Abstract
Experimental allergic encephalomyelitis (EAE), an autoimmune model of multiple sclerosis, is a complex disease influenced by genetic, intrinsic, and environmental factors. In this study, we questioned whether parent-of-origin effects influence EAE, using reciprocal F2 intercross progeny generated between EAE-susceptible SJL/J (S) and EAE-resistant B10.S/SgMcdJ (B) mice. EAE susceptibility and severity were found to be different in female BS × BS intercross mice as compared with females from the three other birth crosses (BS × SB, SB × SB, and SB × BS), and in fact, both traits in female mice resembled those of their male siblings. This masculinization is associated with transmission of the SJL/J Y chromosome and an increased male-to-female sex ratio. Related studies using progeny of C57BL/6J Y-chromosome substitution strains demonstrate that the Y chromosome again influences EAE in both male and female mice, and that the disease course in females resembles that of their male littermates. Importantly, these data provide experimental evidence supporting the existence of a Y-chromosome polymorphism capable of modifying autoimmune disease susceptibility in both males and females.
Keywords: autoimmunity, sex chromosomes, disease susceptibility, parent-of-origin
Multiple sclerosis (MS) is the major inflammatory demyelinating disease of the central nervous system (CNS) (1). The etiology of MS is unknown but is believed to have an immunopathologic basis arising in genetically predisposed individuals as a consequence of environmental insults (2). Evidence for the role of sociocultural, biological, environmental, physical, and parent-of-origin (POO) effects comes from epidemiological studies (3, 4). A recent study of half-siblings detected a significant maternal POO effect (2.35% for shared mother and 1.31% for shared father) in MS (5). The risk for siblings who share only a mother was similar to the risk in full siblings (2.34% vs. 3.11%, P = 0.1), suggesting that the maternal POO effect could be the major component underlying familial aggregation (6). Moreover, findings from twin and half-sibling studies indicate that the gestational and/or neonatal environments influence the risk of MS later in life and that these effects are maternally related (7).
Experimental allergic encephalomyelitis (EAE) is an autoimmune (AI) model of MS induced by immunization with encephalitogenic antigens and adjuvants. Autoreactive T cells infiltrate the CNS and subsequently recruit additional lymphocytes and mononuclear cells, resulting in inflammation and demyelination (8). Susceptibility to EAE in mice is genetically controlled, with different inbred strains exhibiting various degrees of pathology and clinical disease (9). A spectrum of genetically controlled pathologies (10) and clinical (11) disease subtypes recapitulating those observed in MS (1, 12), including benign disease (CNS lesions in the absence of overt clinical signs), are seen in segregating populations. To date, >30 disease susceptibility and modifying loci have been mapped in mice.‖ However, EAE, like MS, is also subject to intrinsic and extrinsic environmental factors (13–17). The present study was therefore undertaken to determine whether the POO effect detected in MS could be documented and investigated in EAE.
Reciprocal F1 hybrid and F2 intercross mice between EAE-susceptible SJL/J (SJL or S) and -resistant B10.S/SgMcdJ (B10.S or B) mice were studied for EAE as described in refs. 10, 11, 18, and 19. Among male and female F2 intercross mice a significant difference in disease susceptibility was observed only in females arising from matings in which the sires and grandsires possessed the SJL Y chromosome (BS × BS), indicating that the Y chromosome may indirectly influence EAE in females. We therefore studied progeny of Y-chromosome-substitution strains, and we found that the Y chromosome significantly affects the clinical disease course of both male and female mice.
Results
EAE susceptibility was assessed in 71 F1 hybrid and 1,152 F2 intercross mice elicited by immunization with mouse spinal cord homogenate plus complete Freund's adjuvant (CFA). Birth cross and sex of the F2 mice were distributed in approximately equal numbers (see Table 5, which is published as supporting information on the PNAS web site). No significant difference was detected in susceptibility to either clinical signs or EAE pathology among the male and female BS and SB F1 hybrid parents of these F2 progeny (see Table 6, which is published as supporting information on the PNAS web site).
The result of a simple logistic regression analysis of the F2 progeny revealed that birth cross was not related to clinical disease (see Table 7, which is published as supporting information on the PNAS web site). A multiple logistic regression examining the effect of birth cross, stratified by sex, and controlling for age and season (17) also did not detect an association between birth cross and clinical EAE. Statistical analyses of POO effects on EAE pathology indicated that females overall were more likely to show CNS lesions (odds ratio = 2.04; P < 0.01) when the dataset was corrected for age and season. Brain and spinal cord (SC) lesions were considered together (CNS lesions); although both showed the same significant trends separately. In addition, birth cross was significantly related to EAE pathology, with BS × SB, SB × SB, and SB × BS progeny more likely to exhibit lesions than BS × BS mice (Table 1). Birth cross was then shown to be significantly associated with CNS lesions in females but not in males. BS × SB, SB × SB, and SB × BS females were on average more than twice as likely to show CNS lesions (odds ratio = 2.58, 2.58, and 2.12, respectively) than BS × BS females.
Table 1.
Association between birth cross and susceptibility to EAE pathology
| Independent variable | Odds ratio | 95% CI* | P |
|---|---|---|---|
| Simple logistic regression model | |||
| Birth cross | |||
| BS × BS | 1.00† | ||
| BS × SB | 2.31 | 1.30, 4.11 | <0.01 |
| SB × SB | 2.41 | 1.40, 4.16 | <0.01 |
| SB × BS | 2.01 | 1.22, 3.32 | <0.01 |
| Post-hoc analysis | |||
| BS × BS < BS × SB = SB × SB = SB × BS | |||
| Multiple logistic regression model‡ | |||
| Birth Cross | |||
| BS × BS | 1.00† | ||
| BS × SB | 1.83 | 1.01, 3.31 | <0.05 |
| SB × SB | 2.10 | 1.20, 3.67 | <0.01 |
| SB × BS | 1.88 | 1.12, 3.14 | <0.05 |
| Post-hoc analysis | |||
| BS × BS < BS × SB = SB × SB = SB × BS | |||
| Multiple logistic regression model stratified by sex§ | |||
| Females | |||
| BS × BS | 1.00† | ||
| BS × SB | 2.58 | 1.02, 6.49 | <0.05 |
| SB × SB | 2.58 | 1.11, 6.02 | <0.05 |
| SB × BS | 2.12 | 1.00, 4.47 | <0.05 |
| Post-hoc analysis | |||
| BS × BS < BS × SB = SB × SB = SB × BS | |||
| Males | |||
| BS × BS | 1.00† | ||
| BS × SB | 1.34 | 0.60, 2.98 | |
| SB × SB | 1.76 | 0.84, 3.72 | |
| SB × BS | 1.64 | 0.79, 3.39 | |
| Post-hoc analysis | |||
| BS × BS = BS × SB = SB × SB = SB × BS | |||
*Confidence interval.
†Reference variable. Both SC and brain showed the same significant trends separately (data not shown).
‡Adjusted for sex, age, and season at immunization.
§Adjusted for age and season at immunization.
The finding that BS × BS females are less likely to develop EAE pathology but nearly as likely to develop clinical signs may be due to differences in central function sensitivity to demyelinating lesions. An analysis of lesions (10), and a comparison of the pathology indices (PIs) for the SC, brain, and overall CNS, support this interpretation (Table 2). The SC, brain, and CNS PIs for BS × BS females are significantly less than those of the other three birth crosses, whereas no significant differences in the PI were detected among the males from the four birth crosses. Furthermore, the PI of the female BS × BS mice is not significantly different from the PI of the males. These results suggest that EAE in BS × BS females is organizationally masculinized and therefore functionally more sensitive to the consequences of AI demyelinating insults. This masculinization may be at the level of the immune system, CNS, or both.
Table 2.
Quantification of lesion severity in F2 intercross progeny
| Sex | Birth cross | PI |
||
|---|---|---|---|---|
| SC | Brain | CNS | ||
| Females | BS × BS | 2.3 | 1.7 | 4.0 |
| BS × SB | 3.5 | 2.5 | 6.0 | |
| SB × BS | 3.5 | 2.6 | 6.0 | |
| SB × SB | 3.2 | 2.5 | 5.7 | |
| 0.009* | 0.04* | 0.002* | ||
| Post-hoc analysis | ||||
| BS × BS < BS × SB = SB × SB = SB × BS for each trait | ||||
| Males | BS × BS | 2.3 | 1.8 | 4.1 |
| BS × SB | 2.2 | 1.4 | 3.6 | |
| SB × BS | 2.3 | 1.8 | 4.1 | |
| SB × SB | 2.5 | 2.0 | 4.4 | |
| Post-hoc analysis | ||||
| BS × BS = BS × SB = SB × SB = SB × BS | ||||
| Females | BS × BS | 2.3 | 1.7 | 4.0 |
| Males | BS × BS | 2.3 | 1.8 | 4.1 |
| BS × SB | 2.2 | 1.4 | 3.6 | |
| SB × BS | 2.3 | 1.8 | 4.1 | |
| SB × SB | 2.5 | 2.0 | 4.4 | |
| Post-hoc analysis | ||||
| BS × BS = BS × SB = SB × SB = SB × BS | ||||
PIs for SC, brain, and total CNS were determined as described in ref. 10. *, P value for BS × BS vs. other birth crosses.
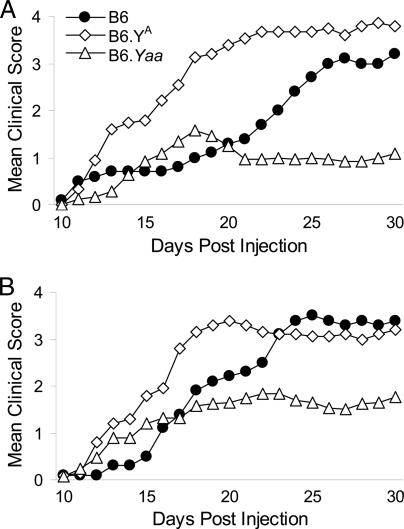
The difference in susceptibility to CNS inflammation was only observed in females arising from matings in which the sires and grand-sires possessed the SJL Y chromosome (BS × BS). In theory the only Y-chromosome dependent mechanisms that can influence EAE in females are those originating in males during the prenatal and/or postnatal periods before weaning. To test this possibility directly, progeny of B6 females × consomic male mice bearing different Y chromosomes [C57BL/6J (B6), B6.A-ChrY/NaJ (B6.YA) and B6.SB-Yaa/J (B6.Yaa) mice] were studied for susceptibility to myelin oligodendrocyte glycoprotein peptide-(35–55) (MOG35–55)-induced EAE (Fig. 1). The clinical disease course in B6 (Fig. 1A) and B6.Yaa (Fig. 1C) progeny increased as a cubic function of time (P = 0.02 and <0.01, respectively), whereas in B6.YA (Fig. 1B) progeny it was consistent with a quadratic function (P < 0.01). In all three strains, the clinical disease courses of male and female mice were not significantly different from one another and no significant sexual dimorphism in disease severity was detected.
Fig. 1.
Clinical EAE course in B6 (♂ = 10, ♀ = 10) (A), B6.YA (♂ = 15, ♀ = 26) (B), and B6.Yaa (♂ = 24, ♀ = 24) (C) progeny. Animals were injected with 100 μg of MOG35–55 emulsified in CFA containing 200 μg of Mycobacterium tuberculosis H37Ra. Immediately thereafter, each animal received 200 ng of pertussis toxin (PTX) by i.v. injection. Mice were scored daily, starting at day 10 after injection.
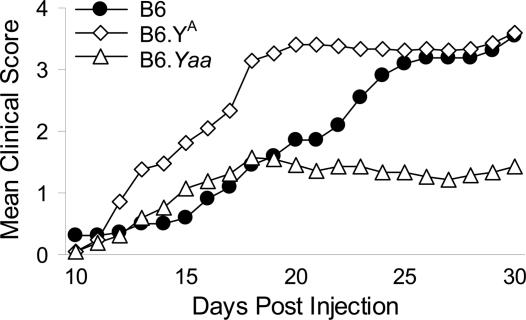
Nevertheless, there was a clear influence of the Y chromosome on EAE in mice of both sexes. In male offspring (Fig. 2A), there were significant differences among the consomic lines (group differences, P < 0.01), and across time (P < 0.01). The interaction term was also significant (P < 0.01), indicating that the clinical disease courses in the progeny of the three strains differed significantly. In female offspring (Fig. 2B), the main effect of group was also statistically significant (P < 0.01), as were the clinical scores across time (P < 0.01), and the interaction term (P < 0.01). Last, because a significant sexual dimorphism was not detected for either disease course or severity of clinical signs in the offspring of the three strains, the data were pooled and reanalyzed (Fig. 3). A significant time effect (P < 0.01), group effect (P ≤ 0.01), and interaction term (P ≤ 0.01) were obtained, indicating that the clinical disease courses differed significantly among the three Y-chromosome-substitution strains.
Fig. 2.
Clinical EAE course in ♂ (A) and ♀ (B) B6, B6.YA, and B6.Yaa progeny elicited by using the single MOG35–55 plus CFA plus PTX induction protocol.
Fig. 3.
Clinical EAE course in male and female B6, B6.YA, and B6.Yaa progeny elicited by using the single MOG35–55 plus CFA plus PTX induction protocol. As summarized in Table 3, no significant difference in the incidence of clinical disease was seen among the progeny of consomic lines; however, significant differences in the mean cumulative disease scores, mean peak scores, mean days affected, and mean severity indices were observed.
Given the dramatic nature of the differences observed, we repeated the study with a double-inoculation protocol (18, 19). Again, significant differences between male and female progeny within each of the Y-chromosome-substitution strains were not detected (see Fig. 7, which is published as supporting information on the PNAS web site), whereas among both male and female offspring (see Fig. 8, which is published as supporting information on the PNAS web site) the main effect of group was significant (♂ and ♀; P < 0.01), as were the differences in clinical scores across time (♂ and ♀; P < 0.01), and the interaction terms (♂ and ♀; P < 0.01). An analysis of the combined male and female data (Fig. 4) revealed a significant time effect (P < 0.01), group effect (P < 0.01), and interaction term (P < 0.01), indicating that the clinical disease courses different significantly as a function of the Y chromosome.
Fig. 4.
Clinical EAE course in male and female B6, B6.YA, and B6.Yaa progeny elicited by using the double MOG35–55 plus CFA induction protocol. As summarized in Table 4, significant difference in the incidence, mean cumulative disease scores, mean peak scores, mean days affected, and mean severity indices were observed among the three lines.
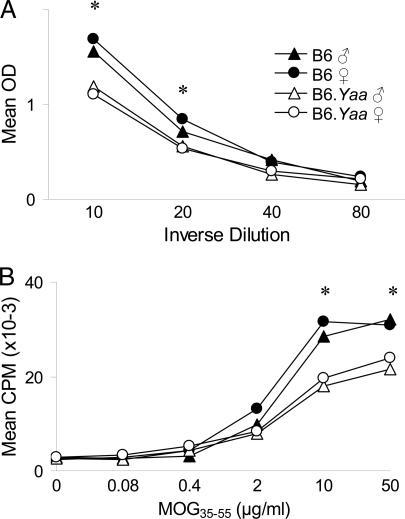
Because the Yaa chromosome had the most profound effect on EAE, we compared the MOG35–55-specific immune responses of B6 and B6.Yaa mice. Serum levels of anti-MOG35–55 IgG were lower in both male and female B6.Yaa mice compared with B6 males and females (Fig. 5A). Similarly, the in vitro proliferative responses (Fig. 5B) and secretion of IFN-γ and IL-4 (Fig. 6) by T cells from male and female B6 mice were significantly greater than those of B6.Yaa male and female T cells.
Fig. 5.
MOG35–55-specific immune responses in B6 and B6.Yaa mice. (A) IgG antibody responses in B6 and B6.Yaa ♂ and ♀ mice. The statistical significance of the differences observed among strains at each of the dilutions was determined by ANOVA (∗, P < 0.05). For the 1:10 and 1:20 dilutions B6 ♂ = B6 ♀ > B6.Yaa ♂ = B6.Yaa ♀. (B) T cell proliferative responses of B6 and B6.Yaa mice (n = 5 males and 5 females for each strain). The statistical significance of the differences observed among strains at each of the antigen concentrations was determined by ANOVA (∗, P < 0.05). For the 10 and 50 μg/ml concentrations B6 ♂ = B6 ♀ > B6.Yaa ♂ = B6.Yaa ♀. No significant differences in the Con A proliferative responses were detected (mean cpm = 70,440 ± 3,111).
Fig. 6.
IFN-γ (A) and IL-4 (B) production by draining lymph node cells from B6 and B6.Yaa mice (n = 5 males and 5 females for each strain) at day 10 after immunization. The statistical significance of the differences observed among strains was determined by ANOVA (∗, P < 0.05). For both IFN-γ and IL-4 B6 ♂ = B6 ♀ > B6.Yaa ♂ = B6.Yaa ♀.
Discussion
In this study, BS × BS F2 females behaved more like their male siblings than like the female mice from the other three birth crosses in both lesion severity and clinical sensitivity to CNS damage. The origin of the POO effect eliciting this organizational masculinization is unknown, but possibilities include both genetic and environmental (neonatal and/or prenatal). However, the mode of inheritance is inconsistent with classical X, Y, or mitochondrial inheritance and genomic imprinting because the observed effect requires a permanent mark in the BS F1 hybrid females that selectively interacts with BS males and not SB males for transmission of the phenotype to the F2 progeny. These interpretations are further supported by the lack of a significant difference in susceptibility to either clinical signs or EAE pathology among male and female bidirectional F1 hybrid mice. This finding suggests that pre- and/or perinatal environmental effects underlie the organizational masculinization of EAE in female BS × BS mice.
Litter composition can significantly influence female behavior during adulthood (20), and differences in maternal behavior generate long-term changes in offspring behavior and physiology (21). Neonatal manipulation of rodents can alter susceptibility to EAE (13), and exposure to testosterone suppresses diabetes and lupus in adult mice (22, 23). In litter-bearing mammals, fetuses developing in utero are subjected to an intrauterine positional (IUP) effect due to differing hormonal environments based on the sex of neighboring fetuses (24). Female fetuses developing between males (2M females) show masculinized traits as adults, whereas female fetuses developing without adjacent males (0M females) are more feminized. As adults, 2M females give birth to more male pups, 0M females give birth to fewer male pups and 1M females produce the expected 1.00 sex ratio (25). Because 0M females give birth to greater numbers of females, these females are more likely to be surrounded by females in utero. This IUP effect serves as a form of nongenetic inheritance from mother to daughter. Therefore, comparing the sex ratios of the four birth crosses in this study will allow us to detect an SJL Y-chromosome-dependent IUP effect.
The sex ratio for all BS × BS F2 progeny generated in the laboratory for which we have sex data at birth is 1.13 (n = 923, P = 0.045), whereas that of the other three birth crosses is ≈1.0 [BS × SB, 1.02 (n = 479); SB × BS, 1.02 (n = 479), and SB × SB, 1.01 (n = 521)]. Notably, the sex ratios of the BS × BS progeny and the BS × SB, SB × BS, and SB × SB birth crosses (average = 1.02) approximate the relative difference in sex ratios between SJL and C57BL/10 mice (1.05 and 0.95, respectively) (26). These data therefore support the existence of an SJL Y-chromosome-associated IUP effect within the BS × BS birth cross and are consistent with a previous study in which the incidence of EAE pathology between B × BS and BS × B males was not significantly different, whereas female B × BS progeny were significantly more susceptible to EAE pathology than were female BS × B mice (61.6% and 36.5%, respectively; P = 1.2 × 10−4) (27).
Significant differences in clinical EAE elicited with MOG35–55 were seen among male and female progeny of B6 female × B6, B6.YA, and B6.Yaa male consomic mice. In all three strains the disease course in female mice reflected that of the males with which they were gestated and maintained until weaning. Importantly, this organizational masculinization persists beyond puberty, indicating that it is resistant to the effects of adult sex hormones. Interestingly, the double-injection protocol (which did not use PTX) resulted in significant differences in disease incidences among the three strains (Table 4), whereas no differences were detected with the single-injection protocol (>90% affected in all three strains, Table 3). This finding suggests a potential interaction between the Y chromosome and PTX; however, with both protocols significant differences were seen for all of the clinical quantitative traits assessed.
Table 4.
Comparison of clinical EAE courses in male and female progeny of B6 females × B6, B6.YA, and B6.Yaa male consomic mice elicited by using the double MOG35–55 plus CFA induction protocol
| Mice | Incidence |
Clinical disease traits |
|||||
|---|---|---|---|---|---|---|---|
| % | n | CDS | PS | DA | SI | ||
| B6 | 100 | 20 | 38.6 | 3.3 | 13.8 | 2.6 | |
| B6.YA | 82 | 16 | 24.5 | 3.1 | 13.8 | 2.2 | |
| B6.Yaa | 53 | 15 | 9.4 | 2.3 | 10.1 | 1.7 | |
| χ2 11.9 | F | 21.1 | 5.9 | 12.1 | 8.6 | ||
| P 0.003 | P | <10−3 | 0.006 | <10−3 | 0.001 | ||
| CDS: B6 > B6.YA > B6.Yaa | |||||||
| PS: B6 = B6.YA > B6.Yaa | |||||||
| DA: B6 = B6.YA > B6.Yaa | |||||||
| SI: B6 > B6.YA = B6.Yaa | |||||||
CDS, mean cumulative disease score; PS, mean peak score; DA, mean days affected; SI, mean severity index.
Table 3.
Comparison of clinical EAE courses in male and female progeny of B6 female × B6, B6.YA, and B6.Yaa male consomic mice elicited by using the single MOG35–55 plus CFA plus PTX induction protocol
| Mice | Incidence |
Clinical disease traits |
|||||
|---|---|---|---|---|---|---|---|
| % | n | CDS | PS | DA | SI | ||
| B6 | 90 | 20 | 38.4 | 3.7 | 12.2 | 2.7 | |
| B6.YA | 95 | 41 | 53.8 | 4.1 | 16.9 | 3.2 | |
| B6.Yaa | 90 | 48 | 23.5 | 2.4 | 11.7 | 1.7 | |
| χ2 0.99 | F | 21.9 | 23.3 | 11.2 | 21.7 | ||
| P >0.61 | P | <10−3 | <10−3 | <10−3 | <10−3 | ||
| CDS: B6.YA > B6 > B6.Yaa | |||||||
| PS: B6.YA = B6 > B6.Yaa | |||||||
| DA: B6.YA > B6 = B6.Yaa | |||||||
| SI: B6.YA = B6 > B6.Yaa | |||||||
CDS, mean cumulative disease score; PS, mean peak score; DA, mean days affected; SI, mean severity index.
Taken together, these results establish that the Y chromosome influences EAE in both male and female mice and provide evidence for the existence of at least one polymorphic locus underlying the effects. The location of the gene(s), i.e., nonpseudoautosomal region (NPAR) versus pseudoautosomal region (PAR), is unknown. There are several NPAR candidate genes of immunologic significance (Hya: histocompatibility Y, Yaa-accelerated autoimmunity, and lymphoproliferation; and Sry: sex-determining region of chromosome Y); in contrast, steroid sulfatase (Sts) is the only full-length functional gene within the PAR (28).
Hya elicits rejection of male tissue by otherwise genetically identical females (29). However, H2s mice such as SJL and B10.S are both HY-nonresponders (30). Consequently, it is unlikely that H2s-restricted anti-HY responses mediate the POO effect observed among the different SJL and B10.S birth crosses.
Yaa selectively leads to monocytosis, B cell hyperactivity, and the acceleration of spontaneous lupus and lymphoproliferative disease in male mice (31). Conversely, in this study and in collagen-induced arthritis (32), Yaa suppressed disease and autoantigen-specific humoral and T cell responses. Yaa has also been implicated in indirectly influencing non-AI phenotypes in female offspring of Yaa-chromosome-bearing males, which exhibit behavioral and neuroanatomic differences not observed when non-Yaa-bearing males are used as sires (33).
Sry is a particularly intriguing candidate for the organizational masculinization of female mice as a consequence of IUP. The SJL Y chromosome is Mus domesticus in origin, whereas B6, A/J, and Yaa are Mus musculus (ref. 34 and http://jaxmice.jax.org/strain/000269.html). Transfer of some M. domesticus Y chromosomes to B6 leads to various degrees of sex reversal, ranging from normal testis development to permanent sex reversal (35) due to Sry enhancer polymorphisms (36). Variation in the timing and/or intensity of the prenatal testosterone surge as a consequence of differences in Sry could lead to variable IUP effects.
Sts encodes an important enzyme in steroid hormone metabolism that catalyzes the hydrolysis of alkyl steroid sulfates (e.g., dehydroepiandrosterone sulfate) and aryl steroid sulfates (e.g., estrone sulfate) to their unconjugated forms (37). The various biologically active steroids derived from this desulfation are known to play an important role in modulating immune responses and AI disease in several animal models, including EAE (38). Importantly, Sts activity levels in mice are known to be genetically controlled (39).
In summary, we present evidence indicating that the Y chromosome possesses at least one polymorphic gene that influences EAE in both male and female mice. Because females do not inherit the NPAR of the Y chromosome from their fathers, in theory EAE should not be influenced by these genes. However, we present evidence indicating that the NPAR of the SJL Y chromosome, like that of the Yaa chromosome, may influence EAE susceptibility in female mice through an IUP effect or postnatally as a consequence of being maintained in the presence of the Y chromosome through weaning. Importantly, our results corroborate the findings from twin and half-sibling studies in MS indicating that the gestational or neonatal environment, or both, influences the risk of MS later in life and that these effects are maternally related (7). The results of studies using Y-chromosome-substitution strains provided direct evidence that the Y chromosome influences the clinical course of EAE in male and female mice equally. Importantly, our results support the recent finding that the sex chromosome complement affects AI response to neuroantigens (40) and extends these findings by providing experimental evidence demonstrating the existence of a Y-chromosome polymorphism that influences AI inflammatory disease of the CNS in both males and females.
Materials and Methods
Animals.
B10.S/SgMcdJ (B10.S or B), SJL/J (SJL or S), C57BL/6J (B6), C57BL/6J-Chr YA/NaJ (B6.YA), and B6.SB-Yaa/J (B6.Yaa) mice were purchased from The Jackson Laboratory. BS and SB F1 hybrid progeny were generated and used to produce the 1,152 BS × BS, BS × SB, SB × BS, and SB × SB F2 intercross mice used in this study (19). B6.YA and B6.Yaa mice are Y-chromosome-substitution strains in which the B6 Y chromosome has been replaced with a Y chromosome from A/J and BXSB/MpJ mice, respectively. Progeny of B6 females × B6, B6.YA, and B6.Yaa male consomic mice were generated in the vivarium at the University of Vermont.
Induction and Evaluation of EAE.
Mice were immunized for the induction of EAE by using either an encephalitogen plus CFA double-inoculation protocol or an encephalitogen plus CFA plus PTX single-inoculation protocol. The encephalitogens used in this study were SJL SC homogenate and MOG35–55. The specific details describing the formulation and preparation of the emulsions as well as the injections are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Mice were scored daily starting at day 10 after injection as previously described (10, 11, 18, 19); F2 animals that exhibited any clinical signs greater than or equal to a flaccid tail and/or hind-leg weakness were considered affected.
Brains and SC were dissected from calvarias and vertebral columns, respectively, and fixed by immersion in phosphate-buffered (pH 7.2) 10% formalin. Representative areas of the brain and SC, including brainstem, cerebrum, cerebellum, and the cervical, thoracic, and lumbar segments of the SC, were selected for histopathological evaluation (see Figs. 9 and 10, which are published as supporting information on the PNAS web site) as previously described (10, 17). Animals exhibiting any CNS lesion were scored as affected in this study.
Proliferation Assay and ELISA for IFN-γ, IL-4, and MOG35–55-Specific Antibody.
Mice were immunized with 100 μg of MOG35–55 and 200 μg of M. tuberculosis H37Ra, and draining lymph node cells prepared from these mice at day 10 after immunization. MOG35–55- specific T cell proliferative responses and quantification of IFN-γ and IL-4 secretion were done as described in ref. 41. Sera were isolated from individual animals and frozen at −80°C until assayed. Anti-MOG35–55 IgG antibody levels were determined by ELISA using anti-mouse IgG conjugated to streptavidin-horseradish peroxidase. The specific details of each assay are published as Supporting Materials and Methods on the PNAS web site.
Statistical Analyses.
A logistic regression analysis was used to examine the relationship between susceptibility to EAE or evidence of CNS inflammation, and cross and sex. This analysis was followed by multiple logistic regression analyses to examine the effects of these variables on disease susceptibility while controlling for each of the other selected variables, including sex and season and age of the animal at the time of injection. The final multiple logistic regression analyses included adjustment for these two variables, with age (in weeks) being considered as a continuous variable. Analyses were performed by using sas, Version 8.1 (SAS Institute, Cary, NC).
A repeated-measures analysis of variance was performed to examine the group changes in the mean clinical score across time, as well as time by group interactions. The time by group interaction term allows an examination of whether the changes in the mean clinical score across time differ among the various groups. Techniques relying on orthogonal polynomials were used to examine whether the mean clinical scores changed in a linear fashion with time, or whether these changes could be better described by some other polynomial function of time [i.e., increased as a multiple of time squared (quadratic function of time) or cubed (cubed function of time)]. Because the goal of this type of analysis is to fit the data with the polynomial of the smallest degree, polynomial functions higher than the cubic function were not considered. Analyses were performed by using bmdp statistical software (BMDP Statistical Software, Los Angeles; 1990).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS36526 (to C.T. and E.P.B), AI4515 (to C.T.), AI41747 (to C.T.), and AI45666 (to C.T.) and National Multiple Sclerosis Society Grant RG-3129 (to E.P.B. and C.T.)
Abbreviations
- AI
autoimmune
- CFA
complete Freund's adjuvant
- EAE
experimental allergic encephalomyelitis
- IUP
intrauterine positional
- MOG35–55
myelin oligodendrocyte glycoprotein peptide-(35–55)
- MS
multiple sclerosis
- NPAR
nonpseudoautosomal region
- POO
parent-of-origin
- PAR
pseudoautosomal region
- PTX
pertussis toxin
- SC
spinal cord.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Prineas J. W., McDonald W. I., Franklin R. J. M. In: Greenfield's Neuropathology. 7th Ed. Graham D. J., Lantos P. I., editors. London: Arnold; 2002. pp. 471–550. [Google Scholar]
- 2.Dyment D. A., Ebers G. C., Sadovnick A. D. Lancet Neurol. 2004;3:104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 3.Marrie R. A. Lancet Neurol. 2004;3:709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- 4.Hupperts R., Broadley S., Mander A., Clayton D., Compston D. A., Robertson N. P. Neurology. 2001;57:290–295. doi: 10.1212/wnl.57.2.290. [DOI] [PubMed] [Google Scholar]
- 5.Ebers G. C., Sadovnick A. D., Dyment D. A., Yee I. M. L., Willer C. J., Risch N. Canadian Collaborative Study Group. Lancet. 2004;363:1773–1774. doi: 10.1016/S0140-6736(04)16304-6. [DOI] [PubMed] [Google Scholar]
- 6.Giordano M., Momigliano-Richiardi P. Lancet. 2004;363:1748–1749. doi: 10.1016/S0140-6736(04)16336-8. [DOI] [PubMed] [Google Scholar]
- 7.Willer C. J., Dyment D. A., Sadovnick A. D., Rothwell P. M., Murray T. J., Ebers G. C. Canadian Collaborative Study Group. Br. Med. J. 2005;330:120–123. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin R., McFarland H. F. Crit. Rev. Clin. Lab. Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 9.Andersson A., Karlsson J. Arch. Immunol. Ther. Exp. (Warsz.) 2004;52:316–325. [PubMed] [Google Scholar]
- 10.Butterfield R. J., Blankenhorn E. P., Roper R. J., Zachary J. F., Doerge R. W., Teuscher C. Am. J. Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield R. J., Blankenhorn E. P., Roper R. J., Zachary J. F., Doerge R. W., Sudweeks J. D., Rose J., Teuscher C. J. Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- 12.Lublin F. D. Neurol. Clin. 2005;23:1–15. doi: 10.1016/j.ncl.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti B., Morale M. C., Testa N., Tirolo C., Caniglia S., Amor S., Dijkstra C. D., Barden N. Brain Res. Rev. 2001;37:259–272. doi: 10.1016/s0165-0173(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 14.Teuscher C., Hickey W. F., Grafer C. M., Tung K. S. K. J. Immunol. 1998;160:2751–2756. [PubMed] [Google Scholar]
- 15.Blankenhorn E. P., Butterfield R. J., Rigby R., Cort L., Giambrone D., McDermott P., McEntee K., Solowski N., Meeker N. D., Zachary J. F., et al. J. Immunol. 2000;164:3420–3425. doi: 10.4049/jimmunol.164.6.3420. [DOI] [PubMed] [Google Scholar]
- 16.Fillmore P. D., Brace M., Troutman S. A., Blankenhorn E. P., Diehl S., Rincon M., Teuscher C. Am. J. Pathol. 2003;163:1623–1632. doi: 10.1016/S0002-9440(10)63519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teuscher C., Bunn J. Y., Fillmore P. D., Butterfield R. J., Zachary J. F., Blankenhorn E. P. Am. J. Pathol. 2004;165:1593–1602. doi: 10.1016/S0002-9440(10)63416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterfield R. J., Sudweeks J. D., Blankenhorn E. P., Korngold R., Marini J. C., Todd J. A., Roper R. J., Teuscher C. J. Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 19.Fillmore P. D., Blankenhorn E. P., Zachary J. F., Teuscher C. Am. J. Pathol. 2004;164:167–175. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crews D., Fuller T., Mirasol E. G., Pfaff D. W., Ogawa S. Exp. Biol. Med. 2004;229:935–939. doi: 10.1177/153537020422900910. [DOI] [PubMed] [Google Scholar]
- 21.Meaney M. J., Szyf M. Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. J. Exp. Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins T., Gala R. R., Dunbar J. C. Proc. Soc. Exp. Biol. Med.; 1993. pp. 201–205. [DOI] [PubMed] [Google Scholar]
- 24.Ryan B. C., Vandenbergh J. G. Neurosci. Biobehav. Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 25.Vandenbergh J. G., Huggett C. L. Proc. Natl. Acad. Sci. USA. 1994;91:11055–11059. doi: 10.1073/pnas.91.23.11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlager G., Roderick T. H. J. Hered. 1968;59:363–365. doi: 10.1093/oxfordjournals.jhered.a107747. [DOI] [PubMed] [Google Scholar]
- 27.Encinas J. A., Lees M. B., Sobel R. A., Symonowicz C., Weiner H. L., Seidman C. E., Seidman J. G., Kuchroo V. K. Int. Immunol. 2001;13:257–264. doi: 10.1093/intimm/13.3.257. [DOI] [PubMed] [Google Scholar]
- 28.Park S. H., Shin Y. K., Suh Y. H., Park W. S., Ban Y. L., Choi H. S., Park H. J., Jung K. C. Gene. 2005;353:177–188. doi: 10.1016/j.gene.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Simpson E., Scott D., Chandler P. Annu. Rev. Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Voskuhl R. R., Pitchekian-Halabi H., MacKenzie-Graham A., McFarland H. F., Raine C. S. Ann. Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 31.Izui S., Ibnou-Zekri N., Fossati-Jimack L., Iwamoto M. Int. Rev. Immunol. 2000;19:447–472. doi: 10.3109/08830180009055507. [DOI] [PubMed] [Google Scholar]
- 32.Jansson L., Holmdahl R. Eur. J. Immunol. 1994;24:1213–1217. doi: 10.1002/eji.1830240531. [DOI] [PubMed] [Google Scholar]
- 33.Hoplight B. J., Mobraaten L., Sherman G. F., Hyde L. A., Harding S., Denenberg V. H. Neuroscience. 2000;96:837–842. doi: 10.1016/s0306-4522(99)00600-4. [DOI] [PubMed] [Google Scholar]
- 34.Wardell B. B., Sudweeks J. D., Meeker N. D., Estes S. S., Woodward S. R., Teuscher C. Mamm. Genome. 1993;4:109–112. doi: 10.1007/BF00290435. [DOI] [PubMed] [Google Scholar]
- 35.McElreavey K., Barbaux S., Ion A., Fellous M. Heredity. 1995;75:599–611. doi: 10.1038/hdy.1995.179. [DOI] [PubMed] [Google Scholar]
- 36.Nagamine C. M., Morohashi K., Carlisle C., Chang D. K. Dev. Biol. 1999;216:182–194. doi: 10.1006/dbio.1999.9436. [DOI] [PubMed] [Google Scholar]
- 37.Reed M. J., Purohit A., Woo L. W., Newman S. P., Potter B. V. Endocr. Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 38.Dillon J. S. Curr. Drug Targets Inflamm. Allergy. 2005;4:377–385. doi: 10.2174/1568010054022079. [DOI] [PubMed] [Google Scholar]
- 39.Le Roy I., Mortaud S., Tordjman S., Donsez-Darcel E., Carlier M., Degrelle H., Roubertoux P. L. Behav. Genet. 1999;29:131–136. doi: 10.1023/a:1021664607131. [DOI] [PubMed] [Google Scholar]
- 40.Palaszynski K. M., Smith D. L., Kamrava S., Burgoyne P. S., Arnold A. P., Voskuhl R. R. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 41.Teuscher C., Poynter M. E., Offner H., Zamora A., Watanabe T., Fillmore P. D., Zachary J. F., Blankenhorn E. P. Am. J. Pathol. 2004;164:883–892. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.