Recently, great technological progress has been achieved in spermatogonial stem cell (SSC) research. In this issue of PNAS, Kanatsu-Shinohara et al. (1) describe yet another important novel use for SSCs. Based on a successful long-term culture protocol for mouse SSCs, this group has designed a way to produce knockout mice from SSCs with an efficiency that is at least comparable with that of embryonic stem (ES) cell-based methods. SSCs were transfected by applying methods used for ES cells and transplanted into recipient mouse testes to produce sperm carrying the desired mutation. This procedure may enable the efficient production of transgenic animals in species from which no ES cells can be made as yet.
Before 1994, spermatogonial stem cell numbers could be assessed only by cell counts (2, 3). Then Brinster and colleagues introduced a functional assay for SSCs, the SSC transplantation technique (4, 5). This method has greatly boosted research on SSCs. However, despite efforts by many groups, it remained problematic to culture SSCs and propagate these cells in vitro, hence limiting SSC availability. The breakthrough came when Kanatsu-Shinohara et al. (6) succeeded in culturing SSCs for at least 5 months, achieving a 1014-fold increase in SSC numbers [called germ-line stem (GS) cells by the authors]. These cultured SSCs remained capable of colonizing recipient mouse testes upon transplantation, giving rise to normal spermatogenesis (6). SSCs could be cultured either without serum or without a feeder layer (7), remained genetically and epigenetically intact (8), and could be cultured also in an anchorage-independent way (9). The culture period could be extended to at least 2 years, and a 1085-fold increase in SSC numbers was achieved in this way (8). The factors leading to this breakthrough in culture possibilities probably lay in the use of a proprietary culture medium of unknown composition and a combination of added growth factors, including glial cell line-derived neurotrophic factor (GDNF) (6). Very large numbers of genetically normal and transplantable mouse SSCs now can be produced in vitro and used as a reliable starting material to make transgenic animals (Fig. 1).
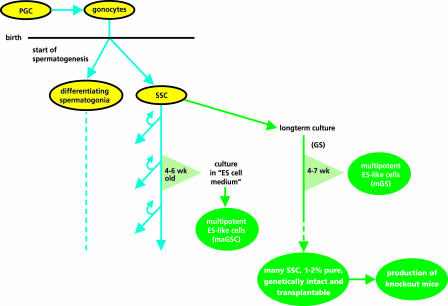
Fig. 1.
Schematic representation of the SSC tool box and the way these tools can be obtained as described by Kanatsu-Shinohara et al. (1, 6–8, 10) and Guan et al. (11). PGC, primordial germ cells; SSC, spermatogonial stem cells; GS, germ-line stem cells (6).
The starting material in the culture experiments was germ cells from newborn mice. In mice, spermatogenesis starts shortly after birth, and the only germ cells present at that time are early differentiating spermatogonia and SSCs (2, 12, 13) (Fig. 1). Therefore, the germ cells already were enriched for SSCs in comparison with the normal adult testis. Interestingly, after 4–7 weeks in culture, colonies of ES-like cells were formed, called mGS cells (10). These mGS cells were multipotential and able to form various types of somatic cells in vitro just like ES cells. The results indicated that the mGS cells were formed by the cultured GS cells themselves at a low frequency and were not some leftover, earlier type of germ cells still present at birth. The formation of ES-like cells by the GS cells may depend on the age of the mice from which the population of SSCs was isolated initially. Kanatsu-Shinohara et al. (10) did not find ES-like cell formation when testes of 4- to 8-week-old WT mice were used to isolate SSCs. This result could point to a differentiation step of SSC shortly after birth, preventing the formation of ES-like cells in culture. However, recently, Guan et al. (11), using a different culture protocol, found multipotent ES-like cell formation, called maGSCs by the authors, from cultured spermatogonia isolated from 4- to 6-week-old mice. In addition, Kanatsu-Shinohara et al. (10) found ES-like cell formation from germ cells isolated from 3- to 8-week-old p53 knockout mice instead of WT mice. Taken together, it seems possible that the transition from SSCs to ES-like cells still can be made in older mice. Further studies are needed to find out whether there is a maximum age of the donor mice, and ES-like cell formation from SSCs also should be studied in other mammals, including humans.
This amazingly fast development in the SSC field now paves the way for important scientific and technological applications for SSCs. First, the propagation of stem cells achieved in the mouse (1085-fold increase) will encourage attempts to in vitro propagate SSCs from other mammals, including humans. Positive results already have been obtained in the rat (14), and we observed a substantial improvement in the success of bovine SSC cultures by using the Kanatsu-Shinohara et al. (6) culture protocol (P. Aponte, personal communication). Extensive in vitro propagation of SSCs will be a necessary step in saving the fertility of young male human cancer patients by way of taking a biopsy before chemotherapy, propagation of SSCs in culture, cryopreservation of the cells, and transplantation back to the patients after a cure and after puberty. When human SSCs can be successfully cultured, this application certainly seems possible. Second, the large number of SSCs grown in vitro can be used to characterize SSCs in terms of genes and proteins expressed. However, one has to keep in mind that the SSCs in the cultures are only 1–2% pure, as suggested by Kanatsu-Shinohara et al. (7), because of differentiation of SSCs in vitro. Indeed, the authors describe the presence of intercellular bridges between cells and, at least in the in vivo situation, the formation of an intercellular bridge is the first visible sign of differentiation (3). Hence, further purification will be required. Using mice with an enhanced testicular expression of GDNF in which SSCs dramatically accumulate might be an alternative source from which to purify SSC (15, 16). Third, it should be sorted out whether SSCs from older mice still can transform into ES-like cells. If so, the next important question will be whether the adult human testis can be a source of ES-like cells. When positive results are obtained, these ES-like cells could be used to produce tissues needed by the donor himself without ethical and immunological problems. Finally, as described by Kanatsu-Shinohara et al. (1), it gives researchers the opportunity to use SSCs to create genetically modified animals without having to make ES cells first. The latter has proven to be a significant problem in all species except for the mouse.
Conflict of interest statement: No conflicts declared.
See companion article on page 8018.
References
- 1.Kanatsu-Shinohara M., Ikawa M., Takehashi M., Ogonuki N., Miki H., Inoue K., Kazuki Y., Lee J., Toyokuni S., Oshimura M., et al. Proc. Natl. Acad. Sci. USA. 2006;103:8018–8023. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij D. G. Int. J. Exp. Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij D. G., Russell L. D. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 4.Brinster R. L., Avarbock M. R. Proc. Natl. Acad. Sci. USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinster R. L., Zimmermann J. W. Proc. Natl. Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. Biol. Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M., Miki H., Inoue K., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Biol. Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 8.Kanatsu-Shinohara M., Ogonuki N., Iwano T., Lee J., Kazuki Y., Inoue K., Miki H., Takehashi M., Toyokuni S., Shinkai Y., et al. Development (Cambridge, U.K.) 2005;132:4155–4163. doi: 10.1242/dev.02004. [DOI] [PubMed] [Google Scholar]
- 9.Kanatsu-Shinohara M., Inoue K., Lee J., Miki H., Ogonuki N., Toyokuni S., Ogura A., Shinohara T. Biol. Reprod. 2006;74:522–529. doi: 10.1095/biolreprod.105.046441. [DOI] [PubMed] [Google Scholar]
- 10.Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., Baba S., Kato T., Kazuki Y., Toyokuni S., et al. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Guan K., Nayernia K., Maier L. S., Wagner S., Dressel R., Lee J. H., Nolte J., Wolf F., Li M., Engel W., Hasenfuss G. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., Suda T., Nabeshima Y. Development (Cambridge, U.K.) 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 13.Kluin P. M., de Rooij D. G. Int. J. Androl. 1981;4:475–493. doi: 10.1111/j.1365-2605.1981.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamra F. K., Chapman K. M., Nguyen D. M., Williams-Stephens A. A., Hammer R. E., Garbers D. L. Proc. Natl. Acad. Sci. USA. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yomogida K., Yagura Y., Tadokoro Y., Nishimune Y. Biol. Reprod. 2003;69:1303–1307. doi: 10.1095/biolreprod.103.015958. [DOI] [PubMed] [Google Scholar]
- 16.Meng X., Lindahl M., Hyvonen M. E., Parvinen M., de Rooij D. G., Hess M. W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M., et al. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]