa1092 to SMa1115 is missing on plasmid pSmeSM11a. Additionally, no DNA sequence homologous to SMa1147, which encodes a conserved hypothetical protein, could be identified on plasmid pSmeSM11a.
FIG. 5.
Analysis of the region homologous to pSymA present in pSmeSM11a compared with the corresponding region in pSymA of S. meliloti strain Rm1021. The homologous region of plasmid pSmeSM11a is designated region V in Fig. 2. (A) Comparison of the genetic organizations of pSymA and pSmeSM11a regions, showing conserved synteny. Coding regions with different organizations (designated regions A, B, C, D, E, and F) in the two homologous regions compared are indicated. Regions which are not present in pSmeSM11a and the position of the Tn5-mob insertion are indicated by open arrows (the drawings are not to scale). Gray boxes indicate a high degree of identity (87 to 100%) between the products of homologous ORFs. Bars 1, 2, 3, and 4 indicate pSmeSM11a amplicons used as probes in hybridization experiments with total DNA preparations from different S. meliloti strains. (B) Comparison of nucleotide sequences in regions A, B, C, D, E, and F of pSmeSM11a and pSymA. The nucleotide sequences differ mostly by 1- or 2-bp deletions. Boldface type indicates the exact positions of the differences. Underlining indicates the sequences in which a single nucleotide is absent. The 232-bp duplicated region in pSmeSM11a that is responsible for a frameshift in the degP4 region (orf125 to orf127) is indicated by the sequence in parentheses (region C).
Most of the pSmeSM11a ORFs in the homologous region exhibit a high degree of similarity (>87%) to corresponding ORFs located on pSymA of S. meliloti strain Rm1021 (Fig. 5A). The predicted functions of genes located in the region homologous to the pSymA region are shown in Table S1 in the supplemental material. To test whether the genes in the homologous region are also duplicated in strain SM11, hybridization experiments were performed with selected probes of the corresponding pSmeSM11a region. Four labeled PCR amplicons that were about 2.7 to 3.0 kb long were generated and hybridized with restricted genomic DNA of S. meliloti strains Rm1021 and SM11, with pSmeSM11a DNA as a control (data not shown). Since the probes were generated to hybridize with either the end portions or nonhomologous portions of the homologous regions (Fig. 5A), different hybridization patterns for the two homologous regions on pSymA and pSmeSM11a were obtained. For strain SM11, the only fragments that hybridized with the four probes were those related to plasmid pSmeSM11a. Neither the pSymA-specific hybridization patterns nor other patterns were found in any of the four hybridizations with strain SM11, which led us to the conclusion that the region homologous to pSymA is not reiterated in the genome of strain SM11.
It might be speculated that part of SM11 plasmid pSymA was transferred to plasmid pSmeSM11a. Searching for pSymA-like sequences on other accessory plasmids might broaden our understanding of the evolution and assembly of symbiotic plasmids.
Single-base-pair deletions in the homologous regions of plasmid pSmeSM11a and S. meliloti strain 1021(pSymA) are responsible for the appearance of nonparalogous ORFs.
Detailed sequence analysis revealed that several base pair deletions or insertions in the segment homologous to pSymA are responsible for local differences compared to pSymA of S. meliloti strain 1021, which resulted in prediction of some nonparalogous coding sequences. Six regions, designated regions A to F (Fig. 5A), encode these nonparalogous coding sequences. Since single-base-pair deletions/insertions could be due to sequencing errors in the pSmeSM11a sequence, as well as in the published pSymA sequence, primers were designed to resequence the corresponding regions of both plasmids. Neither the pSmeSM11a sequence nor the sequence of pSymA contains sequencing errors for the local differences mentioned above. Thus, the single-base-pair deletions/insertions occurred during plasmid evolution.
The genetic configuration for the local difference designated region A in Fig. 5A is described below. Deletion of a single base in the pSmeSM11a nucleotide sequence (Fig. 5B, region A) resulted in prediction of coding sequences orf113 and orf114 covering SMa1084 on pSymA. orf113 encodes the C-terminal part of the SMa1084 gene product, whereas orf114 encodes the SMa1084 product N-terminal portion. Likewise, single-base-pair deletions led to prediction of orf119, orf120, orf129, and orf130 in pSmeSM11a (Fig. 5A and B, regions B and D). The genetic configuration is even more complex for the region covered by pSymA degP4 encoding a putative protease-like protein. The corresponding pSmeSM11a nucleotide sequence includes orf125, orf126, and orf127 (Fig. 5A, region C). This local difference is caused by duplication of an internal 232-bp degP4 region and a single-base-pair deletion in the pSmeSM11a sequence (Fig. 5B, region C). Interestingly, Orf125, Orf126, and Orf127 contain a PDZ-metalloprotease domain (cd00989), a PDZ serine protease domain (cd00987), and a trypsin domain (Pfam0089), respectively. All these domains are fused in pSymA DegP4, which thus might be considered a fusion protein.
In contrast to regions A, B, C, and D, regions E and F on pSmeSM11a each contain only a single coding sequence (Fig. 5A). The corresponding regions on pSymA contain two and three genes, respectively. Interestingly, on pSmeSM11a orf133 of region E encodes a putative response regulator consisting of a CheY-like regulatory module and a helix-turn-helix DNA-binding domain (OmpR, COG0745). Similar domains were found in the nitrogen fixation regulator FixJ (SMa1227) that is involved in oxygen control of nitrogen fixation gene expression (61, 75). A single-base-pair deletion in the pSymA sequence led to prediction of SMa1138 and SMa1139 encoding a truncated regulatory module (homologous to the N-terminal OmpR receiver domain) and an effector DNA-binding domain, respectively. The sensor component interacting with the pSmeSM11a FixJ-like response regulator (Orf133) is currently unknown. However, a gene encoding a FixL-like protein (orf135) is located upstream of orf133 on pSmeSM11a, as well as on pSymA (sma1142).
Finally, region F on pSmeSM11a carrying orf144 covers SMa1159, SMa1160, and SMa1161 on pSymA, which is due to a single-base-pair deletion and a single-base-pair insertion in the pSymA nucleotide sequence (Fig. 5A and B, region F).
In summary, local differences in the regions homologous to pSymA indicate that there was divergent evolution that might have led to modified gene products adapted to function under slightly different environmental conditions, thus enhancing genetic flexibility.
A region on pSmeSM11a carrying the nodPQ and tauABCD genes is predicted to be involved in sulfur metabolism and Nod factor biosynthesis.
Region II of plasmid pSmeSM11a contains genes that are probably involved in sulfur metabolism and Nod factor biosynthesis (Fig. 2). It encodes a predicted incomplete ABC-type nitrate/sulfonate/bicarbonate transport system (TauABC) from coordinates 13548 to 17520. The deduced gene products of tauA, tauB, and tauC are 62% to 81% similar to corresponding gene products of Burkholderia fungorum LB400 (accession no. NZ_AAAJ03000005). TauD, which is encoded upstream of tauA, is 73% identical to the tauD gene product of Bradyrhizobium sp. strain BTAi1 (accession no. ZP_00862508). The gene products of tauA, tauB, and tauC encode an ABC-type transport system required for uptake of aliphatic sulfonates, whereas TauD belongs to the TauD/TfdA family of taurine dioxygenases (COG2175, Pfam02668). The pSmeSM11a-encoded ABC-type transporter consists of a truncated permease component (TauC, COG0600), an ATPase component (TauB, COG1116), and a periplasmic substrate binding protein (TauA, COG0715). TauD from E. coli is an α-ketoglutarate-dependent taurine dioxygenase that catalyzes the oxygenolytic release of sulfite from taurine and enables E. coli to use taurine as a sole source of sulfur (37, 71, 72). A functional taurine transporter has been identified in S. meliloti Rm1021(pSymB). It has been shown that this strain is able to utilize taurine as a sole source of carbon and energy for aerobic growth (57). It has been proposed that in S. meliloti Rm1021 taurine probably is first deaminated by a taurine dehydrogenase (TauXY) and then desulfonated through the action of sulfoacetaldehyde acetyltransferase (Xsc) (9). In contrast, strains possessing a taurine dioxygenase (TauD) are able to desulfonate taurine directly (16).
Since S. meliloti Rm1021 is able to use taurine as a sole source of sulfur, the functionality of the pSmeSM11a-encoded tauD gene product could not be determined by growth assays. Mutants with mutations in the proposed S. meliloti Rm1021 taurine degradation pathway are required to analyze taurine metabolism in S. meliloti.
Two other genes, nodP and nodQ, which are probably involved in sulfur metabolism, are located downstream of the tau region. NodP represents subunit 2 of a possible sulfate adenylate transferase, including the phosphoadenosine phosphosulfate reductase family domain (CysH, COG0175, Pfam01507). NodQ is a bifunctional enzyme combining subunit 1 of sulfate adenylyltransferase (COG2895) and adenylylsulfate kinase (COG0529, Pfam01583). It catalyzes the phosphorylation of adenylylsulfate to 3-phosphoadenylylsulfate. NodP and NodQ of pSmeSM11a exhibit the highest levels of identity to the corresponding enzymes of Mesorhizobium loti (71% identity) and Rhizobium sp. strain N33 (62% identity), respectively. NodP and NodQ are involved in sulfation of the oligosaccharide Nod factor that triggers the symbiotic response of the specific host plant. Two copies of nodPQ, both involved in Nod factor sulfation, have been identified in the S. meliloti genome (60). A third nodPQ copy, located on pSmeSM11a, is thought to increase the ratio of sulfated Nod factor, since the formation of the sulfate donor molecule phosphoadenosine 5′-phosphosulfate might be the limiting step in Nod factor sulfation (31, 52).
pSmeSM11a-encoded ACC deaminase is predicted to modulate the level of the phytohormone ethylene.
The acdS gene, which encodes an ACC deaminase, and a gene encoding a leucine-responsive regulator (LrpL) are located between coordinates 69290 and 70932 on pSmeSM11a (orf75 and orf76), representing region III of plasmid pSmeSM11a (Fig. 2). The deduced gene products, AcdS (ACC deaminase) and LrpL, are 99% and 98% identical, respectively, to the corresponding gene products of Rhizobium leguminosarum bv. viciae 128C53K (accession no. AF421376 for AcdS and accession no. AY172673 for LrpL). Rhizobacteria possessing ACC deaminase activity are capable of stimulating plant growth and can be considered plant growth-promoting rhizobacteria (27, 28, 51). Ma et al. (43, 44) described an ACC deaminase of R. leguminosarum bv. viciae 128C53K that promotes nodulation of pea plants. Recently, the R. leguminosarum bv. viciae asdS-lrpL gene region was introduced into S. meliloti Rm1021 to test the influence of these genes on the ability of S. meliloti to nodulate alfalfa (42). The resulting ACC deaminase-producing S. meliloti derivatives formed approximately 40% more nodules in symbiosis with M. sativa (alfalfa) and were much more competitive in nodulating alfalfa than the wild-type strain. Here we describe for the first time acdS and lrpL homologous genes that are present on an accessory S. meliloti plasmid.
The functionality of the ACC deaminase was initially tested by growing the indigenous strain S. meliloti SM11 and strains Rm1021(pACC1) and Rm1021(pSmeSM11aT), as well as control strains Rm1021 and Rm1021(pJP2), in Vincent minimal medium containing 2 mM ACC as the sole nitrogen source. It was found that only strains containing acdS and lrpL were able to grow with ACC as the sole nitrogen source (Table 2). To prove these results, an ACC deaminase activity assay was carried out with the strains mentioned above. As expected, no activity was detected in parental strains Rm1021 and Rm1021(pJP2), whereas strains Rm1021(pACC1), Rm1021(pSmeSM11aT), and SM11 exhibited ACC deaminase activity (Table 2). The level of ACC deaminase activity in S. meliloti Rm1021(pACC1) was similar to the level in S. meliloti Rm1021(pSmeSM11aT), which could have been the result of identical copy numbers of the plasmids in the host strains, whereas the ACC deaminase activity of the indigenous strain S. meliloti SM11 was significantly higher. It could be assumed that there is a different mode of regulation of the Lrp-like protein and/or uptake of ACC in the indigenous strain S. meliloti SM11, resulting in a higher level of expression of ACC deaminase.
TABLE 2.
Plasmid pSmeSM11a-encoded ACC deaminase activity
| Strain | Relevant genotype | Growth assaya | ACC deaminase activity (nmol α-ketobutyrate · h−1 · mg of protein−1)b |
|---|---|---|---|
| Rm1021 | − | ND | |
| Rm1021(pJP2) | − | ND | |
| Rm1021(pACC1) | acdS lrpL | + | 59 |
| Rm1021(pSmeSM11aT) | acdS lrpL | + | 75 |
| SM11 | acdS lrpL | + | 355 |
Growth was determined in Vincent minimal medium with 2 mM ACC as the sole nitrogen source.
ND, not detectable.
Plasmids pSmeSM11a and pSmeBS9a are very closely related.
Plasmids pSmeSM11a and pSmeBS9a were isolated from different indigenous S. meliloti strains found at different release sites, and they produced nearly identical restriction patterns (Fig. 1). Differences in their restriction patterns might have been the result of different Tn5-B10 insertions. To investigate the level of identity between the plasmids, we performed two Southern blot hybridization experiments. One hybridization experiment using labeled pSmeSM11aT DNA with EcoRI-restricted plasmid DNA of both replicons revealed identical hybridization patterns (Fig. 6), emphasizing that the two replicons are very closely related. A further hybridization experiment was done with labeled pSmeBS9aT DNA and 24 pSmeSM11a gene-specific amplicons (Table 3). Positive hybridization signals were obtained for all 24 amplicons. Among the pSmeSM11a amplicons were gene-specific PCR products of acdS, tauD, nodP, nodQ, and genes of both replicons. These results clearly demonstrate the high level of identity between plasmids pSmeSM11a and pSmeBS9a.
FIG. 6.
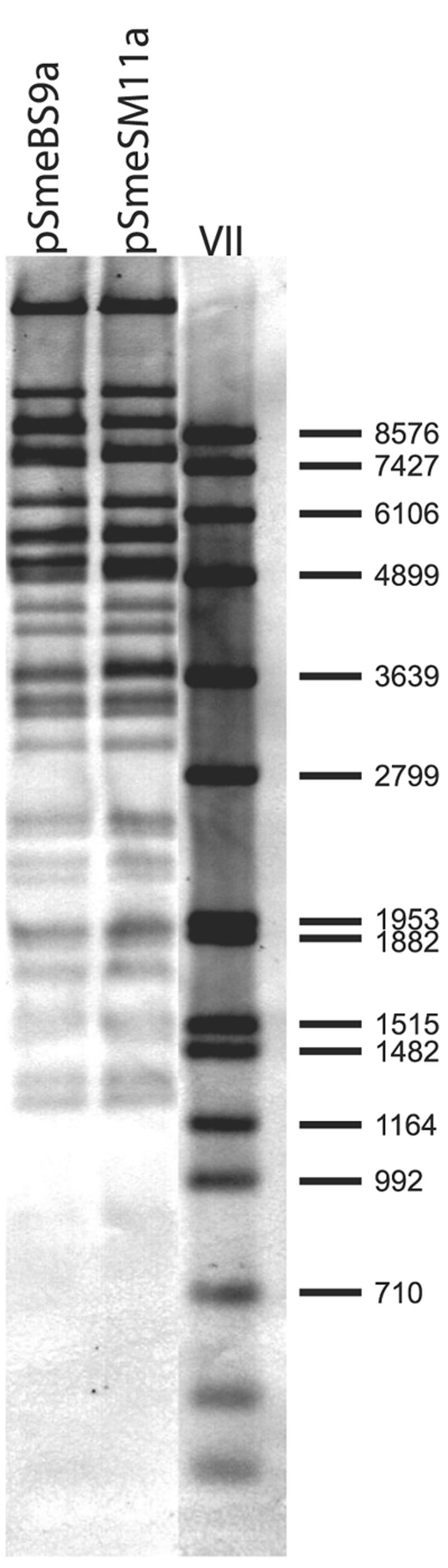
Southern blot hybridization of pSmeSM11a and pSmeBS9a with labeled pSmeSM11aT DNA. Plasmid DNA was restricted with EcoRI and separated on a Tris-acetate-EDTA gel containing 1% agarose. The fragment sizes for digoxigenin-labeled marker VII are indicated on the right.
TABLE 3.
Identification of pSmeSM11a-specific genes in pSmeBS9a by hybridization
| Gene | Amplicon length (bp) | Functiona |
|---|---|---|
| repA | 522 | Active partitioning |
| repB | 450 | Active partitioning |
| repC | 453 | Replication initiation |
| repB2 | 515 | Active partitioning |
| repC2 | 544 | Replication initiation |
| acdS | 468 | Ethylene level modulation |
| lrpL | 453 | Regulation of acdS |
| tauD | 459 | Taurine desulfonation |
| nodP | 583 | Conversion of sulfate to PAPS, Nod factor sulfation |
| nodQ | 479 | Conversion of sulfate to PAPS, Nod factor sulfation |
| traA | 581 | Processing of DNA for conjugal transfer of DNA |
| traG | 551 | Type IV secretion, transfer of plasmid DNA into the host |
| mobC | 209 | Plasmid mobilization |
| orf7 | 544 | Transposition |
| orf11 | 517 | Unknown |
| orf32 | 464 | Unknown |
| orf40 | 419 | Unknown |
| orf50 | 471 | Transposition |
| orf68 | 511 | Transposition |
| orf86 | 567 | Transposition |
| orf105 | 416 | Probably involved in dihydrofolate metabolism |
| orf118 | 438 | Unknown |
| orf135 | 485 | fixL-like, signal transduction mechanisms |
| orf152 | 432 | Lipid transport and metabolism |
PAPS, phosphoadenosine 5′-phosphosulfate.
Concluding remarks.
Sequencing of S. meliloti SM11 accessory plasmid pSmeSM11a extends the S. meliloti genome and exemplifies the mobile gene pool of this species. A detailed pSmeSM11a sequence analysis provided valuable evidence that the accessory genome could provide additional genetic information for adaptation of the bacterium to changing environmental conditions.
Plasmid pSmeSM11a has a modular structure with backbone modules for replication/partitioning and mobilization and adjacent long regions carrying accessory genetic modules. Insertion sequences and transposons obviously played an important role in acquisition of at least some of these modules. Plasmid pSmeSM11a carries two replication modules, a repABC replicon and a type A replicator region, indicating that the plasmid probably resulted from fusion of two formerly individual replicons. The presence of two replicons could be important for the host range of the plasmid, assuming that the two replicons function differently in certain host bacteria. It should be pointed out that host range extension broadens the availability of genetic information. Indeed, analysis of the different accessory genes identified in pSmeSM11a suggests that these genes were acquired from different sources. On the other hand, plasmid mobility is important for transfer of the element within the population and between different species and therefore for the acquisition of additional genetic information.
Approximately two-thirds of pSmeSM11a is occupied by accessory genetic modules that could provide adaptive advantages or broaden the host bacterium's responsive spectrum. Plasmid pSmeSM11a carries genes which do not have counterparts in the tripartite S. meliloti Rm1021 genome, such as tauD encoding a taurine dioxygenase and acdS encoding ACC deaminase involved in modulating the level of the phytohormone ethylene. The presence of these genes in the accessory S. meliloti genome could broaden the catabolic capacity or enhance the nodulation competitiveness of the organism. In this context it should be recalled that the original host strain, SM11 harboring plasmid pSmeSM11a, belongs to a dominant subpopulation of nodulating S. meliloti strains that outcompeted released strains in the long-term field release experiment mentioned above. The presence of pSmeSM11a might be responsible for the dominance of strain SM11. Future functional analyses should show whether pSmeSM11a encodes other gene products that influence the adaptation and survivability of host strains in soil and in the plant rhizosphere.
Nevertheless, it is clear that accessory plasmids extend the S. meliloti genome and could provide additional genetic information important for the population. In addition to plasmid pSmeSM11a, S. meliloti strain SM11 contains another accessory plasmid, the 200-kb plasmid pSmeSM11b. Sequence analysis of this plasmid could certainly increase our knowledge of the S. meliloti plasmid complement.
Supplementary Material
Acknowledgments
We thank Irene Krahn for excellent technical support. We also thank the bioinformatics group at the Lehrstuhl für Genetik for their support.
Michael Stiens received a scholarship from the International NRW Graduate School for Bioinformatics and Genome Research in Bielefeld.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Andronov, E. E., M. L. Rumiantseva, and B. V. Simarov. 2001. Genetic diversity of a natural population of Sinorhizobium meliloti, detected during analysis of a cryptic plasmid and ISRm2011-2 fingerprints. Genetika 37:610-616. [PubMed] [Google Scholar]
- 2.Banfalvi, Z., V. Sakanyan, C. Koncz, A. Kiss, I. Dusha, and A. Kondorosi. 1981. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol. Gen. Genet. 184:318-325. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, M. J., R. F. Fisher, T. Jones, C. Komp, A. P. Abola, F. Barloy-Hubler, L. Bowser, D. Capela, F. Galibert, J. Gouzy, M. Gurjal, A. Hong, L. Huizar, R. W. Hyman, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, C. Palm, M. C. Peck, R. Surzycki, D. H. Wells, K. C. Yeh, R. W. Davis, N. A. Federspiel, and S. R. Long. 2001. Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc. Natl. Acad. Sci. USA 98:9883-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barran, L. R., and E. S. P. Bromfield. 1988. Symbiotic gene probes hybridize to cryptic plasmids of indigenous Rhizobium meliloti. Can. J. Microbiol. 34:703-707. [Google Scholar]
- 5.Bartosik, D., J. Baj, E. Piechucka, E. Waker, and M. Wlodarczyk. 2002. Comparative characterization of repABC-type replicons of Paracoccus pantotrophus composite plasmids. Plasmid 48:130-141. [DOI] [PubMed] [Google Scholar]
- 6.Bartosik, D., J. Baj, and M. Wlodarczyk. 1998. Molecular and functional analysis of pTAV320, a repABC-type replicon of the Paracoccus versutus composite plasmid pTAV1. Microbiology 144:3149-3157. [DOI] [PubMed] [Google Scholar]
- 7.Bartosik, D., M. Szymanik, and E. Wysocka. 2001. Identification of the partitioning site within the repABC-type replicon of the composite Paracoccus versutus plasmid pTAV1. J. Bacteriol. 183:6234-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batut, J., B. Terzaghi, M. Ghérardi, M. Huguet, E. Terzaghi, A. M. Garnerone, P. Boistard, and T. Huguet. 1985. Localization of a symbiotic fix region on Rhizobium meliloti pSym megaplasmid more than 200 kilobases from the nod-nif region. Mol. Gen. Genet. 199:232-239. [Google Scholar]
- 9.Brüggemann, C., K. Denger, A. M. Cook, and J. Ruff. 2004. Enzymes and genes of taurine and isethionate dissimilation in Paracoccus denitrificans. Microbiology 150:805-816. [DOI] [PubMed] [Google Scholar]
- 10.Burgos, P. A., E. Velázquez, and N. Toro. 1996. Identification and distribution of plasmid-type A replicator region in rhizobia. Mol. Plant-Microbe Interact. 9:843-849. [DOI] [PubMed] [Google Scholar]
- 11.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dréano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Pühler, B. Purnelle, U. Ramsperger, C. Renard, P. Thébault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casse, F., C. Boucher, J. S. Julliot, M. Michel, and J. Dénarié. 1979. Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J. Gen. Microbiol. 113:229-242. [Google Scholar]
- 13.Cevallos, M. A., H. Porta, J. Izquierdo, C. Tun-Garrido, A. García-de-los-Santos, G. Davila, and S. Brom. 2002. Rhizobium etli CFN42 contains at least three plasmids of the repABC family: a structural and evolutionary analysis. Plasmid 48:104-116. [DOI] [PubMed] [Google Scholar]
- 14.Dénarié, J., F. Debelle, and C. Rosenberg. 1992. Signaling and host range variation in nodulation. Annu. Rev. Microbiol. 46:497-531. [DOI] [PubMed] [Google Scholar]
- 15.Dröge, M., A. Pühler, and W. Selbitschka. 2000. Phenotypic and molecular characterization of conjugative antibiotic resistance plasmids isolated from bacterial communities of activated sludge. Mol. Gen. Genet. 263:471-482. [DOI] [PubMed] [Google Scholar]
- 16.Eichhorn, E., J. R. van der Ploeg, M. A. Kertesz, and T. Leisinger. 1997. Characterization of α-ketoglutarate-dependent taurine dioxygenase from Escherichia coli. J. Biol. Chem. 272:23031-23036. [DOI] [PubMed] [Google Scholar]
- 17.Engelke, T., D. Jording, D. Kapp, and A. Pühler. 1989. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J. Bacteriol. 171:5551-5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewing, B., and P. Green. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8:186-194. [PubMed] [Google Scholar]
- 19.Ewing, B., L. Hillier, M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8:175-185. [DOI] [PubMed] [Google Scholar]
- 20.Farrand, S. K., J. E. Slota, J. S. Shim, and A. Kerr. 1985. Tn5 insertions in the agrocin 84 plasmid: the conjugal nature of pAgK84 and the locations of determinants for transfer and agrocin 84 production. Plasmid 13:106-117. [DOI] [PubMed] [Google Scholar]
- 21.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finan, T. M., B. Kunkel, G. F. De Vos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finan, T. M., S. Weidner, K. Wong, J. Buhrmester, P. Chain, F. J. Vorhölter, I. Hernandez-Lucas, A. Becker, A. Cowie, J. Gouzy, B. Golding, and A. Pühler. 2001. The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 98:9889-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher, R. F., and S. R. Long. 1992. Rhizobium-plant signal exchange. Nature 357:655-660. [DOI] [PubMed] [Google Scholar]
- 26.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dréano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lalaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thébault, M. Vandenbol, F. J. Vorhölter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 27.Glick, B. R., D. M. Karaturovic, and P. C. Newell. 1995. A novel procedure for rapid isolation of plant-growth promoting pseudomonads. Can. J. Microbiol. 41:533-536. [Google Scholar]
- 28.Glick, B. R., D. M. Penrose, and J. Li. 1998. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 190:63-68. [DOI] [PubMed] [Google Scholar]
- 29.Gordon, D., C. Abajian, and P. Green. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:195-202. [DOI] [PubMed] [Google Scholar]
- 30.Gordon, D., C. Desmarais, and P. Green. 2001. Automated finishing with autofinish. Genome Res. 11:614-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gressent, F., J. V. Cullimore, R. Ranjeva, and J. J. Bono. 2004. Radiolabeling of lipo-chitooligosaccharides using the NodH sulfotransferase: a two-step enzymatic procedure. BMC Biochem. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honma, M., and T. Shimomura. 1978. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 42:1825-1831. [Google Scholar]
- 33.Hynes, M. F., R. Simon, P. Müller, K. Niehaus, M. Labes, and A. Pühler. 1986. The 2 megaplasmids of Rhizobium meliloti are involved in the effective nodulation of alfalfa. Mol. Gen. Genet. 202:356-362. [Google Scholar]
- 34.Hynes, M. F., R. Simon, and A. Pühler. 1985. The development of plasmid-free strains of Agrobacterium tumefaciens by using incompatibility with a Rhizobium meliloti plasmid to eliminate pAtC58. Plasmid 13:99-105. [DOI] [PubMed] [Google Scholar]
- 35.Izquierdo, J., T. Venkova-Canova, M. A. Ramírez-Romero, J. Téllez-Sosa, I. Hernandez-Lucas, J. Sanjuán, and M. A. Cevallos. 2005. An antisense RNA plays a central role in the replication control of a repC plasmid. Plasmid 54:259-277. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 37.Kertesz, M. A. 2000. Riding the sulfur cycle—metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol. Rev. 24:135-175. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz, S., J. V. Choudhuri, E. Ohlebusch, C. Schleiermacher, J. Stoye, and R. Giegerich. 2001. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laberge, S., A. T. Middleton, and R. Wheatcroft. 1995. Characterization, nucleotide sequence, and conserved genomic locations of insertion sequence ISRm5 in Rhizobium meliloti. J. Bacteriol. 177:3133-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, P. L., and S. K. Farrand. 2000. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long, S. R. 1989. Rhizobium genetics. Annu. Rev. Genet. 23:483-506. [DOI] [PubMed] [Google Scholar]
- 42.Ma, W., T. C. Charles, and B. R. Glick. 2004. Expression of an exogenous 1-aminocyclopropane-1-carboxylate deaminase gene in Sinorhizobium meliloti increases its ability to nodulate alfalfa. Appl. Environ. Microbiol. 70:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma, W., F. C. Guinel, and B. R. Glick. 2003. Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl. Environ. Microbiol. 69:4396-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma, W., S. B. Sebestianova, J. Sebestian, G. I. Burd, F. C. Guinel, and B. R. Glick. 2003. Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Antonie Leeuwenhoek 83:285-291. [DOI] [PubMed] [Google Scholar]
- 45.MacLellan, S. R., L. A. Smallbone, C. D. Sibley, and T. M. Finan. 2005. The expression of a novel antisense gene mediates incompatibility within the large repABC family of alpha-proteobacterial plasmids. Mol. Microbiol. 55:611-623. [DOI] [PubMed] [Google Scholar]
- 46.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercado-Blanco, J., and J. Olivares. 1994. The large nonsymbiotic plasmid pRmeGR4a of Rhizobium meliloti GR4 encodes a protein involved in replication that has homology with the RepC protein of Agrobacterium plasmids. Plasmid 32:75-79. [DOI] [PubMed] [Google Scholar]
- 48.MercadoBlanco, J., and N. Toro. 1996. Plasmids in rhizobia: the role of nonsymbiotic plasmids. Mol. Plant-Microbe Interact. 9:535-545. [DOI] [PubMed] [Google Scholar]
- 49.Meyer, F., A. Goesmann, A. C. McHardy, D. Bartels, T. Bekel, J. Clausen, J. Kalinowski, B. Linke, O. Rupp, R. Giegerich, and A. Pühler. 2003. GenDB—an open source genome annotation system for prokaryote genomes. Nucleic Acids Res. 31:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishiguchi, R., M. Takanami, and A. Oka. 1987. Characterization and sequence determination of the replicator region in the hairy-root-inducing plasmid pRiA4b. Mol. Gen. Genet. 206:1-8. [Google Scholar]
- 51.Penrose, D. M., and B. R. Glick. 2001. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can. J. Microbiol. 47:368-372. [DOI] [PubMed] [Google Scholar]
- 52.Poupot, R., E. Martínez-Romero, F. Maillet, and J. C. Promé. 1995. Rhizobium tropici nodulation factor sulfation is limited by the quantity of activated form of sulfate. FEBS Lett. 368:536-540. [DOI] [PubMed] [Google Scholar]
- 53.Prell, J., B. Boesten, P. Poole, and U. B. Priefer. 2002. The Rhizobium leguminosarum bv. viciae VF39 gamma-aminobutyrate (GABA) aminotransferase gene (gabT) is induced by GABA and highly expressed in bacteroids. Microbiology 148:615-623. [DOI] [PubMed] [Google Scholar]
- 54.Ramírez-Romero, M. A., N. Soberón, A. Pérez-Oseguera, J. Téllez-Sosa, and M. A. Cevallos. 2000. Structural elements required for replication and incompatibility of the Rhizobium etli symbiotic plasmid. J. Bacteriol. 182:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg, C., P. Boistard, J. Dénarié, and F. Casse-Delbart. 1981. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol. Gen. Genet. 184:326-333. [DOI] [PubMed] [Google Scholar]
- 56.Roumiantseva, M. L., E. E. Andronov, L. A. Sharypova, T. Dammann-Kalinowski, M. Keller, J. P. Young, and B. V. Simarov. 2002. Diversity of Sinorhizobium meliloti from the central Asian alfalfa gene center. Appl. Environ. Microbiol. 68:4694-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruff, J., K. Denger, and A. M. Cook. 2003. Sulphoacetaldehyde acetyltransferase yields acetyl phosphate: purification from Alcaligenes defragrans and gene clusters in taurine degradation. Biochem. J. 369:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanjuán, J., and J. Olivares. 1989. Implication of nifA in regulation of genes located on a Rhizobium meliloti cryptic plasmid that affect nodulation efficiency. J. Bacteriol. 171:4154-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schröder, G., S. Krause, E. L. Zechner, B. Traxler, H. J. Yeo, R. Lurz, G. Waksman, and E. Lanka. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwedock, J. S., and S. R. Long. 1992. Rhizobium meliloti genes involved in sulfate activation—the 2 copies of nodPQ and a new locus, saa. Genetics 132:899-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwieger, F., and C. C. Tebbe. 2000. Effect of field inoculation with Sinorhizobium meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant (Medicago sativa) and a nontarget plant (Chenopodium album)—linking of 16S rRNA gene-based single-strand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl. Environ. Microbiol. 66:3556-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selbitschka, W., W. Arnold, U. B. Priefer, T. Rottschafer, M. Schmidt, R. Simon, and A. Pühler. 1991. Characterization of recA genes and recA mutants of Rhizobium meliloti and Rhizobium leguminosarum biovar viciae. Mol. Gen. Genet. 229:86-95. [DOI] [PubMed] [Google Scholar]
- 63.Selbitschka, W., U. Dresing, M. Hagen, S. Niemann, and A. Pühler. 1995. A biological containment system for Rhizobium meliloti based on the use of recombination-deficient (recA−) strains. FEMS Microbiol. Ecol. 16:223-232. [Google Scholar]
- 64.Selbitschka, W., M. Keller, R. Miethling-Graff, U. Dresing, F. Schwieger, I. Krahn, I. Homann, T. Dammann-Kalinowski, A. Pühler, and C. C. Tebbe. Long-term field release of bioluminescent Sinorhizobium meliloti strains to assess the influence of a recA mutation on the strains' survival. Microbiol. Ecol., in press. [DOI] [PubMed]
- 65.Simon, R. 1984. High-frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-mob transposon. Mol. Gen. Genet. 196:413-420. [DOI] [PubMed] [Google Scholar]
- 66.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic-engineering—transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 67.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 68.Soto, M. J., A. Zorzano, J. Mercado-Blanco, V. Lepek, J. Olivares, and N. Toro. 1993. Nucleotide sequence and characterization of Rhizobium meliloti nodulation competitiveness genes nfe. J. Mol. Biol. 229:570-576. [DOI] [PubMed] [Google Scholar]
- 69.Tabata, S., P. J. Hooykaas, and A. Oka. 1989. Sequence determination and characterization of the replicator region in the tumor-inducing plasmid pTiB6S3. J. Bacteriol. 171:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tennstedt, T., R. Szczepanowski, I. Krahn, A. Pühler, and A. Schlüter. 2005. Sequence of the 68,869 bp IncP-1alpha plasmid pTB11 from a waste-water treatment plant reveals a highly conserved backbone, a Tn402-like integron and other transposable elements. Plasmid 53:218-238. [DOI] [PubMed] [Google Scholar]
- 71.van der Ploeg, J. R., E. Eichhorn, and T. Leisinger. 2001. Sulfonate-sulfur metabolism and its regulation in Escherichia coli. Arch. Microbiol. 176:1-8. [DOI] [PubMed] [Google Scholar]
- 72.van der Ploeg, J. R., M. A. Weiss, E. Saller, H. Nashimoto, N. Saito, M. A. Kertesz, and T. Leisinger. 1996. Identification of sulfate starvation-regulated genes in Escherichia coli: a gene cluster involved in the utilization of taurine as a sulfur source. J. Bacteriol. 178:5438-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Elsas, J. D., and M. J. Bailey. 2002. The ecology of transfer of mobile genetic elements. FEMS Microbiol. Ecol. 42:187-197. [DOI] [PubMed] [Google Scholar]
- 74.van Rhijn, P., and J. Vanderleyden. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59:124-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venkova-Canova, T., N. E. Soberón, M. A. Ramírez-Romero, and M. A. Cevallos. 2004. Two discrete elements are required for the replication of a repABC plasmid: an antisense RNA and a stem-loop structure. Mol. Microbiol. 54:1431-1444. [DOI] [PubMed] [Google Scholar]
- 76.Yarosh, O. K., T. C. Charles, and T. M. Finan. 1989. Analysis of C4-dicarboxylate transport genes in Rhizobium meliloti. Mol. Microbiol. 3:813-823. [DOI] [PubMed] [Google Scholar]
- 77.Zekri, S., and N. Toro. 1998. A new insertion sequence from Sinorhizobium meliloti with homology to IS1357 from Methylobacterium sp. and IS1452 from Acetobacter pasteurianus. FEMS Microbiol. Lett. 158:83-87. [DOI] [PubMed] [Google Scholar]
- 78.Zhong, Z., R. Caspi, D. Helinski, V. Knauf, S. Sykes, C. O'Byrne, T. P. Shea, J. E. Wilkinson, C. DeLoughery, and A. Toukdarian. 2003. Nucleotide sequence based characterizations of two cryptic plasmids from the marine bacterium Ruegeria isolate PR1b. Plasmid 49:233-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.