Abstract
The mechanisms underlying modulation of corticostriatal synaptic transmission by D2-like receptors (D2Rs) have been controversial. A recent study suggested that D2Rs inhibit glutamate release at this synapse, but only during high-frequency synaptic activation. Because the release of postsynaptic endocannabinoids (eCBs), which act as retrograde messengers to inhibit presynaptic glutamate release, can be triggered by D2R activation and intense synaptic activation, such a mechanism could mediate dopaminergic modulation of corticostriatal transmission. Here, we show that D2R activation reduces excitatory transmission onto striatal medium spiny neurons at a stimulation frequency of 20 Hz but not at 1 Hz. This form of inhibition requires CB1 receptor activation, as evidenced by the fact that it is blocked by AM251 [N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide], a CB1 antagonist, and is absent in CB1 knockout mice. It is also blocked by postsynaptic intracellular calcium chelation, by group I metabotropic glutamate receptor antagonism, and by inhibition of postsynaptic phospholipase C. These results demonstrate a previously unrecognized role for retrograde eCB signaling in reversible and frequency-specific inhibition of glutamate release by the activation of striatal D2Rs.
Keywords: anandamide, cannabinoid, dopamine
The sensorimotor or dorsolateral striatum (DLS) plays critical roles in habit formation, addiction, and the control of sequential behavior (1–4). The main inputs to DLS come from primary sensorimotor cortices, and the downstream networks eventually controlled by DLS include the brainstem and the motor thalamocortical network (5–8).
The glutamatergic terminals of cortical pyramidal neurons synapse on the medium spiny neurons (MSNs), the projection cells of the striatum. This massive excitatory input is modulated by dopamine released from terminals arising from neurons in the midbrain, which synapse on the necks of the dendritic spines on the MSNs (9). In particular, the activation of dopamine D2-like receptors (D2Rs) is known to play a critical role in modulating glutamatergic transmission, although the exact mechanism underlying this action remains controversial (10–16). The Gi/o-coupled D2Rs have been implicated in striatum-dependent motor control (17), and these receptors are also the major targets of a variety of drugs for disorders such as Parkinson’s disease, schizophrenia, and depression (18). Thus, the modulation of excitatory transmission by D2Rs is likely to be involved in integrative functions such as motor control, motivation, and affect.
Recently, Bamford et al. (14) reported that D2R activation with the agonist quinpirole inhibits glutamate release in the DLS only at high frequencies (HFs) of afferent activation and that such inhibition appeared to be restricted to the least active terminals (14). This observation suggests that dopamine, acting on D2Rs, can serve as a low-pass filter for the excitatory input from the cortex. These researchers attributed this function to D2Rs on corticostriatal presynaptic terminals.
Anatomical evidence indicates that D2Rs can be found on glutamatergic presynaptic terminals in the striatum (19), so these Gi/o-coupled receptors are in a position to dampen glutamate release (11). This hypothesis, however, is weakened by two observations. First, presynaptic D2Rs are only sparsely distributed in the dorsomedial striatum (19). There is, to our knowledge, no clear evidence for their presence in the DLS. Second, and more importantly, it is not clear why D2Rs on a presynaptic terminal would only depress transmission at HFs of afferent activation; the standard Gi/o-mediated inhibition of glutamate release should be effective at all frequencies. For example, the activation of A1 adenosine receptors exerts a general inhibitory effect by reducing glutamate release (20). To account for the frequency dependence of their inhibitory function, it would therefore be necessary to invoke different mechanisms for presynaptic D2Rs.
However, a ready explanation of such HF-specific inhibition is suggested by previous work on endocannabinoid (eCB) signaling at the corticostriatal synapse. Retrograde eCB signaling is critical for the expression of long-term depression (LTD) in the DLS (21–23). The eCB anandamide is rapidly synthesized and released in MSNs in response to simultaneous depolarization and D2 receptor activation; they then travel back to the presynaptic terminal to activate CB1 receptors, which results in a long-lasting decrease in glutamate release (21, 23–29).
Retrograde eCB signaling as a mechanism for D2R-mediated dampening of excitatory input to the striatum is not only parsimonious but also plausible, given the abundant distribution of D2Rs on postsynaptic elements in the striatum and of CB1 receptors on the presynaptic glutamatergic terminals synapsing on the MSNs (30, 31). In the present study, we tested this hypothesis in acute brain slices by using whole-cell patch-clamp recording.
Results
Frequency Dependence of D2R-Mediated Inhibition of Glutamatergic Transmission.
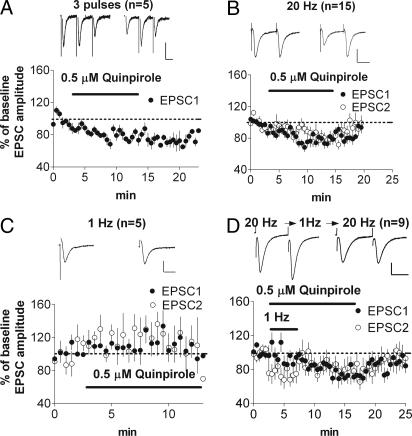
First, we attempted to replicate the basic effect observed by Bamford et al. (14) by using the procedure described by that group. We used trains of three stimuli delivered at a frequency of 20 Hz (30-s intertrain interval), followed by application of 0.5 μM quinpirole in the absence of stimulation. Stimulation at 20 Hz was then resumed, and the effect of quinpirole on excitatory postsynaptic current (EPSC) amplitude was measured. These data were analyzed by comparing the baseline EPSC amplitude with that of the first five sweeps after 5 min of drug application, revealing a significant inhibition by quinpirole (EPSC 1 = 52 ± 4% of baseline, EPSC 2 = 49 ± 6% of baseline, and EPSC 3 = 41 ± 8% of baseline; P < 0.05; Fig. 1B).
Fig. 1.
Activation of D2Rs inhibits glutamate release at the corticostriatal synapse. (A) Schematic illustration of the corticostriatal slice preparation. Note that the stimulating electrode illustrated is placed in the cortex outside of the white matter. (B) Quinpirole inhibits EPSCs when a three-pulse train is used. (C) A two-pulse train is also effective in revealing D2R-mediated inhibition of EPSCs. Inhibition is blocked by pretreatment with sulpiride, a D2R antagonist.
A similar effect was observed when two-pulse stimuli (50-ms interpulse interval and 30 s between pairs) were given before and again 5 min after the onset of quinpirole application (amplitude of EPSC 1 = 74 ± 6% of baseline and EPSC 2 = 80.9 ± 7% of baseline; P < 0.05; Fig. 1C). Because inhibition was effectively elicited by using this protocol, the majority of the remaining experiments were performed using a two-pulse stimulus. Moreover, because higher concentrations of quinpirole (1 μM, 57 ± 7%, n = 4; 2 μM, 63 ± 2%, n = 4) produced inhibition similar to that produced by 0.5 μM quinpirole [the concentration used by Bamford et al. (14)], the 0.5 μM concentration was used in all subsequent experiments.
We found that this form of inhibition was only present when the stimulating electrode was placed outside of the white matter, in the deep layers of the cortex, a stimulation site similar to that used by Bamford et al. (14). When the stimulating electrode was placed directly in the white matter, no inhibition by quinpirole was observed (n = 6, EPSC amplitude = 132 ± 6% of baseline; P > 0.05). Because previous amperometry data showed that stimulation of the white matter causes dopamine release in the striatum (32), the lack of inhibition could be due to activation of intrastriatal dopaminergic fibers, causing dopamine release and the activation of D2Rs in the baseline condition in the absence of quinpirole. To test this possibility, we placed the stimulating electrode in the white matter and compared baseline EPSCs with those during application of 5 μM sulpiride, a D2R antagonist. In support of tonic D2R activation with white matter stimulation, sulpiride increased the magnitude of EPSCs (128 ± 11% of baseline, n = 6; P < 0.05).
We next tested whether the inhibition evoked by quinpirole was indeed mediated by activation of D2Rs by pretreating the slices in 5 μM sulpiride for at least 20 min. As shown in Fig. 1C, no decrease in EPSC amplitude was observed (119 ± 24% of baseline; P > 0.05).
Time Course of HF-Specific Inhibition.
In the experiments described above, HF stimulation was only given during the baseline period and after 5–8 min of drug application. No stimulation was given during the initial drug application period. This procedure allowed us to observe significant inhibition by quinpirole but did not allow us to monitor the detailed time course of the onset of the inhibition. For this reason, we repeated the experiments, this time stimulating and recording continuously for 20–40 min, during which we applied quinpirole after 5 trains and washed out quinpirole after another 20 trains.
First, we demonstrated the frequency dependence of the inhibition. When we used the three-pulse protocol at 20 Hz, we found significant inhibition of the first EPSC (71 ± 5% of baseline; P < 0.05; Fig. 2A). Likewise, by using the two-pulse protocol, significant inhibition was observed after quinpirole application (74 ± 4% of baseline; P < 0.05; Fig. 2B), along with a significant increase in the paired-pulse ratio (PPR), the second EPSC amplitude divided by that of the first EPSC (baseline PPR = 1.05 ± 0.09; after quinpirole, PPR = 1.20 ± 0.09; P < 0.05), suggesting a decrease in the probability of glutamate release. Because the size of inhibition produced by the two-pulse and three-pulse protocols was similar, for all subsequent experiments, we used the two-pulse protocol. Moreover, replicating previous work (14), no decrease in EPSC amplitude was observed at a stimulation frequency of 1 Hz (Fig. 2C; 119 ± 5% of baseline; P > 0.05).
Fig. 2.
Activation of D2 receptors inhibits glutamate transmission only when paired with HF stimulation. (A) Depression is observed after bath application of quinpirole using the three-pulse protocol with 20-Hz afferent stimulation. Only the first EPSC is shown. (B) Depression is observed after bath application of quinpirole using the two-pulse protocol with 20-Hz afferent stimulation. (C) No depression is observed at 1 Hz. After recording EPSCs at 20 Hz during the predrug baseline period, the frequency of stimulation was reduced to 1 Hz during the first 5 min of quinpirole application. (D) When stimulation at 20 Hz was resumed in the presence of quinpirole, inhibition developed gradually, with a time course similar to that seen in A. (Scale bars: 100 pA, 25 ms.)
To see whether the emergence of inhibition requires simultaneous 20-Hz stimulation and D2R activation, we recorded the baseline at 20 Hz, switched to 1 Hz for the first 5 min of quinpirole application, and then switched back to 20 Hz for the rest of the recording. If D2R activation alone is sufficient for inducing inhibition that is only detectable with 20-Hz stimulation, then EPSCs should be immediately depressed compared with baseline when the frequency of stimulation is switched back to 20 Hz after 5 min of quinpirole application at 1 Hz. This pattern, however, was not observed. As shown in Fig. 2D, when stimulation was switched back to 20 Hz, inhibition of EPSC amplitude emerged gradually (no initial decrease, but a decrease to 73 ± 2% of baseline after 5 min; P < 0.05), with a time course similar to that of the 20-Hz-only condition (compare Fig. 2 B and D). There was a significant increase in PPR (baseline PPR = 1.04 ± 0.002; after quinpirole, PPR = 1.21 ± 0.09). Thus, the development of this inhibition also appears to be frequency dependent (i.e., it requires 20-Hz stimulation in conjunction with D2R activation).
HF-Specific Inhibition Blocked by Metabotropic Glutamate Receptor (mGluR) Antagonism and Intracellular Calcium Chelation.
A rise in intracellular calcium is required for eCB synthesis and release in striatum and for eCB-mediated striatal LTD (21, 22, 25, 33, 34). Previous work has shown that activation of group I mGluRs, which can lead to the release of calcium from intracellular stores, is involved in eCB retrograde signaling (35, 36). It follows that HF-specific inhibition might be prevented by (i) blockade of group I mGluRs and (ii) chelation of intracellular calcium.
We tested this hypothesis by continuous bath application of 40 μM 7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide ethyl ester (CPCCOEt), an mGluR1 antagonist. This treatment blocked the quinpirole-induced inhibition of EPSC amplitude (102 ± 3% of baseline; P > 0.05; Fig. 3A). Likewise, chelating intracellular calcium by loading 20 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) into the MSN by means of the patch pipette blocked the HF-specific inhibition (111 ± 5% of baseline; P > 0.05; Fig. 3B). Application of quinpirole along with 20-Hz stimulation did not alter PPR in the presence of extracellular CPCCOEt (baseline PPR = 0.98 ± 0.04; after quinpirole, PPR = 0.93 ± 0.06; P > 0.05) or intracellular BAPTA (baseline PPR = 1.04 ± 0.01; after quinpirole, PPR = 1.05 ± 0.07; P > 0.05).
Fig. 3.
Group I mGluRs are necessary for quinpirole-induced inhibition. Inhibition is blocked by bath application of CPCCOEt, a group I mGluR antagonist (A), postsynaptic loading of BAPTA, a calcium chelator (B), postsynaptic loading of thapsigargin, an inhibitor of intracellular calcium pumps (C), and U73122, an inhibitor of phospholipase C (D). (Scale bars: 100 pA, 25 ms.)
Intracellular postsynaptic loading of 0.5 μM thapsigargin, an inhibitor of intracellular calcium pumps, blocked quinpirole-induced inhibition (Fig. 3C; 94 ± 6%; P > 0.05; baseline PPR = 1.0 ± 0.05; after quinpirole, PPR = 0.99 ± 0.09; P > 0.05). In addition, intracellular loading of 5 μM U73122, a phospholipase C inhibitor, prevented the quinpirole effect (Fig. 3D; 98 ± 3%; P > 0.05; baseline PPR = 1.03 ± 0.07; after quinpirole, PPR = 0.99 ± 0.07; P > 0.05). Vehicle controls showed significant quinpirole-induced inhibition (n = 4, 78 ± 5%; P < 0.05; baseline PPR = 0.94 ± 0.12; after quinpirole, PPR = 1.16 ± 0.16; P < 0.05)
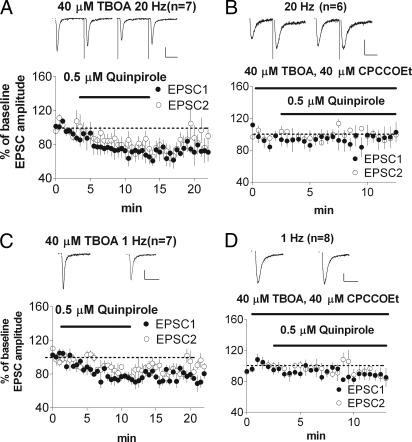
It is possible that stimulation at 20 Hz rather than 1 Hz activates group I mGluRs and that the combination of both group I mGluR activation and D2R activation results in synaptic depression. To determine whether the sensitivity to quinpirole-induced inhibition is increased by enhancing mGluR activation, we used dl-threo-β-benzyloxyaspartic acid (TBOA), an inhibitor of the glutamate transporter, to increase extracellular glutamate. With 40 μM TBOA in the bath, inhibition was observed at both 20 Hz (Fig. 4A; 68.6 ± 8%; P < 0.05; baseline PPR = 0.89 ± 0.04; after quinpirole, PPR = 1.02 ± 0.06; P < 0.05) and 1 Hz (Fig. 4C; 77.9 ± 4%; P < 0.05). Bath application of 40 μM CPCCOEt blocked the inhibition at 20 Hz (Fig. 4B; 102 ± 10%; P > 0.05; baseline PPR = 1.30 ± 0.08; after quinpirole, PPR = 1.22 ± 0.05; P > 0.05) and at 1 Hz (Fig. 4D; 88 ± 6%; P > 0.05; baseline PPR = 0.82 ± 0.04; after quinpirole, PPR = 0.92 ± 0.06; P > 0.05).
Fig. 4.
Shifting the frequency dependence with inhibition of glutamate transport. (A) Quinpirole-induced synaptic depression is observed at 20 Hz with TBOA in the bath. (B) The effect in A is blocked by CPCCOEt. (C) Quinpirole-induced synaptic depression is also observed at 1 Hz with TBOA in the bath. (D) The effect in C is blocked by CPCCOEt. (Scale bars: 100 pA, 25 ms.)
HF-Specific Inhibition Requires eCB Signaling.
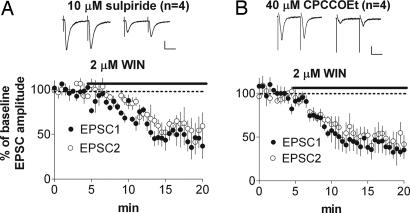
We next tested the hypothesis that eCB signaling is critical for D2R-mediated inhibition. As shown in Fig. 5A, in the presence of 1 μM N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), a CB1 antagonist, no inhibition developed after quinpirole application (EPSC amplitude = 113 ± 1% of baseline; P > 0.05). There was also no change in PPR (baseline PPR = 1.03 ± 0.07; after quinpirole, PPR = 0.93 ± 0.09; P > 0.05). The baseline PPR value in the presence of AM251 (1.03 ± 0.07) was not different from that observed in control recordings (1.07 ± 0.1), indicating that the CB1 antagonist does not alter transmission in the absence of D2 agonist. Moreover, the quinpirole-induced inhibition was occluded by pretreatment with 2 μM WIN552,12-2 (Fig. 5B; 98 ± 10%; P > 0.05; baseline PPR = 1.26 ± 0.04; after quinpirole, PPR = 1.05 ± 0.02; P < 0.05).
Fig. 5.
Activation of CB1 receptors is necessary for HF-specific inhibition. (A) Application of AM251 blocked synaptic depression produced by quinpirole. (B) Pretreatment with WIN552,12-2, a CB1 receptor agonist, occluded the quinpirole-induced inhibition. (C and D) Quinpirole-induced synaptic depression was normal in wild-type mice (C) but absent in CB1 knockout mice (D). (Scale bars: 100 pA, 25 ms.)
To confirm the role of CB1 receptors in D2R-mediated inhibition, we conducted experiments comparing wild-type mice with CB1 knockout mice. In wild-type controls, HF-specific inhibition of EPSC amplitude was observed, just as in rats (Fig. 5C; 72 ± 4% of baseline; P < 0.05; baseline PPR = 0.74 ± 0.05; after quinpirole, PPR = 0.90 ± 0.06; P < 0.05), but no inhibition was observed in CB1 knockout mice (Fig. 5D; 96 ± 3% of baseline; P > 0.05; no change in PPR; baseline PPR = 1.12 ± 0.05; after quinpirole, PPR = 1.04 ± 0.14; P > 0.05).
If CB1 receptor activation occurs downstream of D2R and mGluR activation, then blocking mGluR1 or D2R should not affect inhibition of glutamate release caused by WIN552,12-2. We examined the effect of the CB1 agonist WIN552,12-2 after pretreatment with either CPCCOEt or sulpiride. Pretreatment with 10 μM sulpiride failed to prevent WIN-induced inhibition (Fig. 6A; 46 ± 9%; P < 0.05; baseline PPR = 1.02 ± 0.17; after WIN, PPR = 1.27 ± 0.21; P < 0.05). Likewise, pretreatment with 40 μM CPCCOEt did not block WIN552,12-2-induced inhibition at 20 Hz (Fig. 6B; 39 ± 10%; P < 0.05; baseline PPR = 1.02 ± 0.07; after WIN, PPR = 1.35 ± 0.16; P < 0.05).
Fig. 6.
CB1 activation is downstream of signaling mediated by D2Rs and group I mGluRs. Pretreatment with sulpiride (A) or CPCCOEt (B) did not affect inhibition of glutamate release caused by bath application of WIN552,12-2. (Scale bars: 100 pA, 25 ms.)
Frequency-Specific Quinpirole Inhibition of EPSPs.
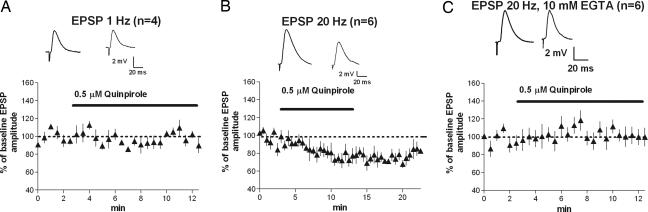
To exclude the possibility that the frequency dependence is restricted to voltage-clamp conditions, we performed the same experiments using current-clamp recording and measured the effect of quinpirole application on EPSPs. As shown in Fig. 7, the results from these experiments agree with our voltage-clamp data. The amplitude of the first EPSP (two-pulse protocol) was significantly reduced after quinpirole application with 20-Hz stimulation (Fig. 7B; 76 ± 5%; P < 0.05). No inhibition was observed with 1-Hz stimulation (Fig. 7A; 93 ± 4%; P > 0.05). Intracellular loading of the calcium chelator EGTA (10 mM) blocked quinpirole-induced inhibition at 20 Hz (Fig. 7C; 108 ± 6%; P > 0.05).
Fig. 7.
Quinpirole-induced, frequency-dependent inhibition is observed under more physiological conditions. (A) No inhibition of EPSPs measured in current-clamp mode was observed with 1-Hz stimulation. (B) Significant inhibition of EPSPs was observed with 20-Hz stimulation. (C) Inhibition of EPSPs at 20 Hz was blocked by intracellular loading of the calcium chelator EGTA.
Discussion
This report provides evidence for a reversible inhibition of glutamate release by retrograde eCB signaling in the striatum. As our results show, activation of D2Rs significantly reduces glutamatergic transmission in the striatum only when receptor activation is combined with stimulation of cortical afferents at a HF (20 Hz). The observed increase in PPR suggests that inhibition involves a presynaptic reduction in glutamate release, consistent with the conclusions of Bamford et al. (14). For this interesting function of dopamine as a low-pass filter, however, we provide evidence for an unexpected mechanism: namely, a retrograde signal resulting from convergent glutamate and dopamine inputs that cooperate to produce eCB synthesis and release from the postsynaptic cell.
Past studies have proposed that dopamine-mediated reduction in glutamate release results from activation of presynaptic D2Rs on the glutamatergic terminals themselves (11, 12, 14). This proposal does not provide a straightforward explanation of the frequency dependence of such inhibition, because one would expect a presynaptic Gi/o-coupled receptor to produce inhibition regardless of stimulus frequency. We hypothesized that D2R-mediated inhibition involves production of a retrograde eCB, perhaps through coordinated activation of D2Rs and group I mGluRs. This hypothesis is supported by our observation that quinpirole-induced inhibition at 20 Hz was abolished by the CB1 receptor antagonist AM251 and by the group I mGluR antagonist CPCCOEt. Furthermore, in accord with the requirement for a rise in intracellular calcium for eCB synthesis and release, HF-specific inhibition was also blocked by intracellular loading of BAPTA or EGTA and by loading thapsigargin into the postsynaptic cell. These observations cannot be readily explained by activation of presynaptic D2Rs.
Without ruling out a role for presynaptic D2Rs in the modulation of corticostriatal transmission, our data implicate a postsynaptic mechanism for D2R-mediated control of synaptic strength in the DLS, but they leave open the question of exactly where the D2Rs that are necessary for HF-specific inhibition are located. These receptors are abundantly expressed in a large population of MSNs, giving rise to the so-called indirect pathway in the striatum, but they are also expressed in other neurons such as cholinergic interneurons (37, 38).
Our results could resolve some of the current controversy surrounding the mixed results from previous work on dopaminergic modulation of glutamate transmission (10–12, 15, 16). If the stimulating electrode is placed close to the MSNs (in the white matter or within the striatum), or if the frequency of stimulation is low, synaptic depression will be difficult to observe. With the stimulating electrode close to the MSNs, dopamine released by stimulation of dopamine terminals (32) may activate D2Rs, occluding further inhibition. In the case of low-frequency stimulation, the amount of glutamate released may be insufficient to consistently activate the extrasynaptically located mGluRs. The optimal location for the placement of stimulating electrodes is therefore in the deep layers of the cortex, which allows for more selective stimulation of cortical afferents.
This account also explains the HF requirement for D2R-mediated synaptic depression: When the terminals are thus stimulated, sufficient glutamate is released to activate group I mGluRs, resulting in the release of calcium from intracellular stores (35, 39). As established by previous studies, the rise in intracellular calcium could cause eCB synthesis and release (25). This model is supported by our observation that intracellular loading of U73122, BAPTA, and thapsigargin all abolished the quinpirole-induced inhibition.
Mechanisms Underlying Retrograde eCB Signaling.
Quinpirole is known to cause an increase in anandamide levels in the rat striatum, and this effect is potentiated by increasing extracellular potassium, which should enhance glutamatergic transmission (24). The mechanisms underlying D2R-mediated stimulation of eCB release are not known. As Piomelli (25) has pointed out, inhibition of cAMP by Gi/o-coupled mechanisms is unlikely to be involved in this case; rather, the main possibilities are through interaction of D2Rs with the Rho family of G proteins to stimulate phospholipase D (which catalyzes conversion of N-arachidonoyl-phosphatidylethanolamine to anandamide) or with βγ-subunits of G proteins to activate phospholipase Cβ (40). An additional possibility is that D2R regulation of the excitability of neurons within the striatum synergizes with mGluR activation to stimulate postsynaptic calcium release and eCB production.
CB1 is the most abundantly expressed G protein-coupled receptor in the mammalian brain, with high levels of expression in the striatum (25, 30). That dopamine modulates excitatory transmission by means of eCB signaling acting on CB1 receptors has a number of important implications for our understanding of transmission at the corticostriatal synapse. First, the rapid synthesis and release of eCBs could serve as a coincidence detector of high levels of presynaptic glutamate release and the activation of D2Rs (41). As a detector of large excitatory drive coupled with dopamine release, it provides a highly specific negative feedback mechanism. Such retrograde signaling is only initiated by converging physiological signals detected by the postsynaptic cell: namely, the amount of glutamate release, reflecting the extent of cortical activity, and the level of dopamine release, reflecting nigrostriatal activity. This type of feedback is impossible in modulation by presynaptic D2Rs, which cannot detect postsynaptic events. Moreover, retrograde eCB signaling appears to be precisely localized to the presynaptic terminals onto a specific postsynaptic target, with the advantage of spatial specificity (42).
The reversible inhibition of excitatory transmission by CB1 receptor activation discovered in the current study has much in common with LTD in the same region. Previous studies have shown that corticostriatal LTD induced by trains of 100-Hz stimulation or by prolonged mGluR activation also requires retrograde eCB signaling (21–23). In addition, Kreitzer and Malenka (22) have shown that quinpirole facilitates LTD induction with a weak stimulation protocol. Like quinpirole-induced inhibition, striatal LTD requires the activation of mGluRs and is blocked by intracellular calcium chelation (43). Unlike striatal LTD, however, the D2R-mediated inhibition described here is reversible and does not require postsynaptic depolarization.
Work from Calabresi and colleagues (44, 45) has shown that, in dopamine-depleted brains, D2R agonists can cause a reduction in presynaptic glutamate release, even at low stimulation frequencies. Whether this effect is also mediated by retrograde eCB signaling is unknown. Dopamine depletion is likely to result in the up-regulation of D2Rs as well as receptor supersensitivity (45), but effects on mGluR activity in the striatum are less clear. It is possible that the retrograde eCB signaling mechanism proposed in the present study also plays a role in the control of glutamate release at the corticostriatal terminal after dopamine depletion.
Conclusions
The corticostriatal projection is of paramount importance in the control of voluntary behavior. The DLS, in particular, is known to play an important role in habit learning and addiction (2, 4), and the D2Rs therein have also been implicated in such phenomena (17, 46, 47). Our results point to the critical role that retrograde eCB signaling plays in the frequency-specific and D2R-mediated inhibition of glutamatergic transmission at the corticostriatal synapse. The question naturally arises as to the significance of this reversible form of inhibition in the control of corticobasal ganglia circuits. Depending on the population of neurons affected, LTD at the corticostriatal synapse could permanently increase or decrease the probability that a certain behavioral repertoire is selected. Particularly strong, convergent, and repetitive inputs from the cortex, coupled with simultaneous dopaminergic input, appear to be the conditions favoring this form of long-term plasticity. In contrast, the D2R-mediated transient inhibition examined in the present study appears to require only HFs of cortical activation and D2R activation and, consequently, may play a role in the online selection of behavioral repertoires rather than their permanent modifications.
Materials and Methods
Brain Slice Preparation.
Brain slices containing both striatum and cortex were prepared as described in ref. 21 from postnatal day 15–19 Sprague–Dawley rats. Animals were killed by decapitation, and the brains were transferred rapidly to ice-cold modified artificial cerebrospinal fluid (aCSF) containing (in mM) 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 d-glucose. Modified aCSF was brought to pH 7.4 by aeration with 95% O2/5% CO2. Coronal sections (350-μm thick) were cut in ice-cold modified aCSF using a Vibratome 1000 sectioning system. Slices were transferred immediately to a nylon net submerged in normal aCSF containing (in mM) 124 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 d-glucose. Normal aCSF was maintained at pH 7.4 by bubbling with 95% O2/5% CO2 at room temperature (19–22°C), and osmolarity was adjusted to 315 milliosmolar (mosM). After at least 1 h of incubation at room temperature, hemislices were transferred to a recording chamber, submerged in normal aCSF containing 50 μM picrotoxin to prevent contamination by GABAA receptor-mediated currents, and maintained at a temperature between 25°C and 28°C (stable within ±1°C during any given experiment).
Whole-Cell Voltage-Clamp Recording.
Whole-cell recordings from MSNs were performed as described in ref. 21. For voltage-clamp recordings, pipettes were filled with an internal solution containing (in mM) 120 cesium methane sulfonate, 5 NaCl, 10 tetraethylammonium chloride, 10 Hepes, 4 lidocaine N-ethyl bromide, 1.1 EGTA, 4 Mg-ATP, and 0.3 Na-GTP, with pH adjusted to 7.2 with CsOH and osmolarity set to 298 mosM with sucrose. For current-clamp recordings, pipettes were filled with an internal solution containing (in mM) 150 K-gluconate, 2 MgCl2, 1.1 EGTA, 10 Hepes, 3 Na-ATP, and 0.2 Na-GTP, with pH adjusted to 7.2 with KOH and osmolarity set to 315 mosM with sucrose.
Stimulation was delivered by a Master-8 stimulator and optical stimulus isolation unit (A.M.P.I., Jerusalem) through a bipolar twisted tungsten wire that was placed in the cortex just above the striatum. Stimulus pulse duration ranged from 0.06 to 0.18 ms, and stimulation intensity ranged from 0.8 to 3.5 mA. With pipette resistance ranging from 2.5 to 6 megaohms (MΩ), recordings were made with the aid of differential interference contrast-enhanced visual guidance. Cells were voltage-clamped at −70 mV. The stimulus intensity was set to the level at which EPSC amplitude was 100–400 pA. The series resistance usually ranged between 8 and 20 MΩ, and if it changed by >20% during the course of an experiment, the cell was discarded. Recordings were only accepted if the series resistance, which was not compensated, remained at <30 MΩ. Series resistance was monitored throughout the experiment by measuring the amplitude of the capacitive transient of current evoked by a 5-mV voltage step.
Synaptic currents and membrane potentials were recorded with an Axopatch 1D amplifier (Axon Instruments, Union City, CA), filtered at 5 kHz, digitized at 10 kHz, and stored on a microcomputer (Dell, Round Rock, TX). The results were analyzed using peak detection software in pclamp8 (Axon Instruments).
Acknowledgments
We thank Drs. Andreas Zimmer (University of Bonn, Bonn) and George Kunos (National Institute on Alcohol Abuse and Alcoholism/National Institutes of Health) for providing CB1 knockout mice. This work was supported by the Division of Intramural Clinical and Basic Research of the National Institute on Alcohol Abuse and Alcoholism/National Institutes of Health.
Abbreviations
- D2R
D2-like receptor
- DLS
dorsolateral striatum
- MSN
medium spiny neuron
- HF
high frequency
- eCB
endocannabinoid
- LTD
long-term depression
- EPSC
excitatory postsynaptic current
- PPR
paired-pulse ratio
- mGluR
metabotropic glutamate receptor
- AM251
N-(piperidin-1-yl)-1-(2,4-dichlorophenyl)-5-(4-chlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CPCCOEt
7-(hydroxylimino)cyclopropa[b]chromen-1a-carboxamide ethyl ester
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
- TBOA
dl-threo-β-benzyloxyaspartic acid
- aCSF
artificial cerebrospinal fluid.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cromwell H. C., Berridge K. C. J. Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin H. H., Knowlton B. J., Balleine B. W. Eur. J. Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 3.Faure A., Haberland U., Conde F., El Massioui N. J. Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everitt B. J., Robbins T. W. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 5.Kemp J. M., Powell T. P. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- 6.Nauta W. J. H. In: Neurology and Psychiatry: A Meeting of Minds. Mueller J., editor. Basel: Karger; 1989. pp. 43–63. [Google Scholar]
- 7.Grillner S., Hellgren J., Menard A., Saitoh K., Wikstrom M. A. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Bolam J. P., Hanley J. J., Booth P. A., Bevan M. D. J. Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicola S. M., Surmeier J., Malenka R. C. Annu. Rev. Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda C., Buchwald N. A., Levine M. S. Proc. Natl. Acad. Sci. USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu K. S., Huang C. C., Yang C. H., Gean P. W. Brain Res. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Hernandez J., Galarraga E., Bargas J. Synapse. 1997;25:185–195. doi: 10.1002/(SICI)1098-2396(199702)25:2<185::AID-SYN9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Bamford N. S., Robinson S., Palmiter R. D., Joyce J. A., Moore C., Meshul C. K. J. Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamford N. S., Zhang H., Schmitz Y., Wu N. P., Cepeda C., Levine M. S., Schmauss C., Zakharenko S. S., Zablow L., Sulzer D. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 15.Nicola S. M., Malenka R. C. J. Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- 16.West A. R., Grace A. A. J. Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packard M. G., White N. M. Behav. Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 18.Bonci A., Hopf F. W. Neuron. 2005;47:335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Pickel V. M. J. Comp. Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- 20.Brambilla D., Chapman D., Greene R. Neuron. 2005;46:275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Gerdeman G. L., Ronesi J., Lovinger D. M. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 22.Kreitzer A. C., Malenka R. C. J. Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronesi J., Gerdeman G. L., Lovinger D. M. J. Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuffrida A., Parsons L. H., Kerr T. M., Rodriguez de Fonseca F., Navarro M., Piomelli D. Nat. Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 25.Piomelli D. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 26.Guo J., Ikeda S. R. Mol. Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- 27.Mackie K., Hille B. Proc. Natl. Acad. Sci. USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C. C., Lo S. W., Hsu K. S. J. Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Marzo V., Melck D., Bisogno T., De Petrocellis L. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 30.Herkenham M., Lynn A. B., de Costa B. R., Richfield E. K. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez J. J., Mackie K., Pickel V. M. J. Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partridge J. G., Apparsundaram S., Gerhardt G. A., Ronesi J., Lovinger D. M. J. Neurosci. 2002;22:2541–2549. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabresi P., Pisani A., Mercuri N. B., Bernardi G. J. Neurosci. 1994;14:4871–4881. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S., Lovinger D. M. J. Neurosci. 1997;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maejima T., Oka S., Hashimotodani Y., Ohno-Shosaku T., Aiba A., Wu D., Waku K., Sugiura T., Kano M. J. Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung K. M., Mangieri R., Stapleton C., Kim J., Fegley D., Wallace M., Mackie K., Piomelli D. Mol. Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 37.Dawson V. L., Dawson T. M., Filloux F. M., Wamsley J. K. Life Sci. 1988;42:1933–1939. doi: 10.1016/0024-3205(88)90492-4. [DOI] [PubMed] [Google Scholar]
- 38.Yan Z., Song W. J., Surmeier J. J. Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 39.Conn P. J., Battaglia G., Marino M. J., Nicoletti F. Nat. Rev. Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 40.Senogles S. E. Mol. Pharmacol. 2000;58:455–462. doi: 10.1124/mol.58.2.455. [DOI] [PubMed] [Google Scholar]
- 41.Gerdeman G. L., Partridge J. G., Lupica C. R., Lovinger D. M. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 42.Chevaleyre V., Castillo P. E. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 43.Calabresi P., Maj R., Pisani A., Mercuri N. B., Bernardi G. J. Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calabresi P., Mercuri N. B., Sancesario G., Bernardi G. Brain. (Part 2) 1993;116:433–452. [PubMed] [Google Scholar]
- 45.Picconi B., Centonze D., Rossi S., Bernardi G., Calabresi P. Brain. 2004;127:1661–1669. doi: 10.1093/brain/awh190. [DOI] [PubMed] [Google Scholar]
- 46.Heinz A., Siessmeier T., Wrase J., Buchholz H. G., Grunder G., Kumakura Y., Cumming P., Schreckenberger M., Smolka M. N., Rosch F., et al. Am. J. Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 47.Martinez D., Broft A., Foltin R. W., Slifstein M., Hwang D. R., Huang Y., Perez A., Frankle W. G., Cooper T., Kleber H. D., et al. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]