Abstract
The callipyge mutation (CLPG) is an A to G transition that affects a muscle-specific long-range control element located in the middle of the 90-kb DLK1-GTL2 intergenic (IG) region. It causes ectopic expression of a 327-kb cluster of imprinted genes in skeletal muscle, resulting in the callipyge muscular hypertrophy and its non-Mendelian inheritance pattern known as polar overdominance. We herein demonstrate that the CLPG mutation alters the muscular epigenotype of the DLK1-GTL2 IG region in cis, including hypomethylation, acquisition of novel DNase-I hypersentivite sites, and, most strikingly, strongly enhanced bidirectional, long-range IG transcription. The callipyge phenotype thus emerges as a unique model to study the functional significance of IG transcription, which recently has proven to be a widespread, yet elusive, feature of the mammalian genome.
Keywords: DNA methylation, DNase-I hypersensitivity, intergenic region, noncoding RNA
The callipyge phenotype is an inherited muscular hypertrophy of sheep. It is characterized by polar overdominance, an unusual mode of inheritance in which only heterozygotes having received the CLPG mutation from their sire express the phenotype (1). The CLPG mutation is an A-to-G transition in a conserved dodecamer motif located in the 90-kb intergenic (IG) region separating the imprinted DLK1 and GTL2 genes on sheep chromosome 18 (refs. 2 and 3; Fig. 1). This motif was assumed to be part of a muscle-specific locus control region (LCR), because the CLPG mutation causes ectopic expression of a core cluster of neighboring genes in postnatal skeletal muscle, a tissue in which these genes are normally silenced (6, 7). Genes whose expression is affected by the CLPG mutation include (i) the paternally expressed protein encoding DLK1 and PEG11 genes, located, respectively, 64 kb proximally and 88 kb distally from the CLPG mutation, and (ii) the maternally expressed noncoding RNA genes GTL2, antiPEG11, MEG8, and MIRG, located between 33 and 262 kb distally from the CLPG mutation, as well as their multiple C/D small nucleolar RNA and microRNA (miRNA) guests (8, 9). With the exception of PEG11, all these genes are transcribed toward the telomere. The effect of the CLPG mutation is cis-restricted and subordinate to imprinting control because it does not perturb the monoallelic expression of the target genes (6).
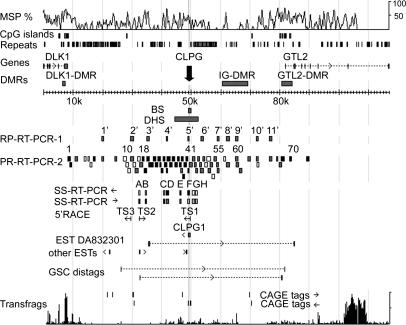
Fig. 1.
Schematic representation of the ovine DLK1-GTL2 IG region. MSP %, multispecies similarity profile; DMRs, differentially methylated regions; BS, bisulfite-sequenced segment (Fig. 2); DHS, segment explored for DNase-I hypersensitive sites (Fig. 3); RP-RT-PCR and SS-RT-PCR, location of amplicons used respectively in random primed (Fig. 4 B and C) or strand-specific RT-PCR experiments (Fig. 4 A and D); ←, targeting D←G transcripts; →, targeting D→G transcripts. For RP-RT-PCR-2 and SS-RT-PCR, which were performed in a +/CLPGPat fetus, amplicons that gave strong RT-PCR products are labeled in black, those yielding weak RT-PCR products are in gray, and those that did not yield any RT-PCR product are in white. 5′ RACE, transcription start sites (TS) identified by 5′ RACE. GSC ditags and CAGE tags, “gene signature cloning” ditags and “cap analysis gene expression” tags identified in ref. 4. Transfrags, a local transfrag profile obtained by microarray analysis (5).
It was recently shown that the callipyge phenotype can be caused by ectopic expression of DLK1 protein in skeletal muscle as observed in +/CPat individuals (10). The lack of phenotypic expression in C/C animals is postulated to be due to translational inhibition of padumnal DLK1 transcripts by noncoding madumnal transcripts (11). A direct role for miRNAs in this trans effect is suggested by the demonstration of RNA interference-mediated degradation of padumnal PEG11 transcripts by miRNAs processed from madumnal antiPEG11 transcripts (12).
How the CLPG mutation operates such profound, tissue-specific influence on the expression of genes, which can be as far as 262 kb away, remains unknown. Intriguingly, Freking et al. (2) detected an RNA species of unknown function (CLPG1) encompassing the mutation and transcribed toward DLK1. Using 5′ RACE, they identified a putative transcription start site at 478 bp from the CLPG site.
To gain additional insight into the mechanisms underlying the cis effect of the CLPG mutation, we studied its effect on three epigenetic features that are known to be correlated with the activation state of other LCRs: DNA methylation, DNase-I hypersensitivity, and IG transcription.
Results
The CLPG Mutation Imposes a Distinct Hypomethylation Mark in Cis.
To test whether the CLPG mutation might affect the methylation status of surrounding DNA, we performed bisulfite sequence analysis of a 777-bp segment encompassing the mutation and the putative CLPG1 transcription start site (TS1 in Fig. 1). As is the case for most of the DLK1-GTL2 IG region (8), this DNA fragment has a high G+C content (60.7%) but a lower than expected number of CpG dinucleotides (28 observed vs. 77 expected). It is characterized by five highly conserved elements, one of which spans the CLPG mutation. We performed the analysis on skeletal muscle DNA of 8-week-old animals, because the CLPG mutation is assumed to act in this tissue and the phenotype is expressed at that age. We studied two animals of each of the four CLPG genotypes. We studied the DNA strand that allows distinction of the CLPG and + allele. The PCR products were cloned, and the sequence of at least 38 independent clones were determined for each animal (65 on average) (Fig. 2A; see also Fig. 5, which is published as supporting information on the PNAS web site). The conversion rate for non-CpG C residues averaged 99.8%, demonstrating the efficacy of the bisulfite treatment.
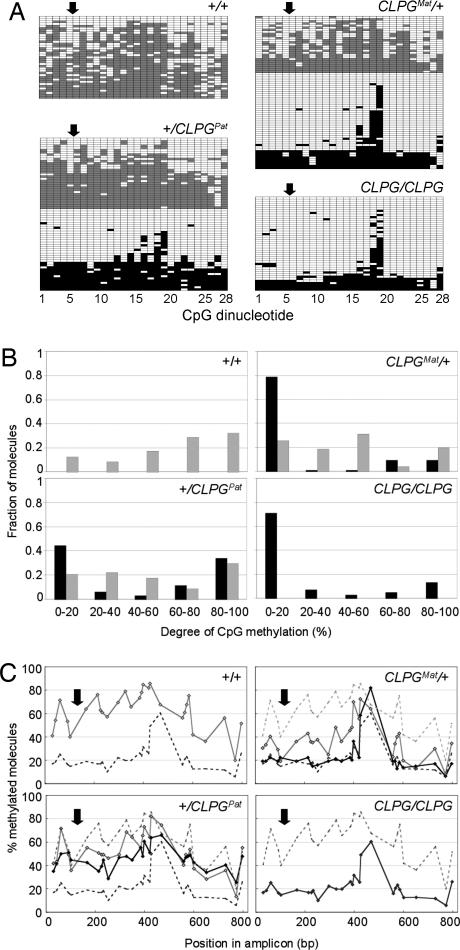
Fig. 2.
DNA methylation analysis. (A) Representative bisulfite sequencing results for a 828-bp amplicon spanning the CLPG mutation for animals representing the four CLPG genotypes. Each line corresponds to a distinct molecule, each column to one of the 28 CpG dinucleotides in the amplicon. The corresponding coordinate is shaded in gray (+ allele) or black (CLPG allele) when methylated and white when unmethylated. The approximate position of the CLPG mutations is marked by the arrow. (B) Frequency distribution of the proportion of methylated CpG sites per molecule. The distribution for + alleles is shown as gray bars; CLPG alleles as black bars. (C) Percentage methylation for each of the 28 CpG sites across molecules. Results obtained for the + chromosomes are shown by the gray diamonds and CLPG chromosomes by the black diamonds. The curves obtained for the homozygous +/+ and C/C individuals are watermarked on all graphs. The position of the CLPG mutations is marked by the arrow.
We first examined the effect of CLPG genotype on the proportion of methylated CpG sites per molecule (Fig. 2B). The major conclusions from this analysis are as follows:
CLPG chromosomes clearly distinguish themselves from + chromosomes by virtue of a population of molecules with <20% CpG methylation.
This hypomethylated population accounts for >70% of CLPG molecules in C/C and CMat/+ animals but only for 45% in +/CPat animals.
+ molecules exhibit a broad, uniform distribution of methylation, irrespective of CLPG genotype.
We also examined the effect of CLPG genotype on the percentage methylation of individual CpG sites across molecules (Fig. 2C), leading to the following conclusions:
In +/+ animals, the methylation rate exhibits a wave-like pattern with amplitude of 20–30% methylation and wavelength of 100–150 bp. The wave oscillates around a mean that maximizes (80%) at position +314 (counting from the CLPG mutation).
In C/C animals, the methylation rate is flat throughout the molecule averaging at ≈20%, except for two adjacent highly methylated CpG sites (sites 18 and 19).
In heterozygotes (CMat/+ and +/CPat), the maternal chromosome in essence is identical to its counterpart in the corresponding homozygotes, i.e., CMat very much resembles C/C, whereas +Mat very much resembles +/+. The paternal chromosomes, on the other hand, differ from their counterparts in the homozygotes, leaning toward the status of the maternal homologue, i.e., the +Pat allele is less methylated than +/+ and CPat is more methylated than C/C.
The CLPG Allele Exhibits Specific DNase-I Hypersensitive Sites (DHSs) and Increased DNase-I Sensitivity.
One of the hallmarks of LCR is the occurrence of DHSs in their immediate vicinity (13). Assuming that the CLPG mutation perturbs a LCR, we wanted to test for the presence and effect of the CLPG mutation on DHS in its neighborhood. To that effect, we purified nuclei from skeletal muscle of 8-week-old sheep of the four CLPG genotypes. The nuclei were treated with increasing concentrations of DNase-I. DNA was extracted and subjected to Southern blot analysis, focusing on an 8.2-kb SspI restriction fragment encompassing the CLPG mutation. We detected at least three DHSs in +/+ animals located at +690 bp (DHS_+1), −525 bp (DHS_+2), and –810 bp (DHS_+3) from the CLPG SNP. Most interestingly, however, at least two additional DHSs were becoming apparent in C/C animals, located at +100 bp (DHS_C1) and –1,175 bp (DHS_C2) from the CLPG mutation. All DHSs, whether constitutive or CLPG-specific, correspond to regions of high conservation (Fig. 3A). Remarkably, the position of DHS_C1 coincides virtually exactly with the CLPG site and DHS_+1 coincides with TS1. The profiles obtained in heterozygotes were compatible with a superposition of the + and CLPG patterns detected in the respective homozygotes. None of the DHSs were detectable in liver samples (data not shown). These results thus strongly suggest that the CLPG mutation uncovers allele- and tissue-specific DHS in cis.
Fig. 3.
DNase-I hypersensitive analysis. (A) Schematic representation of the 8.2-kb SspI fragment analyzed for the presence of DHS, showing (i) the position of the CLPG mutation (black arrow and vertical line), (ii) a multispecies (human, mouse, and ovine) similarity profile (MSP), (iii) “PhastCons” conserved elements (CEs) as obtained from http://genome.ucsc.edu, (iv) the position of the SspI, BstEII restriction sites, and the TS1 transcription start site identified in ref. 2, (v) the position of the probe used for Southern blot hybridization (black horizontal bar), and (vi) the position estimates of the constitutive (DHS_+x) and CLPG-specific (DHS_Cx) DHS. (B) Detection of DHS in nuclear DNA extracted from skeletal muscle of 8-week-old animals of the four possible CLPG genotypes. Purified nuclei were treated with increasing concentrations of DNase-I (25, 50, 100 and 150 units/ml), digested with SspI, and analyzed by Southern blot by using the probe shown in A. The same Southern blots included genomic DNA digested with SspI (S), SspI and BstEII (S+B), an equimolar mixture of both (S+B/S), and a molecular weight marker (MW). BstEII digests the 8.2-kb SspI fragment at 217 bp proximally from the CLPG mutation. Bands corresponding to the three DHS present on both the + and CLPG allele (DHS_+1, DHS_+2, and DHS_+3) are marked by gray arrows; bands corresponding to the two DHS that are specific for the CLPG allele (DHS_C1 and DHS_C2) are marked by black arrows.
Transcriptionally active chromatin is known to exhibit increased, general sensitivity to DNase-I (14). Because the CLPG allele enhances transcriptional activity in cis in skeletal muscle, the CLPG allele is predicted to be more sensitive to DNase-I than the + allele in this tissue. To test this hypothesis, we used PCR-restriction fragment length polymorphism to measure the CLPG-to-+ allelic ratio in DNA extracted from skeletal muscle and liver nuclei of a CMat/+ animal, incubated for increasing lengths of time with DNase-I. The CLPG-to-+ allelic ratio was clearly reduced in DNase-I-treated skeletal muscle nuclei when compared with genomic DNA extracted by using standard procedures (Fig. 6, which is published as supporting information on the PNAS web site). Note that the effect was apparent even after very short exposure to DNase-I and only modestly enhanced with increased incubation time. It suggests that the observed effect could be due to endogenous nucleases. There was no evidence at all for a comparable effect in liver, demonstrating its tissue specificity and a likely genuine correlation with transcriptional activity.
The CLPG Mutation Enhances Bidirectional Long-Range DLK1-GTL2 IG Transcription in Cis.
To follow up on the CLPG1 findings of Freking et al. (2), we repeated strand-specific RT-PCR experiments encompassing the CLPG mutation by using skeletal muscle RNA extracted from sheep of the four possible CLPG genotypes at two development stages: 2 weeks prenatal and 8 weeks postnatal.
Confirming Freking’s findings, we detected transcripts oriented toward DLK1 [hereafter referred to as D(lk1)←G(tl2) transcripts] in +/+ fetuses, albeit at low levels. The same low level D←G transcripts also were detectable in 8-week-old +/+ animals. In addition, we obtained RT-PCR products corresponding to antisense D→G transcripts from the prenatal +/+ samples at extremely low levels (Fig. 4A).
Fig. 4.
Expression analysis of IG transcripts. The position of the CLPG mutation is marked by the black arrow. The white arrows correspond to the positions of the TS2 and TS1 transcription start sites, respectively. MW, molecular weight marker. (A) Results of strand-specific RT-PCR experiments by using a 593-bp amplicon spanning the CLPG site and gluteus medius RNA from animals of the four possible CLPG genotypes at 2 weeks before and 8 weeks after birth. The amplicon was amplified from the cognate genomic DNA extracted from skeletal muscle as positive control, gluteus medius cDNA synthesized by using either one (specific for D←G transcripts) or the other (specific for D→G transcripts) primer, and RT-treated cDNA in the absence of primers. A 428-bp β-actin amplicon was amplified to control for the quality of the RNA. The CLPG amplicons were directly sequenced; the portions of the electropherograms spanning the CLPG site are shown, revealing the preferential expression of the CLPG allele in CMat/+ and +/CPat animals. (B) Results of RT-PCR experiments for 11 amplicons spanning the DLK1-GTL2 IG region (1′–11′ in Fig. 1) by using random primed gluteus medius cDNA (RP cDNA) from animals of the four possible CLPG genotypes at 2 weeks before and 8 weeks after birth. Amplicon 5′ is marked by an arrow as it spans the CLPG site. The same amplicons were amplified from the cognate genomic DNA (gDNA) and randomly primed cDNA with or without RT. The latter were all negative and are not shown. The cDNA amplicons were directly sequenced, and SNP markers in the region were used to determine the parental origin of the transcripts when possible. Biallelically expressed amplicons are marked by both a maternal (M) and a paternal (P) of equal size. Preferential expression of one allele is reflected by the relative size of the corresponding symbols. Monoallelically expressed amplicons are marked by M or P if the allele is madumnal or padumnal, respectively. In the absence of informative polymorphisms, the amplicons are unlabeled. (C) Representative results of PCR experiments performed with 47 overlapping amplicons spanning a 32-kb IG segment (Fig. 1) by using genomic DNA (gDNA) and random primed gluteus medius cDNA (RP cDNA) from a +/CPat fetus. Controls by using cDNA synthesized without RT were all negative and are not shown. (D) Results of strand-specific RT-PCR experiments performed with 11 amplicons, labeled A–H in Fig. 1 (i.e., A and A′ and B and B′, are distinct amplicons with virtually identical position) by using genomic DNA (gDNA), gluteus medius cDNA synthesized by using either one (specific for D←G transcripts) or the other (specific for D→G transcripts) primer, RT-treated cDNA in the absence of primers, and RT minus RNA with both primers.
More remarkably, when compared with +/+ animals, we observed a strong increase in the yield of D←G RT-PCR product from CMat/+, +/CPat, and C/C pre- and postnatal samples. A similar effect also was noticed for the D→G products, albeit more modest. Sequencing the corresponding CMat/+ and +/CPat amplicons indicated that both D←G and D→G transcripts were preferentially transcribed from the CLPG allele (Fig. 4A). These results demonstrate that, in addition to its previously reported effect on the expression of distant imprinted genes, the CLPG mutation enhances bidirectional expression of DLK1-GTL2 IG transcripts in skeletal muscle, irrespective of its parental origin.
To study the extent of this previously undescribed cis effect, we designed 11 amplicons spanning the DLK1-GTL2 IG region (1′-11′ in Fig. 1). They were amplified from genomic DNA and random primed skeletal muscle cDNA from animals of the four CLPG genotypes and the same two developmental stages. All PCR products were sequenced, and SNPs for which the individuals were heterozygous used to determine the allelic origin of the corresponding transcripts. The obtained results can be summarized as follows (Fig. 4B):
In +/+ fetuses, low-level discontinuous transcription is detected throughout the DLK1-GTL2 IG region. More specifically, we obtained RT-PCR products for amplicons 3′, 4′, and 5′, which jointly span ≈15 kb from the CLPG mutation toward DLK1, as well as with the terminally located amplicons 1′, 10′, and 11′. The transcripts were preferentially of paternal origin on the DLK1 side, of maternal origin on the GTL2 side, and biallelic in the center. Single-stranded RT-PCR experiments performed on amplicon 5′ (data not shown) indicated that both the D←G and D→G transcripts are biallelically expressed in +/+ fetuses.
In 8-week-old +/+ animals, IG transcription is further reduced, restricted to the central part, and monoallelic.
At 2 weeks before birth, CMat/+, +/CPat, and C/C animals show a strong enhancement of transcript levels throughout the DLK1-GTL2 IG region. In CMat/+ animals, the IG transcripts are exclusively produced from the maternal CLPG allele. In +/CPat animals, the transcripts are virtually exclusively produced from the paternal CLPG allele, except for the two amplicons nearest GTL2 that show biallelic expression. The effect of the CLPG mutation seems most pronounced for the segment spanned by amplicons 3′-5′, which also were yielding higher amounts of PCR product in +/+ fetuses. Note that transcription proceeds throughout the IG-DMR in these animals, shown to operate as imprinting control element for the DLK1-GTL2 domain (15).
The effect of the CLPG mutation on DLK1-GTL2 IG transcription persists at 8 weeks of age, albeit attenuated. In CMat/+ and +/CPat animals, expression is monoallelic and restricted to the mutant CLPG allele. Expression is concentrated in the central part in the vicinity of the CLPG SNP and distally from the IG-DMR.
To gain additional insight regarding the organization of the detected DLK1-GTL2 IG transcripts, we performed further RT-PCR and RACE experiments by using skeletal muscle RNA from +/CPat fetuses, i.e., a genotype and developmental stage showing pronounced IG activity. These experiments led to the following observations:
Random-primed RT-PCR products could readily be obtained for an uninterrupted chain of 24 overlapping amplicons that jointly span from ≈−15.8 kb to +138 bp from the CLPG site (18–41 in Fig. 4C). This chromosome segment is bounded on the GTL2 side by TS1 and on the DLK1 side by a strong D→G start site (TS2) detected in this work by 5′ RACE at position −16,846 bp (Fig. 7, which is published as supporting information on the PNAS web site). Strand-specific RT-PCR experiments indicate that the transcription proceeds from both strands in this interval (Fig. 4D). The size of the RT-PCR products matched the genomic prediction for all amplicons in the interval, except amplicon 21, yielding a shorter fragment with splice-product compatible sequence.
IG transcripts were detected for 9 of 17 amplicons located between DLK1 and position −16,846, and for 25 of 30 amplicons located between position +478 and GTL2, albeit at markedly lower levels than for the −16.846 to +478 interval (Fig. 4C; see also Fig. 8, which is published as supporting information on the PNAS web site). Strand-specific RT-PCR experiments performed on the GTL2 side of the CLPG mutation (amplicons G and H) indicate that transcription proceeds primarily in the D→G direction in this region (Fig. 4D).
Discussion
We herein demonstrate that the mutant CLPG allele differentiates itself from the wild-type + allele by at least three epigenetic marks: DNA hypomethylation and the emergence of CLPG-specific DHS in the immediate vicinity of the mutation and enhanced bidirectional transcription throughout the DLK1-GTL2 IG region.
Bisulfite sequencing revealed that CLPG alleles obtained from C/C or CMat/+ skeletal muscle were dominated by molecules with a distinct signature, being virtually completely unmethylated with the exception of two adjacent highly methylated CpG dinucleotides (Fig. 2). This pattern contrasted strikingly with the uniformly high level of methylation of + alleles as obtained from +/+ and +/CPat samples. This hypomethylated population only accounts for ≈75% of the CLPG molecules in C/C and CMat/+ individuals. The remaining 25%, in essence, recapitulates the uniformly high methylation pattern typical of the + allele. A simple explanation of this bimodal behavior is tissue heterogeneity. The majority of molecules might originate from muscle tissue in which the mutation exerts its effect and the remainder from unaffected cell types.
It is noteworthy that the position of the two hypermethylated CpG sites in CLPG alleles coincides with the methylation peak of the + alleles. Moreover, sorting CLPG molecules by ascending methylation rate reveals a gradient emanating from these two adjacent sites. One interpretation is that this region acts as a nucleation site for methylation. Spreading of methylation from this nucleation site would be somehow hampered on the CLPG allele. In agreement with this conjecture, Murphy et al. (16) recently observed a postnatal acquisition of methylation in the vicinity of the CLPG site in skeletal muscle of +/+ but not of C/C animals.
Quite surprisingly, in heterozygous CMat/+ and +/CPat animals, the methylation status of the maternal allele, whether CMat or +Mat, recapitulates that of the corresponding allele in homozygotes. However, the paternal allele, whether +Pat or CPat, adopts an intermediate profile leaning toward the methylation status of its maternal homologue. If further confirmed, this observation might reveal the existence of a novel trans-sensing mechanism in the DLK1-GTL2 domain in addition to the previously reported trans interaction between the products of reciprocally imprinted genes (10–12).
Because of its effect on the expression level of genes located within a large chromosomal domain, we hypothesized that the CLPG mutation might perturb a LCR element (6). So far, however, evidence supporting this hypothesis has been only indirect. The presence of DHS is typically considered pathognomonic for LCRs and other distant control elements (13). The identification of multiple tissue-specific DHS in the immediate vicinity of the CLPG mutation, and more specifically the demonstration of DHS that are unique for the CLPG allele, thus directly supports our hypothesis (Fig. 3).
We provide evidence that, when compared to the + allele, the CLPG allele exhibits an increase in general sensitivity to DNase-I in skeletal muscle, which is compatible with it adopting a more open, transcriptionally permissive chromatin configuration in this tissue (Fig. 6).
The latter observation is in good agreement with the most remarkable observation of this study, namely the fact that the CLPG mutation strongly enhances biallelic, long-range transcription throughout the DLK1-GTL2 IG region (Fig. 4). IG transcription has been demonstrated for a number of LCRs, but its role has remained elusive (17–20). More recently, genomewide approaches have revealed that IG transcription is much more widespread than initially suspected (4, 5), but, in these studies as well, the functional significance of these findings was difficult to apprehend. The callipyge phenomenon might offer a unique opportunity to study the role of noncoding IG transcripts.
Murphy et al. (16) recently reported results focusing on D←G transcript in the immediate vicinity of the CLPG mutation. Their most important message, namely that the CLPG mutation cis enhances CLPG1 transcription in skeletal muscle, agrees with our findings (Fig. 4A and ref. 21). Minor differences include the fact that, in CMat/+ and +/CPat, we find preferential expression from the CLPG allele not only after (8 weeks) but also before (2 weeks) birth. This difference could be due to the fact that their fetuses were at an earlier stage of development, before down-regulation of IG transcription from the + allele. Other discrepancies are the fact that Murphy et al. (16) do not report the detection of antisense D→G transcripts, nor of D←G transcripts in adult +/+ animals. It is likely due to a difference in sensitivity between the PCR assays used.
The major difference between the two studies is the fact that we herein demonstrate that the cis effect of the CLPG mutation on IG transcription is not limited to its immediate vicinity but extends throughout the entire 90-kb DLK1-GTL2 IG region. Unraveling the precise organization of the corresponding transcripts will require additional work, but the following statements can be made. The CLPG mutation enhances D←G transcription from TS1 and D→G transcription from TS2, generating long complementary transcripts that have the potential to form double-stranded RNA molecules. It is worthwhile noting that this segment overlaps, in part, with a region of enhanced transcriptional activity detected by microarray (“transfrag”) analysis (5). CLPG chromosomes also produce IG transcripts on both sides of this central 17-kb segment. It remains uncertain, however, whether these transcripts are physically connected with those originating from the central segment or whether they are the products of independent initiations, and, in that case, from which strand they originate. The simplest model assumes that they are just extensions of the TS1 and TS2 transcripts. However, the detection of an additional D←G initiation site at position –19,683 bp (TS3) (Fig. 7), reports of multiple CAGE tags, and GSC ditags corresponding potentially to alternative transcription initiation sites throughout the region (4) hints toward a more complex transcript network.
It remains an open question whether the detected transcripts are just innocent bystanders or play an active role in mediating the effects of the CLPG mutation on its target genes. Some observations, however, are intriguing and, in our opinion, suggest an active function. The first is the fact that the IG transcription induced by the CLPG mutation seems to bridge the gaps between the mutation and at least two of its major targets: DLK1 and GTL2. The IG transcripts, thus, might physically connect the mutation and the genes that are affected by it, thus directly mediating the effect of the mutation. It is very intriguing that a spliced EST and GSC ditags are actually directly connecting IG with GTL2 sequences, as if some GTL2 transcripts actually are initiated within the IG region (Fig. 1). The second is the observation that enhanced transcription in the DLK1-GTL2 IG region is an early event when compared with phenotypic expression. We demonstrated in this work that IG transcripts are more abundant 2 weeks before birth than 8 weeks after birth. This finding contrasts with the observation that ectopic expression of DLK1 protein, and, hence, expression of the callipyge phenotype is only manifest several weeks after birth (10).
Our results allow us to propose the following model (Fig. 9, which is published as supporting information on the PNAS web site). The CLPG mutation would inactivate a silencer element that normally operates in fetal skeletal muscle to control the level of DLK1-GTL2 IG transcription. Increased IG transcription would alter the chromatin epigenotype throughout the region. This alteration, for instance, could be achieved by promoting the incorporation of variant histone molecules (22), by preventing PcG mediated silencing (23), by preventing regional spreading of DNA methylation, or by going through an RNA interference-dependent mechanism (24). This permissive chromatin status would be maintained epigenetically in skeletal muscle throughout development, promoting high-level transcription of the genes known to be influenced by the CLPG mutation. In +/CPat animals, competence to translate the DLK1 mRNAs would be acquired only in muscles of the hindquarters later in development, possibly as a result of the down-regulation of specific miRNAs or any other translational control. In C/C animals, this competence never would be acquired because of the persistent expression of anti-DLK1 miRNAs from the CMat allele.
The recent generation of transgenic mice with a deletion of the dodecamer motif, which are recapitulating the callipyge phenomenon (D. Pirottin, M.G., and C.C., unpublished data), should facilitate testing of the hypotheses that result from this work. Easy access to tissue at multiple developmental stages, combined with tiling arrays of the region, will allow a more extensive characterization of the regional epigenotype and structure of the IG transcripts. Targeted mutagenesis of the IG transcripts combined or not combined with the dodecamer deletion will directly test their functional relevance.
Methods
Bioinformatic analyses were conducted as described in ref. 25. Bisulfite sequencing was performed by using the CpGenome DNA modification kit (Chemicon International, Temecula, California). Detection of DHS and probing general DNase-I sensitivity was performed by following, respectively, Gregory et al. (26) and Gregory and Feil (27) with some modifications. Random primed and strand-specific RT-PCR experiments were performed by using cDNA synthesized, respectively, with the SuperScript-III First-Strand Synthesis System (Invitrogen) and EndoFree RT Kit (Ambion, Austin, TX), and RNA treated with the Turbo DNA-free kit (Ambion) to remove contaminating genomic DNA. 5′ RACE was performed by using the GeneRacer kit (Invitrogen). Detailed descriptions of the used procedures and primer sequences are provided in Supporting Text and Table 1, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
This project was supported by Fund for Collective Fundamental Research Grant 2.4525.96; National Foundation for Scientific Research (FNRS) Crédit aux Chercheurs Grant 1.5.134.00; grants from the Crédit à la Recherche from the Université de Liège and the “GAME” Action de Recherche Concertée from the Communauté Française de Belgique; PAI P5/25 from the Belgian Ministry for Science, Technology, and Culture Grant R.SSTC.0135; grants from the European Union “Callimir” Specific Targeted Research Project and the Utah Center of Excellence Program; U.S. Department of Agriculture/National Research Initiative Competitive Grants Program Grants 94-04358, 96-35205, and 98-03455; and a grant from the Utah Agricultural Experiment Station, Utah State University. H.T. benefits from a European Union–Marie Curie Postdoctoral Fellowship. C.C. is chercheur qualifié from the FNRS.
Abbreviations
- DHS
DNase-I hypersensitive site
- IG
intergenic
- LCR
locus control region
- miRNA
microRNA
- RT
reverse transcriptase.
Footnotes
References
- 1.Cockett N. E., Jackson S. P., Shay T. L., Farnir F., Berghmans S., Snowder G. D., Nielsen D. M., Georges M. Science. 1996;273:236–238. doi: 10.1126/science.273.5272.236. [DOI] [PubMed] [Google Scholar]
- 2.Freking B. A., Murphy S. K., Wylie A. A., Rhodes S. J., Keele J. W., Leymaster K. A., Jirtle R. L., Smith T. P. Genome Res. 2002;12:1496–1506. doi: 10.1101/gr.571002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit M., Segers K., Carrascosa L. G., Shay T., Baraldi F., Gyapay G., Snowder G., Georges M., Cockett N., Charlier C. Genetics. 2003;163:453–456. doi: 10.1093/genetics/163.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M. C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., et al. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J., Kapranov P., Drenkow J., Dike S., Brubaker S., Patel S., Long J., Stern D., Tammana H., Helt G., et al. Science. 2005;308:1149–1154. doi: 10.1126/science.1108625. [DOI] [PubMed] [Google Scholar]
- 6.Charlier C., Segers K., Karim L., Shay T., Gyapay G., Cockett N., Georges M. Nat. Genet. 2001;27:367–369. doi: 10.1038/86856. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S. K., Freking B. A., Smith T. P., Leymaster K., Nolan C. M., Wylie A. A., Evans H. K., Jirtle R. L. Mamm. Genome. 2005;16:171–183. doi: 10.1007/s00335-004-2421-1. [DOI] [PubMed] [Google Scholar]
- 8.Charlier C., Segers K., Wagenaar D., Karim L., Berghmans S., Jaillon O., Shay T., Weissenbach J., Cockett N., Gyapay G., et al. Genome Res. 2001;11:850–862. doi: 10.1101/gr.172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seitz H., Royo H., Bortolin M. L., Lin S. P., Ferguson-Smith A. C., Cavaille J. Genome Res. 2004;14:1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis E., Jensen C. H., Schroder H. D., Farnir F., Shay-Hadfield T., Kliem A., Cockett N., Georges M., Charlier C. Curr. Biol. 2004;14:1858–1862. doi: 10.1016/j.cub.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 11.Georges M., Charlier C., Cockett N. Trends Genet. 2003;19:248–252. doi: 10.1016/S0168-9525(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 12.Davis E., Caiment F., Tordoir X., Cavaille J., Ferguson-Smith A., Cockett N., Georges M., Charlier C. Curr. Biol. 2005;15:743–749. doi: 10.1016/j.cub.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Peterson K. R., Fang X., Stamatoyannopoulos G. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Cell. 1980;20:451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- 15.Lin S. P., Youngson N., Takada S., Seitz H., Reik W., Paulsen M., Cavaille J., Ferguson-Smith A. C. Nat. Genet. 2003;35:97–102. doi: 10.1038/ng1233. [DOI] [PubMed] [Google Scholar]
- 16.Murphy S. K., Nolan C. M., Huang Z., Kucera K. S., Freking B. A., Smith T. P., Leymaster K. A., Weidman J. R., Jirtle R. L. Genome Res. 2006;16:340–346. doi: 10.1101/gr.4389306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gribnau J., Diderich K., Pruzina S., Calzolari R., Fraser P. Mol. Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- 18.Masternak K., Peyraud N., Krawczyk M., Barras E., Reith W. Nat. Immunol. 2003;4:132–137. doi: 10.1038/ni883. [DOI] [PubMed] [Google Scholar]
- 19.Rogan D. F., Cousins D. J., Santangelo S., Ioannou P. A., Antoniou M., Lee T. H., Staynov D. Z. Proc. Natl. Acad. Sci. USA. 2004;101:2446–2451. doi: 10.1073/pnas.0308327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean A. Trends Genet. 2006;22:38–45. doi: 10.1016/j.tig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Georges M., Charlier C., Smit M., Davis E., Shay T., Tordoir X., Takeda H., Caiment F., Cockett N. Cold Spring Harb. Symp. Quant. Biol. 2004;69:477–483. doi: 10.1101/sqb.2004.69.477. [DOI] [PubMed] [Google Scholar]
- 22.Henikoff S., Ahmad K. Annu. Rev. Cell Dev. Biol. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt S., Prestel M., Paro R. Genes Dev. 2005;19:697–708. doi: 10.1101/gad.326205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haussecker D., Proudfoot N. J. Mol. Cell. Biol. 2005;25:9724–9733. doi: 10.1128/MCB.25.21.9724-9733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smit M. A., Tordoir X., Gyapay G., Cockett N. E., Georges M., Charlier C. Mamm. Genome. 2005;16:801–814. doi: 10.1007/s00335-004-2415-z. [DOI] [PubMed] [Google Scholar]
- 26.Gregory R. I., Khosla S., Feil R. Methods Mol. Biol. 2001;181:269–284. doi: 10.1385/1-59259-211-2:269. [DOI] [PubMed] [Google Scholar]
- 27.Gregory R. I., Feil R. Nucleic Acids Res. 1999;27:e32. doi: 10.1093/nar/27.22.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.