Abstract
The TodS and TodT proteins form a previously unrecognized and highly specific two-component regulatory system in which the TodS sensor protein contains two input domains, each of which are coupled to a histidine kinase domain. This system regulates the expression of the genes involved in the degradation of toluene, benzene, and ethylbenzene through the toluene dioxygenase pathway. In contrast to the narrow substrate range of this catabolic pathway, the TodS effector profile is broad. TodS has basal autophosphorylation activity in vitro, which is enhanced by the presence of effectors. Toluene binds to TodS with high affinity (Kd = 684 ± 13 nM) and 1:1 stoichiometry. The analysis of the truncated variants of TodS reveals that toluene binds to the N-terminal input domain (Kd = 2.3 ± 0.1 μM) but not to the C-terminal half. TodS transphosphorylates TodT, which binds to two highly similar DNA binding sites at base pairs −107 and −85 of the promoter. Integration host factor (IHF) plays a crucial role in the activation process and binds between the upstream TodT boxes and the −10 hexamer region. In an IHF-deficient background, expression from the tod promoter drops 8-fold. In vitro transcription assays confirmed the role determined in vivo for TodS, TodT, and IHF. A functional model is presented in which IHF favors the contact between the TodT activator, bound further upstream, and the α-subunit of RNA polymerase bound to the downstream promoter element. Once these contacts are established, the tod operon is efficiently transcribed.
Keywords: Pseudomonas, sensor kinase, toluene dioxygenase, transcriptional regulator
Many Pseudomonas putida strains are able to use benzene, toluene, and ethylbenzene as the sole carbon and energy source through the toluene dioxygenase (TOD) pathway (1). In this pathway, the aromatic hydrocarbons are oxidized to their corresponding substituted catechols, which are further metabolized to Krebs cycle intermediates (1, 2). The catabolic genes of the TOD pathway form the operon todXFC1C2BADEGIH, which is transcribed from a single promoter called PtodX, located upstream from the todX gene (1–3). The todST genes are found downstream and form an independent operon that is expressed constitutively (2, 3).
TodS and TodT have been proposed to form a two-component regulatory system (TCS) that regulates the tod catabolic operon in P. putida F1 (3). TodT shows all of the characteristics of a response regulator, whereas sequence-based domain predictions indicate that the 108-kDa TodS belongs to a family of sensor histidine kinases that have not been studied at the biochemical level. TodS is predicted to comprise two supradomains, each containing a PAS/PAC sensory domain and a histidine kinase domain. The supradomains are separated by the receiver domain of a response regulator. In contrast to other histidine kinases, TodS apparently lacks transmembrane regions (3). The mode of action of this previously unrecognized type of histidine kinase has yet to be established. On the basis of moderate sequence similarity with the heme-binding oxygen sensor FixL, the C-terminal supradomain of TodS was proposed to be involved in oxygen sensing (3). Choi et al. (4) isolated TodS mutants that recognized aromatic compounds that are not effectors of the wild-type protein. Mutations were scattered along the C-terminal half of TodS.
The TodT protein was shown to bind to the Tod box centered at base pair −107 in the PtodX promoter (3). This site is located far from the downstream RNA polymerase-binding site. The PtodX promoter exhibits a well defined −10 base pair region, although there is no base pair −35 consensus region, consistent with the fact that PtodX belongs to the “extended promoter” type that resembles P. putida σ70-dependent promoters (5). Because TodT binds far from the RNA polymerase-binding site, we hypothesize that DNA bending is required for transcription activation, which implies that the activation mechanism involves a direct contact between TodT and the RNA polymerase. This type of enhancer-activation system is well documented for regulators of the NtrC family working in conjunction with RNA polymerase/σ54 (6, 7).
This study was undertaken to characterize this previously unrecognized TCS. We first addressed questions related to signal sensing and transmission, such as the definition of the effector profile of TodS, the thermodynamic characteristics of the interaction of TodS with aromatic hydrocarbons, the modulatory effect of effector binding on the autophosphorylation activity of TodS, and the detection of TodS–TodT transphosphorylation activity. We then explored the interaction of TodT with PtodX and the involvement of DNA-bending proteins such as IHF in the mechanism of transcriptional activation. A functional model for transcription activation mediated by this TCS is discussed.
Results
P. putida DOT-T1E grows on toluene, ethylbenzene, and benzene. We had previously generated todT and todS mutants of this strain, which failed to grow on the above hydrocarbons as the sole carbon source (2). The chromosomal todT mutation was complemented by the todT gene supplied in trans in pJLC1. However, the todS mutant was complemented by pMIR66 (bearing todST) only. These results indicated that both TodS and TodT are needed for induction of the TOD pathway.
P. putida KT2440 does not have the tod genes on its chromosome and cannot grow on toluene. We transferred pMIR77 (PtodX::′lacZ) to this strain, and, regardless of the presence of toluene, no β-galactosidase expression took place. When KT2440 (pMIR77) was transformed with pMIR66, induction took place in response to toluene, and β-galactosidase levels reached 4,500 Miller units. However, when the plasmid bore a mutation in todS (pJLC1) or todT (pJLC2), no induction from PtodX occurred (Table 1). Therefore, although P. putida codifies for a large number of TCSs encoded in its chromosome (8, 9), our results indicate that the TodS–TodT interaction is highly specific and that the Tod regulatory proteins cannot be replaced by proteins of any other TCS.
Table 1.
Bacterial strains and plasmids used in this study
| Strains/Plasmids | Relevant characteristics | Refs. |
|---|---|---|
| E. coli DH5αF′ | F′/hsdR17, recA1, gyrA | 28 |
| E. coli BL21 (DE3) | F−, ompI, hsdSB (r−B m−B) | 28 |
| P. putida DOT-T1E | Tol+, wild type | 29 |
| P. putida DOT-T1E todST | DOT-T1E, todST::Km, Tol− | 30 |
| P. putida DOT-T1E ΔtodT | DOT-T1E, todT::Km, Tol− | This work |
| P. putida KT2440 | Tol−, CmR | 31 |
| P. putida KT2440-IHF3 | KT2440, ihfA::Km | 31 |
| pBSaphA | ApR, aphA3 cassette from pU18K | 32 |
| pET-28b+ | KmR, protein expression vector | 28 |
| pET11c-ihfABhis | ApR, ihfA and His-tagged ihfB genes | 33 |
| pMIR77 | TcR, PtodX::′lacZ | 30 |
| pJLC77 | TcR, mutant PtodX::′lacZ | This work |
| pMIR66 | GmR, containing the todST genes | 30 |
| pJLC1 | pMIR66, todS::aphA3 | This work |
| pJLC2 | pMIR66, todT::aphA3 | This work |
| pJLTodT | pET28b containing todT gene | This work |
| pTodS | pET28b containing todS gene | This work |
| pCTodS | pET28+: todS; encoding CTodS | This work |
| pNTodS | pET28+: todS; encoding NTodS | This work |
| pREIIα | ApR, ropA | 19, 20 |
| pREIIα-Δ235 | ApR, RpoA Δ235 residues | 19, 20 |
| pREIIα-289A | ApR, codifies RpoAL289A | 19, 20 |
| pREIIα-291A | ApR, codifies RpoAK291A | 19, 20 |
ApR, CmR, GmR, KmR, RifR, TcR indicate resistance to ampicillin, chloramphenicol, gentamicin, kanamycin, rifampicin, and tetracycline, respectively. Tol+ and Tol− indicate either that the strain grows or fails to grow on toluene, respectively.
Effector Profile of the TodS–TodT System.
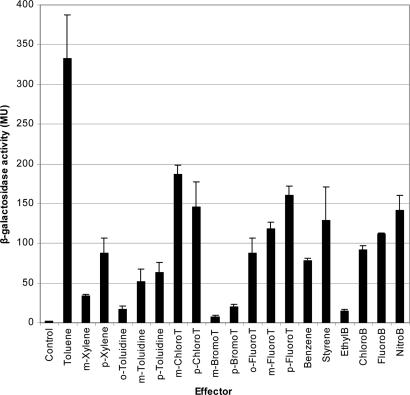
The range of effectors recognized by catabolic pathway regulators is typically larger than the range of substrates metabolized by the pathway (10–13). To identify the range of aromatic compounds that activate transcription from PtodX we measured induction by using the PtodX::lacZ fusion. Basal activity was 1.9 ± 0.7 Miller units, but the addition of benzene to the culture led to an almost 50-fold increase in activity (Fig. 1). Then we analyzed benzene derivatives with different substituents. The data indicated that a short lateral chain, such as in toluene, increased the activation of PtodX significantly, whereas longer alkyl chains resulted in a significant decrease in the inducing capability of PtodX. This finding is exemplified by ethylbenzene, which was a poor effector, whereas n-propylbenzene showed no inducer activity (Fig. 1). Chloro-, fluoro-, and nitrobenzene significantly induced expression from PtodX (Fig. 1).
Fig. 1.
Induction of PtodX by TodS–TodT in response to a wide range of aromatics. P. putida DOT-T1E bearing pMIR77 (PtodX:′lacZ) was grown on M9 medium with 1 mM of the indicated effector. When turbidity of the cultures was 0.8, β-galactosidase activity was determined. Tested compounds that did not induce were as follows: o-xylene, m- and p-ethyltoluene; o-, m-, and p-nitrotoluene; o-chloro-, o-, m-, and p-iodotoluene; propyl-, butyl-, and isobutylbenzene; 1,2,3-, 1,3,5-, 1,2,4-trimethylbenzene; and benzamide. T, toluene; B, benzene.
Because toluene was the best tested effector, we evaluated different substituents in the aromatic ring at C2, C3, and C4. A number of substituents were permissible at positions 3 and 4 in the aromatic ring of toluene, allowing for methyl, amino, fluoro, bromo, and chloro substitutions (Fig. 1). In contrast, in position 2, only fluoro and amino substituents were permitted. This result evidenced that the regulator profile was significantly broader than the substrate range accepted by the pathway.
Isothermal Titration Calorimetry.
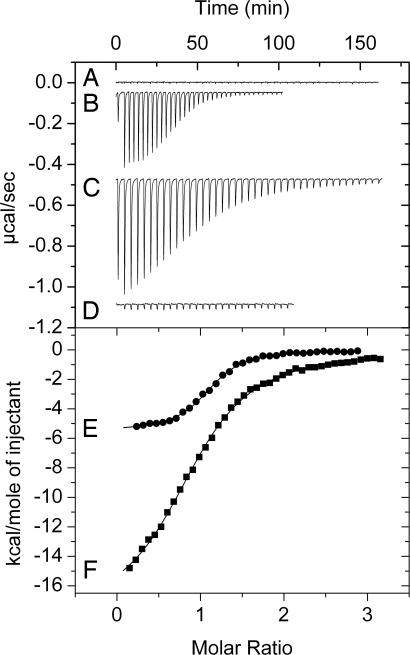
Purified His-tag TodS protein was used to determine the thermodynamic parameters for the binding of toluene and ethylbenzene. Fig. 2 shows that the heat changes obtained for the injection of toluene into buffer were negligible. Binding of toluene to TodS (Fig. 2B) was driven by favorable enthalpy (ΔH = −5.5 ± 0.1 kcal/mol) and entropy (TΔS = 2.9 ± 0.1 kcal/mol) changes. Binding was tight, with a dissociation constant of 684 ± 13 nM, and the TodS:toluene binding stoichiometry was 1:1. Ethylbenzene binds to TodS in a similar fashion (ΔH = −3.6 ± 0.1 kcal/mol; 1 kcal = 4.18 kJ) but with lower affinity (Kd = 3.1 ± 0.2 μM).
Fig. 2.
Isothermal titration calorimetry data for the binding of toluene to TodS and its recombinant fragments NTodS and CTodS. (Upper) Heat changes are shown. (Lower) Integrated peak areas are shown. (A) Titration of buffer with 1 mM toluene. (B) Titration of 15 μM TodS with 1 mM toluene. (C) Titration of 12 μM NTodS with 0.8 mM toluene. (D) Titration of 8 μM CTodS with 1 mM toluene. Integrated peak areas are shown for heat changes in B (E) and C (F).
Because TodS contains two PAS/PAC-type input domains, to identify the toluene-binding domain, two truncated variants of TodS were created. The N-terminal TodS fragment (NTodS), comprising residues 1–584, contained the N-terminal PAS/PAC-histidine kinase domains and the receiver domain of a response regulator, whereas the C-terminal TodS fragment (CTodS), comprising residues 452–978, contained the receiver domain of a response regulator followed by the PAS/PAC-histidine kinase domains located at the C terminus of TodS. We purified both TodS variants and found that toluene bound to NTodS with a very favorable enthalpy change (ΔH = −18.3 ± 0.2 kcal/mol), an affinity of 2.3 ± 0.1 μM, and a 1:1 stoichiometry (Fig. 2C), whereas purified CTodS did not interact with the effector (Fig. 2D).
Modulation of Basal Autophosphorylation Activity of TodS by Toluene and Rate of Dephosphorylation.
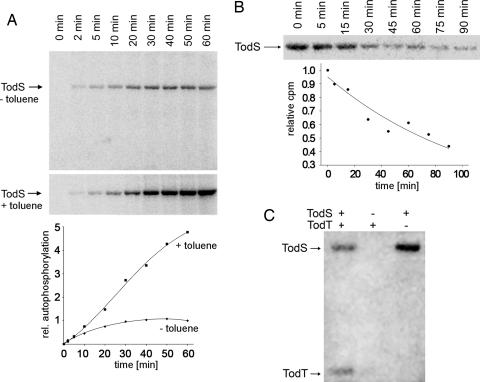
A typical property of histidine kinases is the capacity to autophosphorylate in the presence of ATP. TodS was incubated with 50 μM [γ-32P]ATP, and samples were taken at different times and analyzed (Fig. 3A Upper). Densitometric analysis revealed that phosphorylation in the absence of effectors reached a plateau at ≈30 min, indicative of an equilibrium between phosphorylation and dephosphorylation (Fig. 3A Bottom). In a parallel assay, phosphorylation was done in the presence of 100 μM toluene. After 50 min of incubation, autophosphorylation activity was ≈5 times as high as the basal level (Fig. 3A).
Fig. 3.
The catalytic properties of TodS. (A Upper) Autophosphorylation in the presence and absence of toluene. Experiments were carried out in parallel, and the graph (A Lower) shows the densitometric analysis of the gels. (B) Dephosphorylation kinetics. (C) Transphosphorylation to TodT. Conditions for these assays are described in Materials and Methods.
In a subsequent experiment, TodS was autophosphorylated in the presence of toluene, and, after 50 min, a 500-fold molar excess of unlabeled ATP was added. Samples were taken at different times and analyzed by SDS/PAGE to measure the rate of dephosphorylation. TodS dephosphorylates with a half-life of ≈70 min (Fig. 3B), which is similar to the half-life of other sensor kinases (14). As mentioned above, TodS contains two sensor domains that probably sense two different signals. In this study, we demonstrate that the addition of a single signal, i.e., toluene, suffices to activate this regulatory system.
TodS–TodT Transphosphorylation Activity.
The in vivo data suggested that TodS–TodT forms a TCS. Attempts were thus made to detect transphosphorylation in vitro. Purified TodS and TodT and a mixture of both proteins were incubated in the presence of radioactive ATP for 50 min. As illustrated in Fig. 3C, when alone, TodS was autophosphorylated by ATP, which was not the case for TodT when it was alone. However, a band corresponding to TodT appeared in the mixture of both proteins, demonstrating phosphorylation through TodS. This phosphorylation was confirmed by the fact that the band corresponding to TodS had become fainter (Fig. 3C).
IHF Participates in the Activation of PtodX.
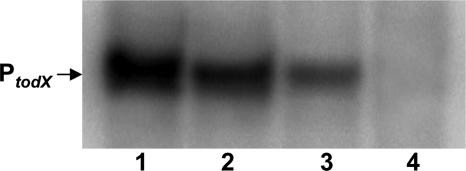
Lau et al. (3) demonstrated that the TodT-binding box was located far upstream from the RNA polymerase-binding site and was centered at base pair −107 with respect to the transcription start point. This finding implies that activation requires DNA bending to allow productive contacts between the transcriptional regulator and RNA polymerase bound in the downstream element. IHF is involved in DNA bending and in the activation and repression of a number of promoters in which the regulator-binding site is located far from the transcription start point (6, 7). A potential IHF site (AAAAACAATA) was detected between −43 and −34 in PtodX, which matched the consensus defined for IHF (6). To study the involvement of IHF in transcription activation, we transferred pMIR66 and pMIR77 into the wild-type KT2440 strain and its isogenic IHF mutant strain and measured the expression from PtodX in response to toluene. In both backgrounds, basal expression was similarly low (≈2 Miller units). In response to toluene in the culture medium, β-galactosidase levels in KT2440 reached values of ≈4,500 units; however, induction in the IHF mutant strain was 1/8 of that found in the wild type, which supports a direct role for IHF in the activation of PtodX.
To verify the role of IHF, we constructed pJLC77 carrying a PtodX* mutant with an altered IHF site fused to ′lacZ, andβ-galactosidase activity was measured in P. putida DOT-T1E in the exponential phase after induction with toluene. Activity levels with the mutant promoter were 8-fold lower than those obtained with the wild-type promoter (data not shown).
IHF Binds to PtodX in Vitro.
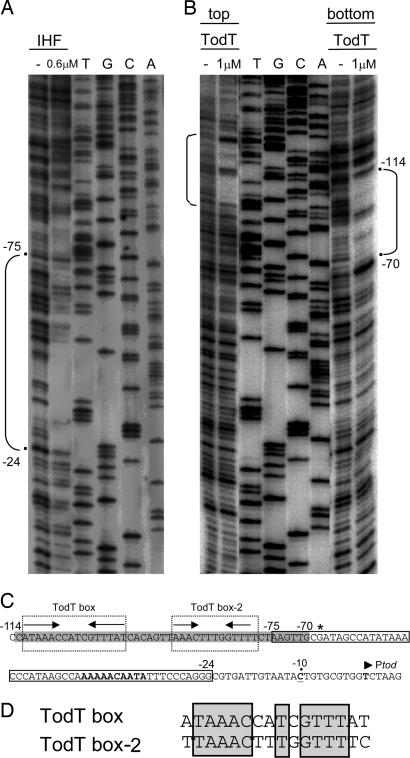
To confirm that IHF binds PtodX in vitro, we carried out EMSA with increasing IHF concentrations and a 352-bp32P-labeled PtodX promoter or the PtodX* with an altered IHF site. No binding of IHF to the mutant PtodX* promoter was found (data not shown). In contrast, 100 nM IHF retarded 80% of the wild-type PtodX promoter. From the EMSA, we estimated that the apparent affinity of IHF for its target was ≈70 nM (data not shown). DNase I footprint assays revealed that IHF protected the −24 to −75 base pair region of PtodX (Fig. 4A and C), which includes the region identified in silico.
Fig. 4.
Identification of TodT and of IHF-binding sites at PtodX. DNase I footprints of IHF (A) and TodT (B). Brackets indicate the protected area. (C) Sequence of PtodX. The gray shading indicates the region protected by TodT. The dotted outline boxes indicate the two TodT-binding sites. Arrows represent palindromic sequences; the asterisk marks a hyperreactive G; sequences matching the IHF consensus sequence are in bold. The narrow box indicates the fragment protected by IHF. The transcription start site is indicated by an arrowhead, and the −10 base pair position in the promoter is marked with a dot and underlined. (D) Sequence alignment of the two TodT binding sites. Conserved nucleotides are boxed.
Redefining the TodT-Binding Region in PtodX.
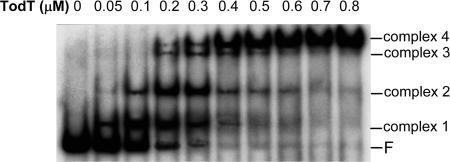
We used EMSA to study the binding of TodT to PtodX (Fig. 5). We observed four bands corresponding to TodT–DNA complexes, which might be indicative of the existence of multiple TodT-binding sites. To identify the TodT-binding site(s), DNase I footprint assays were carried out with 1 μM TodT. As shown in Fig. 4 B and C, the region between base pairs −114 and −70 was protected by TodT. This fragment contained the TodT box centered at base pair −107, as described in ref. 3. However, there was clear evidence that protection went beyond this site. Inspection of the sequence downstream from the TodT box revealed a second palindromic fragment, termed “TodT box-2” (Fig. 4C). Sequence alignment of the two TodT boxes revealed a high degree of identity (Fig. 4D), and we hypothesize that TodT box-2 represents a second binding site for TodT. The consensus sequence between the two TodT boxes (AAACNNTNGTTT) can be considered a specific TodT-binding motif.
Fig. 5.
Specific binding of TodT to PtodX EMSA for the binding of TodT to a 352-bp DNA fragment containing the PtodX promoter. F, free DNA.
Activation from the PtodX Promoter Requires Contacts with the α-Subunit of RNA Polymerase.
A number of positive transcriptional regulators interact with the α-subunit of RNA polymerase (15, 16). To test whether this interaction occurs in the case for TodT, experiments were carried out in Escherichia coli with defects in the α-subunits bearing pMIR66 and pMIR77. This approach was based on an experimental set-up in which most of the RNA polymerase pool contained a mutant subunit (15–17). The assays were designed to primarily detect a negative effect on transcription. We first tested activation in a mutant background in which α-C-terminal domain (CTD) lacked 235 residues and found that activity from PtodX was ≈50% of that in the wild type. This observation is consistent with data obtained for other promoters, such as mtr regulated by TyrR (18), the TOL plasmid Pm promoter (17), and the rha promoter of E. coli (15). Subsequently, we used a series of point mutants exhibiting alanine substitutions (19, 20). TodT-dependent transcription from PtodX was significantly affected in a background in which α-CTDL289A or α-CTDK291A mutants were overproduced, because activity decreased by ≈40% with respect to the wild type. Overproduction of α-CTD carrying other mutations did not affect the level of expression from PtodX. Therefore, the drop in activity in the two α-CTD mutants seems to result from decreased interactions of α-CTD with TodT. This finding is in agreement with previous studies showing that the patch of residues around position 289 are involved in interactions between the α-CTD and the MetR regulator of E. coli and the bacteriophage P2 Ogr protein (21).
In Vitro Transcription Assays.
The results obtained in vitro and in vivo indicated that expression from PtodX requires that TodT be activated by TodS through transphosphorylation in response to toluene. Expression from PtodX also involves DNA bending in a process assisted by IHF. To provide unequivocal evidence, we performed in vitro transcription assays with linear PtodX DNA. Fig. 6 shows that efficient transcription from PtodX took place in the presence of RNA polymerase, TodS, TodT, IHF, and toluene (Fig. 6, lane 1). However, when TodS, TodT, or toluene was omitted, hardly any or no transcription from PtodX occurred (for absence of toluene, see Fig. 6, lane 3). In the absence of IHF, expression from PtodX was ≈20–30% of that observed in the complete system (Fig. 6, lane 2). To further confirm that the phosphorylated TodT protein is the activating form of the response regulator, we replaced, in the in vitro transcription assay, the TodT protein by a mutant variant that retained the ability to bind target DNA but in which the phosphoaccepting Asp-57 was replaced by alanine. When this mutant regulator was used in in vitro transcription assays, no transcript was seen (Fig. 6, lane 4).
Fig. 6.
In vitro transcription from PtodX. Transcription assays were performed as described in Materials and Methods. The assay performed in the presence of 1.5 μM TodS and TodT, 50 nM IHF, and 150 μM toluene is shown in lane 1. In lane 4, TodTD57A replaced TodT. IHF and toluene were omitted in lanes 2 and 3, respectively.
Discussion
Soil bacteria sense and respond to environmental cues through a number of TCSs (9). Analyses of annotated bacterial genomes revealed that soil bacteria possess ≈50 TCSs per genome, which is far greater than the average for other bacteria (22). The soil bacterium P. putida KT2440 was shown to have 93 TCSs (8). The large number of structurally similar proteins in a single organism raises the question of specificity of each of these regulatory systems. Here we report assays with todS and todT mutants that demonstrate that there is no functional interaction between either of the two proteins with other histidine kinases or with transcriptional regulators. We have concluded that the interaction of TodS with TodT is highly specific.
The TOD catabolic enzymes metabolize benzene, toluene, and ethylbenzene. These substrates were found to induce PtodX (Fig. 1); however, they induce it to a different degree. Toluene was the most efficient effector of all of the molecules tested, benzene was found to be a good inducer, and the inducing effect of ethylbenzene was modest. Our results showed that there was a high degree of tolerance to different substituents on the aromatic ring and that substitutions in the 3 and 4 positions were better tolerated than substitutions at position 2. This finding suggests that TodS is a protein with broad effector-recognition properties, which is unusual for sensor histidine kinases.
The affinity of TodS for toluene (≈0.7 μM) is significantly higher than the binding of effector molecules to other histidine kinase sensors. For instance, CitA has a Kd of 5.5 μM for the binding of citrate (23), and NarX and PhoQ have apparent affinities of ≈35 μM for nitrate and ≈300 μM for Mg2+ ions, respectively (24, 25). The high affinity of TodS for toluene assures that bacteria activate transcription and use it as a carbon source at very low concentrations, as expected for a volatile chemical.
TodS contains two signal-sensing domains. Isothermal titration calorimetry showed that toluene bound to NTodS with an affinity of 2.3 ± 0.1 μM, whereas no binding was observed with CTodS. This observation provided direct proof that toluene binds to the N-terminal sensor domain. At first sight, our data may appear inconsistent with the findings by Choi et al. (4), who showed that TodS mutants within the C-terminal half had a wider effector specificity range. Although we demonstrated that toluene binds to the N-terminal supradomain, an indirect role of the C-terminal region in toluene sensing is likely. This proposal is deduced from the larger enthalpy changes observed for toluene binding to NTodS (ΔH = −18.3 ± 0.2 kcal/mol) than for the wild-type TodS (ΔH = −5.5 ± 0.1 kcal/mol).
On the basis of sequence similarity to the heme-binding, oxygen-sensing domain of FixL, it was suggested that the C terminus of TodS was also a heme-containing sensor domain (3). Heme-binding proteins, recombinantly produced in E. coli, copurify with heme and show characteristic absorption peaks between 400 and 600 nm (26). Spectral analysis of purified and active TodS showed no maximum peaks in this wavelength range, indicating that it is unlikely that TodS exhibits a heme group.
The TodS protein has a low but measurable basal level of autophosphorylation. We showed that the addition of toluene increases autophosphorylation and results in TodS phosphotransfer to TodT. Phosphorylated TodT functions as the positive regulator. This phenomenon has been corroborated in in vitro transcription assays, because, in the absence of TodT or in the presence of mutant TodTD57A, no transcription took place (Fig. 6).
EMSA studies revealed the presence of four retarded bands, indicating that multiple TodT monomers interact with their target DNA. The footprint analyses revealed the existence of two binding sites of similar size and sequence (Fig. 4). The four bands observed in EMSA may be the result of the binding of one TodT monomer per half site in each of the two TodT boxes identified here. Our EMSA and footprint results differ from those originally reported by Lau et al. (3), which showed only two retarded bands corresponding to only one TodT binding site. This discrepancy could be due to the fact that, in the initial study, a recombinant TodT-GST fusion protein was used (3). Because GST is similar in size to TodT, this fusion protein may have caused steric hindrance, preventing other TodT molecules from binding to the proximal Tod box-2.
Expression from PtodX in an IHF-deficient background was very low and probably reflected the limitations imposed by the absence of the bending protein. Indeed, IHF binds to PtodX and protects the region between base pairs −24 and −75, which is consistent with IHF-mediated DNA bending, allowing a direct interaction between TodT bound to the −85 to −107 boxes and RNA polymerase bound to the downstream promoter site (6). On the basis of the observation that expression from PtodX decreased in mutant backgrounds that express RpoA variants, the structure of α-CTD was resolved and shown to be compactly folded and to contain four α-helices (27). TodT seems to interact with residues in α-helix 3 (residues 286–292), a subset of residues also identified in interactions with other positive regulators (21).
Our results support the following model for the mechanism of transcription activation from PtodX by the TodS–TodT TCS. TodS exhibits basal autophosphorylation activity, which increases in the presence of toluene. Increased autophosphorylation increases the rate of transphosphorylation of TodT, which is persistently bound to its target sequences in the PtodX promoter. Phosphorylation of TodT probably induces conformational changes, which allow or alter the interaction with RNA polymerase in a process assisted by IHF. Once the RNA polymerase–IHF–TodT–P scaffold is formed, the tod operon is read.
Materials and Methods
Bacterial Strains, Culture Media, and Plasmids.
The bacterial strains and plasmids used are shown in Table 1 (28–33). E. coli strains were grown at 30°C in LB or 2× YT medium (for recombinant protein expression) with shaking. P. putida was grown on M9 minimal medium with glucose.
β-Galactosidase Assays.
To quantify the expression from the TOD pathway promoter, we used pMIR77 [PtodX::lacZ (30)] and a mutant PtodX* promoter carrying an altered IHF site (AAAGAGGATA, mutations are underlined) in pJLC77 (PtodX*::lacZ). Bacterial strains were grown on M9 minimal medium with the appropriate antibiotics without or with the inducer. β-galactosidase activity was determined in permeabilized cells (34).
Construction of todS and todT Mutants in pMIR66.
The todS and todT genes in plasmid pMIR66 were disrupted by using the aphA3 kanamycin (Km)-resistance cassette, which produced no polar effects (32). The resulting plasmids were called pJLC1 (todS:Km) and pJLC2 (todT:Km).
Overexpression and Purification of TodT, TodS, CTodS, NTodS, and IHF.
todS, todT, and the todS mutant variants were cloned independently in pET28b+ to yield plasmids pTodS, pJLTodT, pCTodS, and pNTodS, which allow the overexpression of these proteins with a His-6 tag. These proteins were purified by using HisTrap columns (Amersham Biosciences). IHF was purified after overexpression of the ihfA and ihfB genes, as described by Ilves et al. (33).
EMSA.
A 352-bp DNA fragment containing the PtodX promoter was amplified by PCR from pMIR77 and end-labeled with 32P as described. Approximately 2 nM labeled DNA (≈1.5 × 104 cpm) was incubated with the indicated amounts of purified TodT or IHF for 30 min at 30°C in 10 μl of binding buffer [50 mM Tris·HCl, pH 8.0/100 mM KCl/1 mM DTT/10 mM MgCl2/10% (vol/vol) glycerol] containing 20 μg/ml poly(dI-dC) and 200 μg/ml BSA. Electrophoresis in PAGE was done as described in ref. 35.
DNase I Footprint.
The DNA fragment and buffer used for EMSA was also used for footprinting assays. Approximately 10 nM (≈105 cpm) labeled probe was incubated in the presence and absence of IHF (0.6 μM) or TodT (1 μM). Reactions were incubated for 30 min before treatment with DNase I (10−5 units/μl). Then DNA was precipitated, suspended in 10 mM Tris/0.1 mM EDTA (pH 8), heated at 90°C for 3 min, and analyzed by using 6.5% (wt/vol) PAGE.
In Vitro Transcription Assays.
Reactions (20 μl) were performed in 25 mM Tris·HCl/9 mM MgCl2/100 mM KCl (pH 7.5) containing 50 nM σ70-holoenzyme, 20 units of RNasin (Promega), and 5 nM linear PtodX DNA template (680 bp). The mixtures were incubated for 10 min at 30°C before the addition of 0.1 mM ATP, CTP, and GTP, 0.05 mM UTP, and 3.6 μCi of [α-32P]UTP (10 μCi/μl) (1 Ci = 37 GBq). After a 20-min incubation, the reactions were chilled at 4°C, and 4 μl of formamide sequencing dye was added. Samples were separated in a 6.5% (wt/vol) PAGE.
Isothermal Titration Calorimetry.
Measurements were done on a VP microcalorimeter (Microcal, Amherst, MA) at 25°C. TodS was dialyzed against 50 mM Tris·HCl/250 mM KCl/2 mM MgCl2/2 mM DTT/0.1 mM EDTA, pH 7.5. One millimole toluene and ethylbenzene solutions were made up in dialysis buffer. The titrations involved 1.6-μl injections of toluene or ethylbenzene into a solution of 12–15 μM TodS. Titration curves were fitted by a nonlinear least-squares method (origin software; Microcal) to a function for the binding of a ligand to a macromolecule (36).
In Vitro Phosphorylation Assays.
Autophosphorylation of TodS.
Assays were done in 50 mM Tris·HCl, pH 7.5/200 mM KCl/2 mM MgCl2/0.1 mM EDTA/10% (vol/vol) glycerol/10 mM DTT. The autophosphorylation assay was performed at 24°C with 85 pmol purified TodS in a final reaction volume of 100 μl in the presence and absence of toluene (100 μM). Reactions were initiated by adding radiolabeled ATP (5 nmol ATP containing 1 μCi [γ-32P]ATP), and 7.5-μl samples were removed at different times. The reaction was stopped by adding 2× SDS sample buffer and transferring onto ice. All samples were run on SDS/7.5% (wt/vol) PAGE.
Dephosphorylation of TodS.
Protein was autophosphorylated in the presence of 100 μM toluene, as detailed above, for 50 min. A 500-fold molar excess of ATP was added, and samples were removed at different times before analysis by SDS/PAGE.
Transphosphorylation of TodS–TodT.
A mixture of TodS (11 μM) and TodT (55 μM) was incubated in a final volume of 20 μl, as described above, for autophosphorylation of TodS. In parallel, TodS and TodT were individually submitted to the same treatment. After 50 min, samples were analyzed by SDS/10% (wt/vol) PAGE.
Acknowledgments
We thank K. Shashok for improving our use of English and C. Lorente for secretarial assistance. This work was supported by Ministry of Science and Education Grants BIO2003-00515 and GEN2001-4698-CO5-03 and Ministry of the Environment Grant VEM2004-08560.
Abbreviations
- CTD
C-terminal domain
- CTodS
C-terminal TodS fragment
- IHF
integration host factor
- NTodS
N-terminal TodS fragment
- TCS
two-component regulatory system
- TOD
toluene dioxygenase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Zylstra G. J., Gibson D. T. J. Biol. Chem. 1989;264:14940–14941. [PubMed] [Google Scholar]
- 2.Mosqueda G., Ramos-González M. I., Ramos J. L. Gene. 1999;232:69–76. doi: 10.1016/s0378-1119(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 3.Lau P. C. K., Wang Y., Patel A., Labbé D., Bergeron H., Brousseau R., Konishi Y., Rawlings M. Proc. Natl. Acad. Sci. USA. 1997;94:1453–1458. doi: 10.1073/pnas.94.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi E. N., Cho M. C., Kim Y., Kim C.-K., Lee K. Microbiology. 2003;149:795–805. doi: 10.1099/mic.0.26046-0. [DOI] [PubMed] [Google Scholar]
- 5.Domínguez-Cuevas & Marqués S. Pseudomonas. Vol. 2. Kluwer, Dordrecht: The Netherlands; 2004. pp. 319–345. [Google Scholar]
- 6.Pérez-Martín J., de Lorenzo V. Annu. Rev. Microbiol. 1997;51:593–628. doi: 10.1146/annurev.micro.51.1.593. [DOI] [PubMed] [Google Scholar]
- 7.Berger D., Naberhaus F., Kustu S. Proc. Natl. Acad. Sci. USA. 1994;91:103–107. doi: 10.1073/pnas.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson K. E., Weinel C., Paulsen I. T., Dodson R. J., Hilbert H., dos Santos V. A. P., Fouts D. E., Gill S. R., Pop M., Holmes M., et al. Environ. Microbiol. 2002;4:799–808. doi: 10.1046/j.1462-2920.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 9.Ventre I., Filloux A., Lazdunski A. Pseudomonas. Vol. 2. Kluwer, Dordrecht: The Netherlands; 2004. pp. 257–289. [Google Scholar]
- 10.Ramos J. L., Michán C., Rojo F., Dwyer D., Timmis K. N. J. Mol. Biol. 1990;211:373–382. doi: 10.1016/0022-2836(90)90358-S. [DOI] [PubMed] [Google Scholar]
- 11.Shingler V., Pavel H. Mol. Microbiol. 1995;17:505–513. doi: 10.1111/j.1365-2958.1995.mmi_17030505.x. [DOI] [PubMed] [Google Scholar]
- 12.Delgado A., Salto R., Marqués S., Ramos J. L. J. Biol. Chem. 1995;270:5144–5150. doi: 10.1074/jbc.270.10.5144. [DOI] [PubMed] [Google Scholar]
- 13.Tropel D., van der Meer J. R. Microbiol. Mol. Biol. Rev. 2004;68:474–500. doi: 10.1128/MMBR.68.3.474-500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter C. A., Ward A., Laguri C., Williamson M. P., Henderson P., Phillips-Jones M. K. J. Mol. Biol. 2002;320:201–213. doi: 10.1016/S0022-2836(02)00424-2. [DOI] [PubMed] [Google Scholar]
- 15.Holcroft C. C., Egan S. M. J. Bacteriol. 2000;182:3529–3535. doi: 10.1128/jb.182.12.3529-3535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busby S., Ebright R. H. Mol. Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruíz R., Marqués S., Ramos J. L. J. Bacteriol. 2003;185:3036–3041. doi: 10.1128/JB.185.10.3036-3041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Murakami K., Camakaris H., Fujita N., Ishihama A., Pittard A. J. J. Bacteriol. 1997;179:6187–6191. doi: 10.1128/jb.179.19.6187-6191.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H., Severinov K., Goldfarb A., Fenyo D., Chait B., Ebright R. H. Genes Dev. 1994;8:3058–3067. doi: 10.1101/gad.8.24.3058. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y., Merkel T. J., Ebright R. H. J. Mol. Biol. 1994;243:603–610. doi: 10.1016/0022-2836(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 21.Savery N. J., Rhodus V. A., Wing H. J., Busby S. J. W. Biochem. J. 1995;309:77–83. doi: 10.1042/bj3090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashby M. K. FEMS Microbiol. Lett. 2004;231:277–281. doi: 10.1016/S0378-1097(04)00004-7. [DOI] [PubMed] [Google Scholar]
- 23.Gerharz T., Reinelt S., Kaspar S., Scapozza L., Bott M. Biochemistry. 2003;42:5917–5924. doi: 10.1021/bi0340595. [DOI] [PubMed] [Google Scholar]
- 24.Lee A. I., Delgado A., Gunsalus R. P. J. Bacteriol. 1999;181:5309–5316. doi: 10.1128/jb.181.17.5309-5316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesley J. A., Waldburger C. D. J. Biol. Chem. 2001;276:30827–30833. doi: 10.1074/jbc.M104262200. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H., Kumita H., Imai K., Iizuka T., Shiro Y. Proc. Natl. Acad. Sci. USA. 2004;101:2742–2746. doi: 10.1073/pnas.0305795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon Y. H., Negishi T., Yamazaki T., Fujita N., Ishihama A. S., Kyoguku Y. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 28.Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1991. [Google Scholar]
- 29.Ramos J. L., Duque E., Huertas M. J., Haïdour A. J. Bacteriol. 1995;177:3911–3916. doi: 10.1128/jb.177.14.3911-3916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-González M. I., Olson M., Gatenby A. A., Mosqueda G., Manzanera. M., Campos M. J., Vílchez S., Ramos J. L. J. Bacteriol. 2002;184:7062–7067. doi: 10.1128/JB.184.24.7062-7067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marqués S., Gallegos M. T., Manzanera M., Holtel A., Timmis K. N., Ramos J. L. J. Bacteriol. 1998;180:2889–2894. doi: 10.1128/jb.180.11.2889-2894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménard R., Sansonetti P. J., Parsot C. J. Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilves H., Horak R., Teras R., Kivisaar M. Mol. Microbiol. 2004;51:1773–1785. doi: 10.1111/j.1365-2958.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 34.Platt T., Meueler-Hill B., Miller J. Miller J. Experiments in Molecular Genetics. Woodbury, New York: Cold Spring Harbor Lab. Press; 1972. pp. 352–355. [Google Scholar]
- 35.Guazzaroni M. E., Terán W., Zhang X., Gallegos M. T., Ramos J. L. J. Bacteriol. 2004;186:2921–2927. doi: 10.1128/JB.186.10.2921-2927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiseman T., Williston S., Brandts J. F., Lin L. N. Anal. Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]