Abstract
Dietary carbohydrates have the potential to influence diverse functional groups of bacteria within the human large intestine. Of 12 Bifidobacterium strains of human gut origin from seven species tested, four grew in pure culture on starch and nine on fructo-oligosaccharides. The potential for metabolic cross-feeding between Bifidobacterium adolescentis and lactate-utilizing, butyrate-producing Firmicute bacteria related to Eubacterium hallii and Anaerostipes caccae was investigated in vitro. E. hallii L2-7 and A. caccae L1-92 failed to grow on starch in pure culture, but in coculture with B. adolescentis L2-32 butyrate was formed, indicating cross-feeding of metabolites to the lactate utilizers. Studies with [13C]lactate confirmed carbon flow from lactate, via acetyl coenzyme A, to butyrate both in pure cultures of E. hallii and in cocultures with B. adolescentis. Similar results were obtained in cocultures involving B. adolescentis DSM 20083 with fructo-oligosaccharides as the substrate. Butyrate formation was also stimulated, however, in cocultures of B. adolescentis L2-32 grown on starch or fructo-oligosaccharides with Roseburia sp. strain A2-183, which produces butyrate but does not utilize lactate. This is probably a consequence of the release by B. adolescentis of oligosaccharides that are available to Roseburia sp. strain A2-183. We conclude that two distinct mechanisms of metabolic cross-feeding between B. adolescentis and butyrate-forming bacteria may operate in gut ecosystems, one due to consumption of fermentation end products (lactate and acetate) and the other due to cross-feeding of partial breakdown products from complex substrates.
Microbial growth and metabolism in the human large intestine depend to a large extent on the supply of dietary carbohydrates that resist digestion in the upper gut. The fermentation of these compounds, which include plant cell wall polysaccharides and some storage polysaccharides and oligosaccharides, has a major influence on health (9, 20, 43). Indeed, specific carbohydrates are now widely used as functional foods and as prebiotics, based on the concept that they stimulate particular gut bacteria that promote gut health (18) and, at the same time, reduce the populations of nonutilizing bacteria through competition. Inulin and fructo-oligosaccharides (FOS), for example, were originally proposed as prebiotics that selectively stimulate bifidobacteria. While there is evidence that this occurs (11, 19, 26, 45), other studies, using molecular techniques, have revealed that a variety of other bacterial groups, including clostridium-related species, also respond to inulin or FOS supplied as prebiotics in either fermentor experiments or animal models (13, 25).
Among the possible explanations for this diversity in response to prebiotics is that complex gut microbial communities involve extensive metabolic interactions (10, 46). Metabolic products produced from dietary prebiotics by one bacterial species may then provide substrates to support growth of other populations, and this is termed cross-feeding. Such cross-feeding can result in metabolic consequences that would not be predicted simply from the substrate preferences of isolated bacteria. For example, both resistant starch and FOS can be butyrogenic in vivo (23, 42, 43), although the main utilizers of such substrates so far identified have been lactic acid bacteria (31, 43). This may be due to compositional changes of bacterial communities within the colon following the reduction in pH that results from rapid carbohydrate fermentation (44) together with the fact that some butyrate producers are able to utilize those substrates (2, 37), but it is also possible that lactate (produced by bifidobacteria, for example) can be converted to butyrate by other species (24). The latter possibility is supported by the recent isolation from human feces of bacteria that convert lactate and acetate to butyrate (14) and by the observation that butyrate can be the main product formed from lactate by mixed human fecal bacteria (5).
This paper examines the potential role of metabolic cross-feeding between strains of Bifidobacterium adolescentis that are able to utilize starch or FOS as growth substrates and strains of butyrate-producing bacteria that cannot themselves utilize starch or FOS but can potentially utilize the lactate and acetate formed by B. adolescentis. Using isotopically labeled substrates, we confirmed that cross-feeding of lactate can occur in cocultures. These experiments also reveal a second mechanism of metabolic cross-feeding, however, that may boost butyrate formation by non-lactate-utilizing species found in the human colon.
MATERIALS AND METHODS
Bacterial strains and maintenance.
All bacterial strains included in this study were of human origin. Anaerostipes caccae L1-92 (DSM14662T) (41), the Eubacterium hallii-like strain L2-7 (DSM 17630) (14), and Roseburia sp. strain A2-183 (DSM 16839) (2) are available from the Deutsche Sammlung von Mikroorganismen (DSMZ). Bifidobacterium adolescentis L2-32 was isolated from an infant (14), while strain 70/18, which shares 99% 16S rRNA sequence homology with Bifidobacterium bifidum (S. H. Duncan and H. J. Flint, unpublished data), was isolated from an adult human fecal sample. Other Bifidobacterium strains were obtained from the DSMZ (B. adolescentis DSM 20083 and DSM 20086, B. angulatum DSM 20098, B. breve DSM 20213, B. longum biotype longum DSM 20219 [40], B. longum biotype infantis DSM 20088 [40], B. pseudocatenulatum DSM 20438, and B. bifidum DSM 20456) or from the National Collection of Industrial and Marine Biology (NCIMB) (Aberdeen, United Kingdom) (B. breve NCIMB 8807 and B. longum biotype longum NCIMB 8809). All strains were routinely maintained in M2GSC broths and stored in medium containing 0.75% agar (35).
Growth rates of Bifidobacterium strains.
The growth rates of 12 strains with either potato starch (BDH, Poole, United Kingdom) or FOS (Trouw International B.V., Holland) as a substrate were tested in anaerobically prepared yeast extract-Casitone-fatty acid (YCFA) medium (12) adjusted to pH 5.7. FOS (filter sterilized) or starch (sterilized by autoclaving) was added to give a final concentration of 0.2%. Specific growth rates (h−1) were calculated from absorbance readings (optical density [OD] at 650 nm) during the exponential phase of growth at 37°C.
Coculture studies.
Two types of coculture incubations were conducted. First, a known lactate producer, B. adolescentis L2-32 or DSM 20083 was incubated on medium containing either starch or FOS with a known lactate utilizer (either A. caccae or E. hallii) that is incapable of using either of the carbon substrates directly. Second, the use of nonlactate products of starch digestion from B. adolescentis metabolism was tested by coculture with another butyrate producer, Roseburia sp. strain A2-183, which lacks the ability to grow on lactate. In all cases, replicate tubes of anaerobic YCFA medium with the appropriate added carbon source (potato starch or FOS) were inoculated with each strain individually and with identical inocula of the two strains in combination. Cultures providing the inoculum were pregrown overnight in M2GSC medium (35). Duplicate experiments were performed in media that had been adjusted to two different initial pH values (5.7 ± 0.2 and 6.5 ± 0.2).
For the flux studies involving growth of E. hallii in monoculture, the strain was grown in the presence of acetate (33 mM) and lactate (45 mM), containing either [1-13C]acetate or [U-13C]lactate to give 10 molar percent excess (MPE). Replicate tubes were processed at 0, 3, 8, and 24 h to measure short-chain fatty acid (SCFA) and lactate concentrations and 13C enrichments. For the flux studies involving cocultures, a filter-sterilized solution of [1-13C]acetate or [3-13C]lactate was added after 3 h of incubation to give 10 MPE. Samples were taken for estimation of SCFA and lactate concentrations and 13C enrichments at the times indicated (see Results).
Analysis of short-chain fatty acids and [13C]acetate, [13C]butyrate, and [13C]lactate enrichments.
Replicate derivatized samples were routinely prepared for estimation of concentrations of SCFA and lactate by capillary gas chromatography (38). In experiments involving stable isotopes, lactate and SCFA enrichments and concentrations, estimated by isotope dilution, were measured by gas chromatography-mass spectrometry (GC-MS) analysis of the tert-butyldimethylsilyl derivatives. Procedures were as described previously (7, 15), except that 10 μl trypan blue was added to the initial sample to provide a visual aid in the transfer of the ether layer. GC-MS analyses were performed as described previously (15) with the following exceptions. The temperatures of the injector and the interface line were both 250°C. The GC separation was with an EC-1 capillary column (3 m by 0.25 mm by 0.25 μm) (Alltech, Carnforth, Lancs., United Kingdom) under the following conditions: 60°C for 3 min and then 25°C/min to 210°C for 4 min. Injections (1 μl) were made in the split mode with a 40:1 split and a 2-cm plug of silanized fused silica wool in the glass liner of the injector. The MS was operated under electron impact ionization conditions. For acetate, the ions M+, M + 1, and M + 2 at mass/charge (m/z) ratios of 117, 118, and 119 were monitored; for butyrate, M+, M + 1, M + 2, and M + 4 (i.e., m/z 145, 146, 147, and 149) were determined, the latter two to quantify butyrate formation from two [1-13C]acetate and two [1,2-13C]acetate molecules; while for lactate, M+, M + 1, and M + 3 ion fragments (m/z 261, 262, and 264) were analyzed. In practice, the amounts of M + 2 or M + 4 labeled butyrate formed were close to those predicted by the laws of probability from precursor enrichments. For the concentration determinations, appropriate corrections were applied for the enrichments in the sample.
Kinetic modeling of SCFA and lactate metabolism.
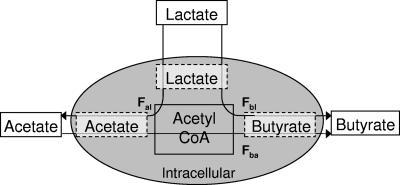
For simplicity, all units are expressed as C2 units (thus, butyrate concentrations were doubled, while butyrate enrichments were halved [15]). Let C and E denote concentration (mM) and enrichment (MPE), respectively. Subscripts a, b, and l refer to acetate, butyrate, and lactate, respectively. Let i denote the interval between any two times t0 and t1, and let F(i) denote the flow of labeled plus unlabeled material during i, while F = ΣiF(i) denotes the cumulative flow. Flows to pool y from pool x are denoted by Fyx. Flows of labeled material are denoted by f. E(i) denotes the average enrichment during i. As the system was not in steady state, inflows to (subscript “in”) and outflows from (subscript “out”) the acetate and lactate pools were calculated separately. Therefore, at any time point, inflows and outflows may differ. Butyrate was assumed as an end product with inflow Fb. A schematic representation of the model is given in Fig. 1.
FIG. 1.
Schematic representation of the model used for the C2 flows. Fbl, flow of C2 from lactate to butyrate via acetyl-CoA without exchange with exogenous acetate; Fal, flow of C2 from lactate to acetate; Fba, flow of C2 from acetate to butyrate.
For the [1-13C]acetate batch monocultures, Fa.out(i), Fa.in(i), Fb(i), and Fba(i) are obtained from Ea(t1)Ca(t1) = Ea(t0)Ca(t0) − Ea(i)Fa.out(i), Ca(t1) = Ca(t0) − Fa.out(i) + Fa.in(i), Cb(t1) = Cb(t0) + Fb(i), and Eb(t1)Cb(t1) = Eb(t0)Cb(t0) + Ea(i)Fba(i).
Occasionally Fa.out(i) was less than Fba(i), and here Fa.out(i) was set equal to Fba(i). Let p = Fba/Fb and q = Fba/Fa.out, based on cumulative flows.
For the [U-13C]lactate batch monocultures, Fb(i) was obtained from Cb(t1) = Cb(t0) + Fb(i), as given above. Assuming that the relative flows were similar for both the [U-13C]lactate and [1-13C]acetate studies, then Fba(i) = pFb(i) and Fa.out(i) = Fba(i)/q. Furthermore, fb(i) = Eb(t1)Cb(t1) − Eb(t0)Cb(t0). Then, fba(i) = pfb(i), and fa.out(i) = fba(i)/q. Also, fbl(i) = fb(i) − fba(i), and fa.in(i) = Ea(t1)Ca(t1) − Ea(t0)Ca(t0) + fa.out(i). Assuming that fal(i) = fa.in(i), then Fbl(i) = fbl(i)/El(i) and Fal(i) = fal(i)/El(i).
For the batch cocultures, calculations based on [1-13C]acetate data are identical to those for the monoculture, but those for the [U-13C]lactate data are based on El(t1)Cl(t1) = El(t0)Cl(t0) − El(i)Fl.out(i) and Cl(t1) = Cl(t0) + Fl.in(i) − Fl.out(i), giving Fl.out(i) and Fl.in(i). In contrast to the case for the monoculture, Ea and Eb were close to the detection limits, so lactate outflows to acetate (Fal) and butyrate (Fbl) could not be calculated directly. Therefore, using data from the [1-13C]acetate coculture study, it was assumed that Fbl(i) = Fb(i) − Fba(i) and Fal(i) = Fl.out(i) − Fbl(i).
Results from the cocultures were analyzed by analysis of variance with substrate, pH, and their interaction as treatment effects, using Genstat release 8.1, 8th ed. (VSN International Ltd., Hemel Hempstead, Herts., United Kingdom).
Quantitative real-time PCR.
The abundances of B. adolescentis L2-32 and Roseburia sp. strain A2-183 alone and in coculture were determined by quantitative real-time PCR. Equal volumes of cocultures grown in triplicate were combined and centrifuged at 10,000 × g for 5 min. For comparison, two sets of triplicate monocultures, grown for the same length of time, were combined and treated in the same way. Cell pellets were resuspended in 25 μl of sterile distilled H2O and DNA extracted using the Fast DNA spin kit for soil (Qbiogene). DNA was diluted to 0.5 ng μl−1 in 5 μg ml−1 herring sperm DNA (Promega) and amplified with primers BifF (TCGCGTCYGGTGTGAAAG) (39) and g-Bifid-R (GGTGTTCTTCCCGATATCTACA) (34) for the quantification of B. adolescentis L2-32 and with primers Cclos99modF (TGAGTGGCGGACGGGTGAG, modified) (3) and CcmodRosR (TACCACCGGAGTTTTTCACAC, modified) (3) for the quantification of Roseburia sp. strain A2-183. Primers were checked for their specificity with the Probe Match function of the Ribosomal Database Project II (8). Standard template DNA was prepared from the 16S rRNA gene of Roseburia sp. strain A2-183 by amplification with primers 27F and RP2 and purification as described previously (30). Standard curves were prepared with five standard concentrations of 107 to 103 gene copies μl−1 in 5 μg ml−1 herring sperm DNA, with universal primers UniF (GTGSTGCAYGGYYGTCGTCA, modified) (33) and UniR (ACGTCRTCCMCNCCTTCCTC, modified) (33).
PCRs were performed in triplicate with iQ SYBR Green Supermix (Bio-Rad) in a total volume of 25 μl with primers at 500 nM in optical-grade 96-well plates sealed with optical sealing tape. Amplification was performed with an iCycler (Bio-Rad) with the following protocol: one cycle of 95°C for 3 min, 40 cycles of 95°C and 60°C for 30 s each, one cycle of 95°C for 1 min, one cycle of 55°C for 1 min, and a stepwise increase of the temperature from 55 to 95°C (at 10 s per 0.5°C) to obtain melt curve data. Data were analyzed using the iCycler IQ software version 3.1.
RESULTS
Growth and metabolism of Bifidobacterium strains with starch or FOS as a substrate.
There was wide variation in the abilities of different Bifidobacterium strains isolated from the human gut to utilize potato starch and Trouw FOS for growth. Out of 12 strains that were tested, belonging to seven species, nine showed measurable rates of growth on FOS and four showed significant growth on starch (Table 1). Acetate, lactate, and formate were the major acid products formed. Lactate concentrations in growing cultures after 24 h ranged from 0.7 to 9 mM, accounting for 10 to 30% of the organic acids formed. In agreement with previous findings (22), the proportion of lactate tended to increase with increasing growth rate. Two strains of B. adolescentis were chosen for further study. These were B. adolescentis L2-32, which was used previously in cross-feeding experiments (14) and grows well on potato starch, and B. adolescentis DSM 20083, which showed the highest growth rate on FOS.
TABLE 1.
Specific growth rates of Bifidobacterium strains in YCFA medium containing 0.2% potato starch or fructo-oligosaccharides
| Strain | Specific growth rate (h−1)a on:
|
|
|---|---|---|
| Starch | Fructo- oligosaccharides | |
| B. adolescentis L2-32 | 0.40 ± 0.007 | 0.20 ± 0.012 |
| B. adolescentis DSM 20083 | —b | 0.56 ± 0.045 |
| B. adolescentis DSM 20086 | — | 0.42 ± 0.011 |
| B. angulatum DSM 20098 | 0.41 ± 0.024 | 0.45 ± 0.085 |
| B. bifidum 70/18 | 0.26 ± 0.090 | — |
| B. breve DSM 20213 | — | 0.21 ± 0.006 |
| B. longum (biotype longum) DSM 20219 | — | 0.30 ± 0.062 |
| B. longum (biotype longum) NCIMB 8809 | — | 0.15 ± 0.026 |
| B. longum (biotype infantis) 20088 | — | 0.54 ± 0.041 |
| B. pseudocatenulatum DSM 20438 | 0.16 ± 0.053 | 0.45 ± 0.042 |
Values are means of three replicates ± standard deviations.
—, poor growth (final ΔOD of <0.13). B. bifidum DSM 20456 and B. breve NCIMB 8807 were also tested but gave final ΔODs of <0.1 on both substrates.
Coculture of B. adolescentis L2-32 and lactate-utilizing butyrate-producing E. hallii and A. caccae strains with starch as a substrate.
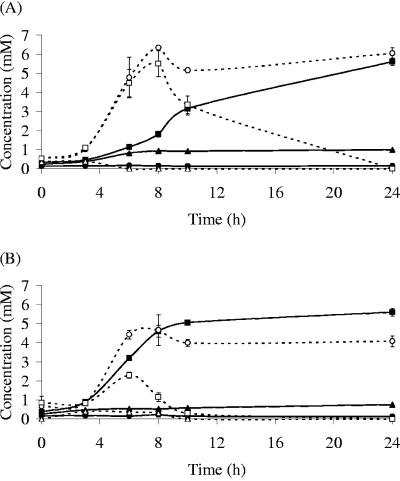
Lactate accumulated in B. adolescentis L2-32 cultures grown on potato starch (Fig. 2). The butyrate-producing bacterium E. hallii L2-7 was unable to grow on starch in pure culture but can utilize lactate (14). In cocultures of E. hallii L2-7 and B. adolescentis L2-32, lactate concentrations decreased after the initial rise and there was a progressive increase in butyrate formation. This effect was seen both at pH 5.7 and 6.5, although utilization of lactate was less efficient at the lower pH (Fig. 2). Experiments with another lactate utilizer, A. caccae L1-92, gave similar results (not shown), except that lactate utilization was incomplete at pH 5.7 after 24 h.
FIG. 2.
Changes in butyrate (closed symbols) and lactate (open symbols) concentrations during incubation of monocultures of B. adolescentis L2-32 (circles), E. hallii L2-7 (triangles), and their cocultures (squares) on potato starch at either pH 5.7 (A) or 6.5 (B).
Conversion of acetate and lactate to butyrate by E. hallii.
The mechanism proposed previously for the cross-feeding phenomenon illustrated in Fig. 2 is that l-lactate and acetate produced by B. adolescentis drive butyrate formation by E. hallii (14). To clarify the carbon flows involved, E. hallii was first grown in pure culture in the presence of unlabeled acetate plus [U-13C]lactate, or in the presence of [1-13C]acetate plus unlabeled lactate, in duplicate experiments at two initial pH values (Table 2). The carbon flows through lactate, acetate, and butyrate pools were estimated by kinetic modeling (see Materials and Methods) (Fig. 1). As found previously for related Roseburia species, E. hallii showed active interchange between internal and external C2 pools (15). The lactate was initially converted intracellularly by E. hallii to acetyl coenzyme A (acetyl-CoA), which then rapidly interconverted with exogenous acetate. Consequently, a high proportion of butyrate carbon was derived through that acetate pool (endogenous plus exogenous). Overall, lactate contributed between 57 to 62% to butyrate carbon, with the majority (95 and 80% at pH 5.7 and 6.5, respectively) via the acetate pool (Table 2).
TABLE 2.
Conversion of lactate and acetate to butyrate by Eubacterium hallii L2-7 incubated in YCFA in the presence of acetate (33 mM) and lactate (45 mM) and with the addition of [1-13C]acetate or [U-13C]lactate
| Parametera | Value with the following addition and initial pH:
|
|||
|---|---|---|---|---|
| [13C]acetate
|
[13C]lactate
|
|||
| 5.7 | 6.5 | 5.7 | 6.5 | |
| Acetate outflow (Fa.out) | ||||
| Total | 25.95 | 44.48 | 26.27 | 52.00 |
| To butyrate (Fba) | 22.99 | 40.16 | 23.27 | 46.63 |
| Acetate production (Fa.in) | 15.45 | 25.26 | 16.06 | 28.44 |
| p1 (%) | 88 | 74 | ||
| Lactate outflow | ||||
| To butyrate (Fbl) | 0.77 | 7.57 | ||
| To acetate (Fal) | 16.30 | 34.06 | ||
| p2 (%) | 57 | 62 | ||
| Butyrate production (Fb) | 26.27 | 53.96 | 26.59 | 62.67 |
p1, percentage of butyrate carbon (C) coming from acetate C, estimated as Fba/Fb; p2, percentage of butyrate C coming from lactate C (either directly or indirectly via conversion to acetate), calculated as [(q × Fal) + Fbl]/Fb, with q being the proportion of acetate C going to butyrate C (Fba/Fa.out). All flows are expressed in terms of C2 units (mmol/liter per 24 h).
Carbon flow of lactate and acetate to butyrate in cocultures.
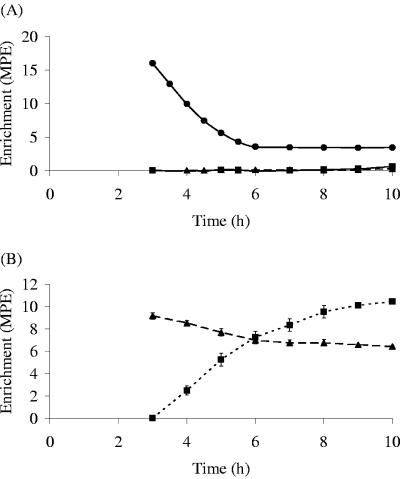
Carbon flow was next studied in coculture experiments involving either B. adolescentis L2-32 and E. hallii L2-7 grown on starch or B. adolescentis DSM 20083 and E. hallii L2-7 grown on FOS. Each experiment was performed at two initial pH values, 5.7 and 6.5. [3-13C]lactate or [1-13C]acetate was added as a tracer after 3 h of growth, and their incorporation into acetate and butyrate was followed (as shown for the experiment involving B. adolescentis L2-32 and E. hallii L2-7, at initial pH 5.7, in Fig. 3). The flows of carbon in the two coculture experiments are presented in Table 3. In the experiment involving B. adolescentis L2-32 with starch as a substrate, lactate production was approximately 30% of the value for acetate production at pH 5.7 but was only 10% at pH 6.5, due mainly to a 70% decline in lactate formation. All lactate formed was metabolized, however, with most entering the exogenous acetate pool. Only 11 to 21% was converted to butyrate without exchange with exogenous acetate. In the experiment performed with B. adolescentis DSM 20083 and FOS as the substrate, lactate production was slightly lower at pH 6.5 than at pH 5.7. Again all the lactate produced was metabolized, with the majority (61 to 77%) entering the exogenous acetate pool. In both experiments, the estimated contribution of lactate to butyrate carbon (p2 in Tables 2 and 3) was somewhat lower in the cocultures (44 to 48%) than in the pure culture of E. hallii grown on lactate and acetate (57%) at pH 5.7 but was markedly lower at pH 6.5 (25 to 28%, compared with 62% in the pure culture).
FIG. 3.
Enrichments of lactate (circles), acetate (triangles), and butyrate (squares) in cocultures of Bifidobacterium adolescentis L2-32 and Eubacterium hallii L2-7 on starch at pH 5.7 following [3-13C]lactate (A) or [1-13C]acetate (B) injection.
TABLE 3.
Conversion of lactate and acetate to butyrate in the cocultures between Bifidobacterium strains and Eubacterium hallii L2-7 with potato starch or fructo-oligosaccharides as the substrate
| Parametera | Value with the following substrate and initial pH:
|
SEMd |
P valued
|
|||||
|---|---|---|---|---|---|---|---|---|
| Starchb
|
FOSc
|
|||||||
| 5.7 | 6.5 | 5.7 | 6.5 | Substrate | pH | Substrate · pH | ||
| Lactate production (Fl.in) | 6.11 | 1.78 | 4.79 | 3.19 | 0.145 | 0.761 | <0.001 | <0.001 |
| Lactate outflow (Fl.out) | 7.67 | 2.96 | 6.01 | 4.12 | 0.162 | 0.203 | <0.001 | <0.001 |
| To acetate (Fal) | 6.07 | 2.61 | 4.63 | 2.53 | ||||
| To butyrate (Fbl) | 1.60 | 0.34 | 1.37 | 1.60 | ||||
| Acetate production (Fa.in) | 21.81 | 17.53 | 21.59 | 20.02 | 0.843 | 0.248 | 0.026 | 0.184 |
| Acetate outflow (Fa.out) | ||||||||
| Total | 15.69 | 12.25 | 13.32 | 15.36 | 0.790 | 0.660 | 0.427 | 0.026 |
| To butyrate (Fba) | 8.67 | 7.00 | 7.97 | 10.21 | 0.307 | 0.015 | 0.397 | 0.003 |
| Butyrate production (Fb) | 10.26 | 7.34 | 9.34 | 11.81 | 0.276 | <0.001 | 0.433 | <0.001 |
| p1 | 89 | 90 | 85 | 87 | 0.431 | 0.002 | 0.086 | 0.192 |
| p2 | 48 | 25 | 44 | 28 | ||||
p1, percentage of butyrate carbon (C) coming from acetate C, estimated from 13C acetate studies as Fba/Fb; p2, percentage of butyrate C coming from lactate C (either directly or indirectly via conversion to acetate), calculated as [(q × Fal) + Fbl]/Fb, with q being the proportion of acetate C going to butyrate C (Fba/Fa.out). All flows are expressed in terms of C2 units (mmol/liter per 21 h).
Incubation with B. adolescentis L2-32.
Incubation with B. adolescentis DSM 20083.
From analysis of variance with substrate, pH, and their interaction (substrate · pH) as treatment effects. Values are based on 8 observations (4 residual df), except for Fb, which is based on 16 observations (12 residual df). Fal, Fbl, and p2 were calculated from combinations of mean values obtained from [13C]acetate and [13C]lactate studies, which did not allow for statistical analysis.
Evidence for a second mechanism of cross-feeding.
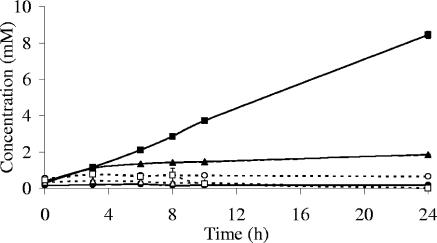
Coculture studies of B. adolescentis L2-32 with E. hallii L2-7 or A. caccae L1-92 were also conducted with Trouw FOS as the substrate. As noted above, B. adolescentis L2-32 grew poorly on this substrate (Table 1). Although only low concentrations of lactate were detected with the pure culture of B. adolescentis L2-32 on FOS, the coculture with E. hallii L2-7 or A. caccae L1-92 nevertheless gave rise to substantial butyrate (Fig. 4). This observation suggested that another mechanism apart from lactate cross-feeding might be responsible for the stimulation of butyrate in this case. In order to explore this possibility further, we chose to examine cocultures involving a butyrate producer, Roseburia sp. strain A2-183, which cannot utilize lactate. Table 4 shows that butyrate formation was also observed when Roseburia sp. strain A2-183 was cocultured with L2-32 on FOS or starch, although A2-183 was unable to grow significantly on FOS or starch in monoculture. The relative abundances of B. adolescentis L2-32 and Roseburia sp. strain A2-183 were estimated by 16S rRNA-based real-time PCR in these cocultures and compared with results for mixtures of the control pure cultures incubated for the same period of time (see Materials and Methods). This revealed significant stimulation of the Roseburia rRNA gene copy number in the cocultures on FOS at an initial pH of 6.5, the pH that produced the greatest butyrate formation, and on starch at both initial pH values (Table 4). The presence of the B. adolescentis L2-32 therefore appeared to stimulate growth and butyrate production by the Roseburia strain. This effect must be due to a mechanism that is independent of lactate utilization, and it is probably the result of cross-feeding of partially degraded carbohydrate substrate. This mechanism is assumed to account also for most of the butyrate formation seen in cocultures of B. adolescentis L2-32 and A. caccae L1-92 (Fig. 4) or E. hallii L2-7 on medium containing FOS.
FIG. 4.
Changes in butyrate (closed symbols) and lactate (open symbols) concentrations during incubation of monocultures of B. adolescentis L2-32 (circles), A. caccae L1-92 (triangles), and their cocultures (squares) on fructo-oligosaccharides at pH 5.7.
TABLE 4.
Influence of pH on SCFA concentrations and relative proportions of each strain in monocultures of B. adolescentis L2-32 and Roseburia sp. strain A2-183 and their cocultures when incubated in YCFA medium containing 0.2% potato starch or fructo-oligosaccharides
| SCFA | Concn change (mM) with the following substrate, pH, and inoculuma
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starch
|
FOS
|
|||||||||||||||
| 5.7
|
6.5
|
5.7
|
6.5
|
|||||||||||||
| B | R | R + B | B | R | R + B | B | R | R + B | B | R | R + B | |||||
| Acetate | 15.2 ± 2.2 | −0.8 ± 0.6 | 0.02 ± 3.2 | 18.0 ± 1.4 | 2.4 ± 1.4 | 5.3 ± 1.2 | 6.3 ± 2.1 | −1.3 ± 0.7 | 7.3 ± 1.9 | 12.1 ± 2.4 | −1.1 ± 1.4 | 11.5 ± 3.1 | ||||
| Butyrate | 2.5 ± 0.1 | 8.1 ± 1.8 | 2.6 ± 0.2 | 6.1 ± 0.9 | 1.5 ± 0.1 | 5.1 ± 0.4 | 1.5 ± 0.1 | 9.9 ± 2.1 | ||||||||
| Lactate | 6.5 ± 0.6 | 1.9 ± 0.7 | 3.2 ± 0.2 | 0.4 ± 0.1 | 1.4 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 | 0.7 ± 0.1 | 1.8 ± 0.7 | |||||||
| R/Bb | 0.60 | 2.07 | 0.25 | 1.34 | 0.31 | 0.41 | 0.41 | 1.69 | ||||||||
Values are means of three replicates ± standard deviations. B, B. adolescentis L2-32; R, Roseburia sp. strain A2-183; R + B, coculture.
R/B, ratio of abundances of Roseburia sp. strain A2-183 and B. adolescentis L2-32 in pellets obtained from the combination of equal volumes of the cocultures or the monocultures of both strains.
DISCUSSION
There is much interest in the impact of nondigestible but fermentable dietary carbohydrates, including prebiotics (18), on gut metabolism and health in humans (43). The effects of resistant starch and FOS on microbial metabolism and bacterial populations have been studied in humans and in animal models (4, 16, 17, 29), and several studies have reported the stimulation of human fecal bifidobacteria by FOS or inulin (11, 19, 26). Previous work has also indicated varied capability among bifidobacterial strains to use FOS and starch (22). In the present study, of 12 Bifidobacterium strains of human gut origin examined, four grew well on potato starch and nine on Trouw FOS, although growth rates varied. This suggests that prebiotic stimulation of bifidobacterial populations might prove to be both strain and substrate specific. B. adolescentis was selected for these studies because it is one of the most abundant species of bifidobacteria in the human colon (1) and thus has the potential to play a significant role in diet utilization and colonic health.
It has been proposed that cross-feeding of lactate produced by bifidobacteria can stimulate the formation of butyrate by other bacteria within the gut community (14, 24). This proposal arose from the observation that the same substrates that probably promote bifidobacterial populations in vivo can also be butyrogenic (28). The recent isolation of butyrate-producing species such as E. hallii and A. caccae that are able to utilize lactate (14) offered the chance to investigate the potential significance of lactate cross-feeding in defined cocultures. The stable-isotope experiments showed that E. hallii L2-7 converts l-lactate to acetyl-CoA, and this is rapidly exchanged with exogenous acetate, thus providing precursors for butyrate synthesis. The fate of labeled lactate was entirely consistent with conversion of lactate to pyruvate via lactate dehydrogenase, as proposed previously (14). No evidence was found for the conversion of lactate to butyrate through a distinct pathway. In cocultures involving B. adolescentis L2-32 on starch or B. adolescentis DSM 20083 on FOS, the Bifidobacterium strain was shown to produce lactate in the presence of E. hallii, with the latter organism being responsible for conversion of the lactate into butyrate. The pH of the proximal colonic lumen is reported to fall below pH 6.0 as a result of active microbial fermentation of certain dietary substrates (6, 36). The ability of E. hallii to utilize lactate in cocultures with B. adolescentis both at pH 5.7 and at pH 6.5 could therefore have important implications for the supply of butyrate to various regions of the colon.
E. hallii and its relatives can account for 4% of human bacteria (21), and such bacteria may play a significant role in preventing lactate accumulation in vivo. Indeed, a recent study examined the fate of [13C]lactate in human fecal slurries, maintained at pH 5.8, and found that for two out of the three donors lactate was mainly converted to butyrate via acetyl-CoA (5). Bifidobacterium spp. can account for up to 15% of fecal bacteria (27) and therefore make a potentially important contribution to lactate production in vivo. Further work is needed, however, to determine rates of lactate formation and disposal in the complete gut community under conditions that operate within the colon in vivo. The molar proportion of lactate in pure cultures of bifidobacteria was found here to range from 30% down to 10% of total SCFA, and lactate production by B. breve is known to decrease under carbon limitation (32). On the other hand, bacteria other than Bifidobacterium spp. also have the potential to be major producers of lactate in vivo.
A second form of cross-feeding was also inferred from the increased production of butyrate by Roseburia sp. strain A2-183 when in coculture with B. adolescentis L2-32. In pure culture Roseburia sp. strain A2-183 is unable to utilize lactate or to grow on potato starch or Trouw FOS. The butyrate production observed in these cocultures is probably due to cross-feeding of products released by partial hydrolysis of FOS or starch by enzymes from B. adolescentis, most likely in the form of small fructo-oligosaccharides or malto-oligosaccharides. Indeed, the ability of Roseburia sp. strain A2-183 to survive in fermentor systems inoculated with mixed human fecal bacteria and supplied with different polysaccharide substrates was previously attributed to this type of cross-feeding (13). This mechanism probably operated in combination with lactate utilization to account for the butyrate formation in the cocultures involving A. caccae and E. hallii strains; indeed, this is a likely explanation for the observation (Tables 2 and 3) that lactate contributed less to butyrate carbon in the coculture experiments than in the pure-culture experiments with E. hallii. Since the majority of butyrate producers in the human gut are not lactate utilizers (2), such “substrate spillover” in fact represents a more generic mechanism of metabolic cross-feeding with the ability to also stimulate butyrate production. Cross-feeding of breakdown products between primary polysaccharide-degrading and oligosaccharide-utilizing gut bacteria has been recognized as a wide-ranging phenomenon in gut microbial ecosystems (10).
In conclusion, several mechanisms may contribute to the butyrogenic effects of dietary substrates such as FOS and starch. First, active fermentation tends to decrease the pH of the colonic lumen (6). This may have the effect of reducing competition for carbohydrate substrates from nonbutyrogenic species such as Bacteroides when the pH is decreased from 6.7 to 5.7, as suggested by a recent study in vitro (44). Butyrate-producing bacteria that are able to directly utilize FOS and starch (13) therefore may be expected to compete better for these substrates and to contribute to increased butyrate production at the lower pH (44). Second, the current data demonstrate two potential indirect mechanisms that involve metabolic cross-feeding. The importance of specific cross-feeding in vivo via lactate needs to be assessed further by determining the rate of lactate production and utilization in the complete ecosystem. This may depend partly on the abundances of lactate utilizers in different individuals. In a wider context, cross-feeding of polysaccharide breakdown products released by bifidobacteria has the potential to stimulate butyrate production regardless of the ability of butyrate producers to utilize lactate.
The relative importance of these various mechanisms has yet to be established in vivo but will probably vary between individuals and between different dietary regimens. In particular, the pH of the colonic lumen is likely to be a key factor in determining both the competition between different groups of polysaccharide-utilizing bacteria and the nature and extent of metabolic cross-feeding.
Acknowledgments
The Rowett Research Institute and Biomathematics and Statistics Scotland are supported by the Scottish Environment and Rural Affairs Department. A. Belenguer received financial support from Comisión Mixta Caja Inmaculada-Consejo Superior de Investigación y Desarrollo de la D.G.A. and from Secretaría de Estado de Universidades e Investigación of the Spanish Ministry of Education and Science.
REFERENCES
- 1.Apajalahti, J. H. A., A. Kettunen, P. H. Nurminen, H. Jatila, and W. E. Holben. 2003. Selective plating underestimates abundance and shows differential recovery of bifidobacterial species from human feces. Appl. Environ. Microbiol. 69:5731-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, and H. J. Flint. 2000. Phylogenetic relationships of dominant butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosch, S., A. Fite, G. T. Macfarlane, and M. E. T. McMurdo. 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hositalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 70:3575-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosch, S., E. J. Woodmansey, J. C. M. Paterson, M. E. T. McMurdo, and G. T. Macfarlane. 2005. Microbiological effects of consuming a symbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 40:28-37. [DOI] [PubMed] [Google Scholar]
- 5.Bourriaud, C., R. J. Robins, L. Martin, F. Kozlowski, E. Tenailleau, C. Cherbut, and C. Michel. 2005. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 99:201-212. [DOI] [PubMed] [Google Scholar]
- 6.Bowns, R. L., J. A. Gibson, G. E. Sladen, B. Hicks, and A. M. Dawson. 1974. Effects of lactulose and other laxatives on ileal and colonic pH as measured by a radiotelemetry device. Gut 15:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder, A. G., K. E. Garden, S. E. Anderson, and G. E. Lobley. 1999. Quantitation of blood and plasma amino acids using isotope dilution electron impact gas chromatography/mass spectrometry with U-13C amino acids as internal standards. Rapid Commun. Mass Spectrom 13:2080-2083. [DOI] [PubMed] [Google Scholar]
- 8.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, S. A. Kulam, D. M. McGarrell, G. M. Garrity, and J. M. Tiedje. 2005. The Ribosomal Database Project (RDP-II): sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 33:D294-D296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings, J. H., and H. J. Englyst. 1987. Fermentation in the human large intestine and the available substrates. Am. J. Clin. Nutr. 45:1243-1255. [DOI] [PubMed] [Google Scholar]
- 10.Dehority, B. A. 1991. Effects of microbial synergism on fiber digestion in the rumen. Proc. Nutr. Soc. 50:149-159. [DOI] [PubMed] [Google Scholar]
- 11.DeWiele, T. V., N. Boon, S. Possemiers, H. Jacobs, and W. Verstraete. 2005. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 51:143-153. [DOI] [PubMed] [Google Scholar]
- 12.Duncan, S. H., A. Barcenilla, C. S. Stewart, S. E. Pryde, and H. J. Flint. 2002. Acetate utilization and butyryl coenzyme A:acetate coenzyme A transferase in human colonic bacteria. Appl. Environ. Microbiol. 68:5186-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan, S. H., K. P. Scott, A. G. Ramsay, H. J. M. Harmsen, G. W. Welling, C. S. Stewart, and H. J. Flint. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan, S. H., P. Louis, and H. J. Flint. 2004. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan, S. H., G. Holtrop, G. E. Lobley, G. Calder, C. S. Stewart, and H. J. Flint. 2004. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 91:915-923. [DOI] [PubMed] [Google Scholar]
- 16.Fooks, L. J., R. Fuller, and G. R. Gibson. 1999. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 9:53-61. [Google Scholar]
- 17.Furrie, E., S. Macfarlane, A. Kennedy, J. H. Cummings, S. V. Walsh, D. A. O'Neil, and G. T. Macfarlane. 2005. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot study. Gut 54:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 19.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 20.Gill, C. I. R., and I. R. Rowland. 2002. Diet and cancer: assessing the risk. Br. J. Nutr. 88(Suppl. 1):S73-S87. [DOI] [PubMed] [Google Scholar]
- 21.Harmsen, H. J. M., G. C. Raangs, T. He, J. E. Degener, and G. E. Welling. 2002. Extensive set of rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopkins, M. J., J. H. Cummings, and G. T. Macfarlane. 1998. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 85:381-386. [Google Scholar]
- 23.Jacobasch, G., M. Schiedl, M. Kruschewski, and K. Schmel. 1999. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Colorectal Dis. 14:201-211. [DOI] [PubMed] [Google Scholar]
- 24.Kanauchi, O., Y. Fujiyama, K. Mitsuyama, Y. Araki, T. Ishii, T. Nakamura, K. Hitomi, K. Agata, T. Saiki, A. Andoh, A. Toyonaga, and T. Bamba. 1999. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int. J. Mol. Med. 3:175-179. [DOI] [PubMed] [Google Scholar]
- 25.Kleesen, B., L. Hartmann, and M. Blaut. 2001. Oligofructose and long chain inulin: influence on the gut microbial ecology of rats associated with a human fecal flora. Br. J. Nutr. 86:375-382. [DOI] [PubMed] [Google Scholar]
- 26.Kruse, H. P., B. Kleesen, and M. Blaut. 1999. Effects of inulin on faecal bifidobacteria in human subjects. Br. J. Nutr. 82:375-382. [DOI] [PubMed] [Google Scholar]
- 27.Lay, C., M. Sutren, V. Rochet, K. Saunier, J. Dore, and L. Rigottier-Gois. 2005. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 7:933-946. [DOI] [PubMed] [Google Scholar]
- 28.LeBlay, G., C. Michel, H. M. Blottiere, and C. Cherbut. 1999. Prolonged intake of fructooligosaccharides induces a short-term elevation of lactic acid producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 129:2231-2235. [DOI] [PubMed] [Google Scholar]
- 29.LeBlay, G. M., C. D. Michel, H. M. Blottiere, and C. J. Cherbut. 2003. Raw potato starch and short-chain fructo-oligosaccharides affect the composition and metabolic activity of rat intestinal microbiota differently depending on the caecocolonic segment involved. J. Appl. Microbiol. 94:312-320. [DOI] [PubMed] [Google Scholar]
- 30.Louis, P., S. H. Duncan, S. I. McCrae, J. Millar, M. S. Jackson, and H. J. Flint. 2004. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 186:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macfarlane, G. T., and H. N. Englyst. 1986. Starch utilization by the human large intestinal microflora. J. Appl. Bacteriol. 60:195-201. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane, S., and G. T. Macfarlane. 2003. Regulation of short chain fatty acid production. Proc. Nutr. Soc. 62:67-72. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, H., C. Fujimoto, Y. Haruki, T. Maeda, S. Kokeguchi, M. Petelin, H. Arai, I. Tanimoto, F. Nishimura, and S. Takashiba. 2003. Quantitative real-time PCR using TaqMan and SYBR green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ and total bacteria. FEMS Immunol. Med. Microbiol. 39:81-86. [DOI] [PubMed] [Google Scholar]
- 34.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68:5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki, K., J. C. Martin, R. Marinsek-Logar, and H. J. Flint. 1997. Degradation and utilization of xylans by the rumen anaerobe P. bryantii (formerly P. ruminicola subsp. brevis B14). Anaerobe 3:373-381. [DOI] [PubMed] [Google Scholar]
- 36.Nugent, S. G., D. Kumar, D. S. Rampton, and D. F. Evans. 2001. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryde, S. E., S. H. Duncan, G. L. Hold, C. S. Stewart, and H. J. Flint. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217:133-139. [DOI] [PubMed] [Google Scholar]
- 38.Richardson, A. J., A. G. Calder, C. S. Stewart, and A. Smith. 1987. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 9:5-8. [Google Scholar]
- 39.Rinttilä, T., A. Kassinen, E. Malinen, L. Krogius, and A. Palva. 2004. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97:1166-1177. [DOI] [PubMed] [Google Scholar]
- 40.Sakata, S., M. Kitahara, M. Sakmoto, H. Hayashi, M. Fukuyama, and Y. Benno. 2002. Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int. J. Syst. Evol. Microbiol. 52:1945-1951. [DOI] [PubMed] [Google Scholar]
- 41.Schwiertz, A., G. L. Hold, S. H. Duncan, P. A. Lawson, M. D. Collins, H. J. Flint, and M. Blaut. 2001. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 25:46-51. [DOI] [PubMed] [Google Scholar]
- 42.Schwiertz, A., U. Lehmann, G. Jacobasch, and M. Blaut. 2002. Influence of resistant starch on the SCFA production and cell counts of butyrate-producing Eubacteriun spp. in the human intestine. J. Appl. Bacteriol. 93:157-162. [DOI] [PubMed] [Google Scholar]
- 43.Topping, D. L., and P. M. Clifton. 2001. Short chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81:1031-1064. [DOI] [PubMed] [Google Scholar]
- 44.Walker, A. W., S. H. Duncan, E. C. McWilliam Leitch, M. W. Child, and H. J. Flint. 2005. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71:3692-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., and G. R. Gibson. 1993. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 75:373-380. [DOI] [PubMed] [Google Scholar]
- 46.Wolin, M. J., T. L. Miller, S. Yerry, Y. Zhang, S. Bank, and G. A. Weaver. 1991. Changes of fermentation pathways of fecal microbial communities associated with a drug treatment that increases dietary starch in the human colon. Appl. Environ. Microbiol. 65:2807-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]