Abstract
Several regions of the brain (including medial prefrontal cortex, rostral anterior cingulate, posterior cingulate, and precuneus) are known to have high metabolic activity during rest, which is suppressed during cognitively demanding tasks. With functional magnetic resonance imaging (fMRI), this suppression of activity is observed as “deactivations,” which are thought to be indicative of an interruption of the mental activity that persists during rest. Thus, measuring deactivation provides a means by which rest-associated functional activity can be quantitatively examined. Applying this approach to autism, we found that the autism group failed to demonstrate this deactivation effect. Furthermore, there was a strong correlation between a clinical measure of social impairment and functional activity within the ventral medial prefrontal cortex. We speculate that the lack of deactivation in the autism group is indicative of abnormal internally directed processes at rest, which may be an important contribution to the social and emotional deficits of autism.
Keywords: default mode, functional MRI, introspection, medial prefrontal cortex, precuneus
Internally directed processes, such as self-reflective thought and most higher-order social and emotional processes, consistently activate a medial cortical network involving several brain regions, namely, the medial prefrontal cortex (MPFC) and adjacent rostral anterior cingulate cortex (rACC), posterior cingulate cortex (PCC), and precuneus (PrC) (1–4). Interestingly, this network is active when normal subjects are passively resting (5), leading many to speculate that these internally directed thoughts dominate the resting state (6–9). Self-reports from subjects while at rest further support this interpretation, wherein they typically describe “autobiographical reminiscences, either recent or ancient, consisting of familiar faces, scenes, dialogues, stories, and melodies” (8). Conversely, activity in this midline “resting network” is reduced when subjects perform externally directed, attention-demanding, goal-oriented tasks (such as the Stroop task or math calculations), and the resulting “deactivation” of this network is thought to be an indicator of an interruption of ongoing internally directed thought processes (5, 6, 9–11). In this context, the term deactivation simply refers to activity that is greater during rest than during task performance (i.e., the opposite of the more typically reported activations). Thus, an objective method for testing the functioning of this midline resting network is to measure whether there is deactivation in these regions during externally directed tasks as compared with passive rest. Similar approaches of examining this “deactivation effect” have been used in studies of patients with fragile X (12), a developmental disorder with some characteristics that overlap with autism, and in patients with dementia of the Alzheimer type (13) and Alzheimer's disease (14).
There are several lines of evidence that suggest that this resting network might be functioning abnormally in autism. First, the functions it subserves [including emotional processing (15–17), perception of social interactions (18), theory of mind (19–22), experience of joint attention (23), and person familiarity (24, 25)] overlap remarkably well with the social and emotional deficits that characterize autism. Second, in anterior regions of this network, researchers have documented volumetric, metabolic, cellular, and developmental growth abnormalities in this disorder (reviewed in ref. 26). Third, neuroimaging studies of autism have often observed functional abnormalities in these midline cortical regions during a variety of both socioemotional (25, 27, 28) and nonsocioemotional tasks (29–31). These functional abnormalities in nonsocioemotional tasks [e.g., a visually cued motor task (30)] are particularly interesting because they suggest that the results may be due to resting baseline differences between groups, rather than task-related differences.
To objectively determine whether in autism this resting network functions abnormally, we used functional magnetic resonance imaging (fMRI) to measure this deactivation effect in autistic and normal control subjects. To control for task performance across patient and control groups, we used the Stroop task because it is known that autistic patients are unimpaired in this task relative to controls (32, 33). To demonstrate that the magnitude of the deactivation effect in these regions can be modulated in control but not autistic subjects without changing the specific task demands, three conditions of the counting Stroop task were used: one with incongruent-number stimuli, one with emotional stimuli, and one with neutral stimuli. Activity in each condition was compared with activity during passive rest. To examine individual subject and group effects, we used a whole-brain group analysis followed-up with a region-of-activation approach in which the pattern of activity for each autistic and control individual could be characterized and quantified.
We had two main comparisons of interest: First, we predicted that the greatest level of functional deactivation from a passive resting state in control subjects would be seen in the most cognitively demanding task condition, because this would draw the most attention away from internal thought processes that occur during rest. Based on previous research on the counting Stroop task (17, 34), we chose the number condition to contrast with rest, because counting the number of words while reading incongruent numbers would create more interference (as reflected in longer reaction times and lower accuracy) than counting the number of emotional or neutral words. We hypothesized that in subjects with autism, although the number condition will still be the most cognitively demanding condition, this deactivation in the number vs. rest contrast would be absent, thus implicating differences in functional activity (and, therefore, functional processes) during rest. Second, based on previous studies using negatively valenced words (17), we predicted that control subjects would show relatively greater activity in anterior midline regions (MPFC and rACC) in the emotional compared with the neutral condition. However, we hypothesized that autistic subjects would fail to show this activity related to implicit emotional processing of negatively valenced words. Such a pattern of results would suggest that there is abnormal functioning of the midline resting network in autism during rest and emotion processing, which we speculate may reflect a more general failure to engage in the types of internally directed thoughts that normally recruit this network.
Results
Behavior.
As predicted, autism spectrum disorder (ASD) and control participants showed similar behavioral responses while performing the counting Stroop task (see Fig. 3 A and B, which is published as supporting information on the PNAS web site). Reaction time did not differ significantly between groups [ASD, 676.1 ms; control, 664.1 ms; F(1, 23) = 0.097; P = 0.759]; however, there was a main effect of condition [F(2, 22) = 4.811; P = 0.018]. Contrasts corrected for multiple comparisons revealed that this effect was due to significantly longer reaction times in the number condition (692.0 ms) as compared with those in the emotional (659.3 ms) and neutral (659.0 ms) conditions [F(1, 23) = 10.05, P = 0.004]. There was no significant interaction between condition and group for reaction time [F(2, 22) = 0.730, P = 0.493]. In the accuracy data, the ASD subjects did have a significantly lower overall percent correct score than did the control group [ASD, 95.8%; control, 98.8%; F(1, 22) = 6.499; P = 0.018]. However, mean accuracy for both groups was >95% for all three conditions, indicating that, despite group differences, both groups were performing the task with a high level of accuracy. There was also a main effect of condition with number showing a lower percent correct than the other two conditions [neutral, 97.7%; emotional, 97.4%; number, 96.7%; F(2, 21) = 7.08; P = 0.004] but no significant interaction between condition and group [F(2, 21) = 0.679, P = 0.518].
Immediately after image acquisition, subjects were given a word recognition task for which they were asked to mark all of the words they had seen during scanning. Repeated-measures ANOVA revealed a main effect of word type (emotional vs. neutral) [emotional, 86.0%; neutral, 73.8%; F(1, 19) = 11.43; P = 0.003] and a trend toward an interaction between group and word type [F(1, 19) = 4.24, P = 0.062] (Fig. 3C). Follow-up within group t tests revealed a significant effect of word type in control subjects [neutral, 72.2%; emotional, 91.1%; t(1, 8) = −4.02; P = 0.004] but not in ASD subjects [neutral, 75.4%; emotional, 80.8%; t(1, 11) = −1.15; P = 0.274].
Functional Imaging.
Group analysis.
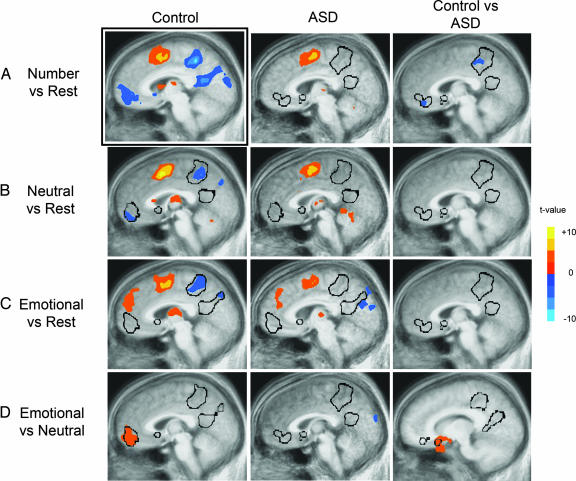
For control subjects, whole-brain analysis of the number vs. rest contrast revealed large regions of deactivation in MPFC∕rACC and PCC∕PrC (P < 0.01, cluster-corrected) (Fig. 1A; see also Table 1, which is published as supporting information on the PNAS web site). However, this deactivation was entirely absent in ASD subjects. Furthermore, a direct group comparison between control and ASD subjects revealed a significant difference between groups in MPFC∕rACC and PrC (Fig. 1A; see also Table 2, which is published as supporting information on the PNAS web site). The right superior temporal sulcus and bilateral angular gyrus also deactivated in control subjects but not in ASD subjects, although these regions were not significantly different in the direct group comparison (see Tables 1 and 2).
Fig. 1.
Significant functional activity derived from group whole-brain analyses (P < 0.01, cluster corrected). t values are displayed, with negative t values representing deactivations. The black outlines correspond to the area of deactivation derived from the number vs. rest condition in controls (A Left) mapped onto the sagittal slices of the images shown. These outlines, which represent regions active during rest, highlight the presence or absence of activations or deactivations in each image. Each sagittal slice location differs slightly because each image was chosen to best represent the midline activity for each group and comparison. The comparisons shown are number vs. rest (A), neutral vs. rest (B), emotional vs. rest (C), and emotional vs. neutral (D).
Similar but weaker effects were seen in the neutral vs. rest and emotional vs. rest comparisons. In the neutral vs. rest contrast (Fig. 1B), cluster volume in control subjects was reduced compared with that seen in the number vs. rest comparison, likely reflecting differences in difficulty between the conditions (10). Again, the autism group showed no significant deactivations in this comparison. Similarly, in the emotional vs. rest contrast (Fig. 1C), significant deactivations were seen in the PCC and PrC in controls but not in ASD subjects. Furthermore, a significant cluster of positive activity was observed in the dorsal MPFC in both groups, although this activity was of a greater extent in control subjects. In both of these comparisons, there were no significant differences in these midline regions in the direct group comparison.
To determine functional activity specific to emotional processing, we contrasted the emotional and neutral conditions (Fig. 1D). As predicted, significantly increased activity was observed in the ventral MPFC∕rACC, extending into the medial portion of the orbital frontal cortex, for control subjects, but this activation was absent in ASD subjects (Table 1). Direct group comparison revealed significantly greater activity in the control subjects in this medial orbital region of the resting network (Fig. 1D and Table 2).
Finally, to ensure that there were no between-group differences in functional activity relating to the interference aspect of the task [namely, in the dorsal anterior cingulate cortex (34)], we examined group differences between the number and neutral conditions. Even at a more relaxed threshold (P < 0.05, uncorrected), dorsal anterior cingulate cortex activity was still not significantly different between groups (see Fig. 4, which is published as supporting information on the PNAS web site), although differences were seen in MPFC and PrC.
Exploratory correlational analyses.
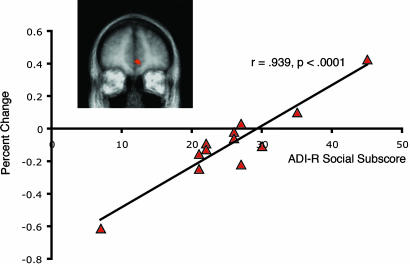
We found a cluster of voxels in the ventral MPFC (P < 0.005, uncorrected) whose activity during the number vs. rest contrast significantly correlated with the social subscale score on the Autism Diagnostic Interview–Revised in ASD individuals [r(11) = 0.939, P < 0.0001)] (Fig. 2). This region was partially overlapping with the region found to be significantly different between the ASD and control groups in the number vs. rest contrast (see Fig. 2 Inset). ASD subjects with greater deactivation had lower social impairment scores, whereas those that showed less deactivation (or, in certain individuals, activation) had greater social impairment scores. Furthermore, this correlation was still significant when the two extreme points (i.e., subjects with the lowest and highest scores on the social subscale) were removed from the analysis [r(9) = 0.669, P = 0.034].
Fig. 2.
Correlation between functional activity in a ventral MPFC cluster (Inset) and score on the social subscale of the Autism Diagnostic Interview–Revised in the ASD group.
Additional Analyses.
Because we noted individual variability in deactivation and activation patterns in the ASD subjects, we chose to examine the data at the individual subject level. These results are published in Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site.
Discussion
Our results demonstrate that ASD subjects fail to show the deactivation effect in resting network regions (MPFC∕rACC and PCC∕PrC) in the number vs. rest comparison (Fig. 1A). These results cannot be accounted for merely by differences in task performance because (i) no differences were seen in reaction time between groups; (ii) slightly reduced accuracy (and, thus, increased difficulty) in the ASD group should lead to greater deactivation (10), although we observed the exact opposite result; (iii) there were no significant correlations between task performance and functional activity in the anterior region-of-activation mask; and (iv) there were no neurofunctional differences in regions involved in Stroop task performance (namely, the dorsal ACC) (see Figs. 1A, 4, and 5B). ASD subjects also failed to show normal behavioral and neurofunctional processing of emotional stimuli. Although control subjects showed a recognition bias for emotional words, the autism group did not show an effect of word type on recognition ability, consistent with previous behavioral reports in autism (35). Furthermore, we demonstrated that autistic subjects failed to show the normal pattern of functional activation in the medial orbital frontal region of the resting network when processing emotional words compared with neutral words (Fig. 1D), demonstrating a failure of emotional task-induced modulation of this region. Finally, in the ASD group, there was a high correlation (r = 0.939) between a clinical measure of social impairment and functional activity in the ventral MPFC (Fig. 2). The subjects with higher social impairment scores had less deactivation in the number vs. rest contrast (i.e., the greater the behavioral abnormality, the greater the neurofunctional abnormality) and vice versa. We should emphasize, however, that we have only identified a correlative relationship between abnormal social behavior and brain activity, rather than a causative one. Thus, although tantalizing, this result cannot be used to argue that this neurofunctional abnormality causes social impairment or that social impairment causes this neurofunctional abnormality.
There are two possible reasons why the ASD group failed to show the typical deactivation effect. One possibility is that midline resting network activity during both rest and task performance is high, and, thus, a subtraction between these conditions would reveal no difference in activity levels. We believe, however, that it is unlikely that high midline network activity was maintained during the cognitively demanding number task in autism for several reasons. First, as mentioned previously, behavioral performance was similar between control and ASD groups. This result, however, would be unexpected if the ASD group were carrying out additional mental processing that control subjects inhibit during cognitively demanding conditions. Second, positron-emission tomography studies of autism, which provide an absolute measure of brain metabolism, have found reduced, as opposed to increased, glucose metabolism in rACC and PCC (36) during task performance, as compared with controls. Furthermore, one positron-emission tomography study found that lower blood flow in MPFC and rACC at rest was correlated with more severe social and communicative impairments in subjects with autism (37), a finding similar to our correlational results. Third, reduced anatomical volumes and neurochemical deficiencies have consistently been observed in MPFC∕rACC in adults with autism (reviewed in ref. 26), likely indicative of a reduced functioning of these regions. Therefore, an alternative explanation, the one to which we attribute the lack of deactivation, is that midline activity is low during rest. We suggest, then, that the absence of deactivation in this network indicates that the mental processes that normally occur at rest are absent or abnormal in autism.
What are these mental processes that dominate during rest? Evidence in the literature to date seems to suggest that tasks that induce certain types of internal processing activate this resting network. Examples of such tasks are self- and other-person judgments (4, 6, 7, 19–22, 38–45), person familiarity judgments (24, 25), emotion processing (15–17, 46), perspective-taking (22, 47), passive observation of social interactions vs. nonsocial interactions (18), relaxation based on interoceptive biofeedback (48, 49), conceptual judgments (based on internal knowledge stores) vs. perceptual judgments (50), and episodic memory tasks (51), among others [moral decision making (52), joint attention experience (23), and pleasantness judgments (53)]. Therefore, the activity in these regions at rest might simply reflect the extent to which these types of internally directed thoughts are engaged at rest. In fact, a particularly intriguing behavioral study found that individuals with ASD report very different internal thoughts than control subjects (54, 55), lending support to our interpretation that an absence of this resting activity in autism may be directly related to abnormal internal thought. Admittedly, this is a speculative hypothesis but one that can be explicitly tested.
The findings in the present study are supported by previous functional imaging studies of autism, which have consistently found abnormalities in these midline regions during a variety of tasks. Although these functional abnormalities would be expected in socioemotional tasks [i.e., personally familiar face processing (25), facial affect processing (28), theory of mind (27)] because these resting network regions support these processes, these abnormalities have also been seen using nonsocioemotional tasks [i.e., spatial working memory (29), visually cued motor task (30), embedded figures task (31)]. Furthermore, within both socioemotional and nonsocioemotional task domains, the direction of abnormality has not been consistent in that some studies report greater activity, whereas others report reduced activity compared with control subjects. Unfortunately, interpretation of these findings is not straightforward, because different studies report results in different ways (e.g., individual group results or direct group comparisons) and use different control conditions. For instance, in our study, if we only reported results from the direct comparison for control vs. ASD subjects in the number vs. rest condition, it would seem like ASD subjects had greater activity in resting network regions (Fig. 1A Right), although such an oversimplified interpretation would be flawed because it actually reflects an absence of deactivation in the autism group. Therefore, although functional abnormality within midline anterior and posterior regions has been repeatedly shown in ASD, the precise nature of and explanation for these abnormalities have been unclear based on past studies. We offer an encompassing explanation for these disparate imaging results by suggesting that the seemingly inconsistent findings can be attributed to a lack of presenting individual group data and to a lack of a common reference to a resting baseline condition.
Although our discussion of resting network regions has been limited to midline resting regions, other regions, including superior temporal sulcus, temporal pole, and angular gyrus, are known to show the same deactivation effect (5, 50). In fact, we observed deactivation in these regions in control subjects in the number vs. rest contrast, and, as expected, these regions failed to deactivate in the ASD group (Table 1). However, functional activity in these regions was not found to be significantly different in our relatively conservative direct group comparison (P < 0.01, whole-brain corrected), likely due to the smaller size and increased anatomic variability of these regions (particularly, the superior temporal sulcus) as compared with midline regions. Interestingly, several functional imaging studies have shown abnormalities in these regions (27, 56–58). Follow-up studies using region of interest approaches or sulcal mapping are needed to determine the functionality of these other resting network regions in autism.
It is particularly remarkable that the autism behavioral phenotype can be caused by a number of different factors (including genetic, infectious, and environmental toxins), suggesting that these factors are likely acting on a common neural substrate that must be particularly vulnerable to developmental insult. In fact, Raichle and colleagues (5) speculated that the PrC and PCC might be highly susceptible to damage because of their high metabolic rate. Furthermore, because these highly active midline regions are richly connected with numerous cortical and subcortical regions (59–62), an early insult affecting any one of these areas may have devastating, widespread consequences on brain connectivity and subsequent functionality. Interestingly, a recent diffusion tensor imaging study on autism identified white matter abnormality in the region underlying the rACC∕MPFC (63). Furthermore, several fMRI studies have suggested reduced functional connectivity in the autistic brain in a variety of tasks (27, 64–66). Functional connectivity studies, which allow for observation of brain activity during rest (67, 68) or naturalistic viewing conditions (69), may be particularly useful in further characterizing resting state abnormalities in autism.
Although purely speculative, it is interesting to hypothesize about the nature of resting thoughts in ASD, if the typical internally directed thoughts are absent or abnormal. One possibility is that their thoughts are directed toward their obsessive interests and preoccupations (55), which are often of a concrete, as opposed to abstract and subjective, nature (for instance, calendars, maps, or schedules), or perhaps hypersensitivity to their external environment constantly interrupts the full emergence and elaboration of internally directed thoughts.
In summary, this study demonstrates that neural activity at rest in ASD is abnormal, which suggests that individuals with ASD might fail to engage in typical internally directed resting thoughts. Furthermore, during an emotion processing task, both behavioral performance and functional activity in a specific region of this resting network (medial orbital frontal cortex) was abnormal in ASD, suggesting that behavioral impairment might persist in tasks that recruit these resting network regions. Finally, the amount of functional abnormality in MPFC correlated with the amount of social impairment in individuals with ASD. Future experiments exploring resting network activity and organization in ASD and those examining specific aspects of resting functional processes, such as self-reflection, may provide valuable insights into the neurocognitive basis of the disorder.
Materials and Methods
Participants.
Fifteen participants with ASD (10 with high-functioning autism, 3 with Asperger's syndrome, and 2 with pervasive developmental disorder–not otherwise specified; 2 were left-handed and 1 was ambidextrous) and 14 healthy controls (three were left-handed) participated in this experiment. Three participants with ASD (two with autism and one with pervasive developmental disorder–not otherwise specified) were excluded from all analyses because of uncorrectable head motion. All participants or their legal guardians gave informed written consent and were monetarily compensated for participation in the experiment. The protocol was approved by the Institutional Review Board of the University of California at San Diego, and Children's Hospital at San Diego. All ASD participants were diagnosed by a clinical psychologist with the Autism Diagnostic Interview–Revised (70) and the Autism Diagnostic Observation Schedule (71) and administered the Wechsler Adult Intelligence Scale or Wechsler Adult Intelligence Scale–Revised. The mean ages of ASD participants (25.49 ± 9.61) and control participants (26.07 ± 7.95) were not significantly different [t(24) = −0.169, P = 0.87]. Clinical data for individual ASD subjects are reported in Table 3, which is published as supporting information on the PNAS web site.
Stimuli.
While in the scanner, subjects performed a counting Stroop task with three conditions of interest (34). This task was based on the classic color–word Stroop (72) but adapted to be more suitable for the fMRI environment (34). Instead of naming the color of a word, subjects were instructed to count the number of words that appeared on the screen and respond as quickly and accurately as possible by pressing the button on the response device corresponding to either two, three, or four words. Subjects were presented with three different types of words: emotional, neutral, and number words. Number words were always incongruent with the number of words that appeared on the screen (e.g., “two” written three times). The emotional words were chosen from a set of negatively valenced emotional words that had been rated by a separate set of participants for degree of emotional arousal. Ten words with the highest ratings of emotional arousal were included in the study. Examples of these words include “murder,” “torture,” and “blood.” The 10 neutral words, each naming a household item, included words like “table,” “curtain,” and “desk.” Stimuli were presented for 1.5 s each in a block design, with each block containing 20 presentations of either emotional, neutral, or number words, for a total of 30 s per block. There were 12 task blocks, interlaced with three 21-s rest periods, wherein subjects were simply instructed to passively view a fixation cross.
Behavioral Data Acquisition and Analysis.
Stimuli were presented using the presentation software package (Neurobehavioral Systems, Albany, CA), whereby reaction time and number of errors were recorded during scanning. Additionally, immediately after scanning, each participant was asked to complete a surprise word recognition test outside of the scanner. The test consisted of two columns; one with 20 neutral words describing household items and one with 20 negatively valenced emotional words. Subjects were instructed to identify the words they recognized from the scanning session.
Subject responses were scored for average reaction time (from stimulus onset until subject response) and percent correct for each condition. Data from the word recognition test were scored for hits, misses, and false alarms and then converted into a percent correct score. All behavioral analyses were conducted with spss 12.0 statistical software package (SPSS, Chicago, IL). Two repeated-measures ANOVAs were run with group (autism, control) as the between-subjects factor and reaction time or percent correct for each of the three conditions (emotional, neutral, and number) as the within-subjects factor. A third repeated-measures ANOVA was run on the posttest word recognition data with group as the between-subjects variable and percent correct for each condition (emotional words or neutral words) as the within-subjects factor.
Functional Imaging Data Acquisition and Analysis.
Images were acquired on a Symphony 1.5 Tesla Scanner (Siemens, Iselin, NJ) at the University of California at San Diego Hillcrest Medical Center. Whole-brain axial slices were collected with a gradient-recalled echo-planar imaging pulse sequence [repetition time, 3,000 ms; echo time, 35 ms; flip angle, 90°; field of view, 256 mm; matrix, 64 × 64 (4-mm2, in-plane resolution); slice thickness, 4 mm; number of slices, 30; number of volumes, 148]. A T1-weighted anatomical image using an mprage sequence was collected during each scan session for coregistration with the functional images. Anatomical scans were collected in the sagittal plane [field of view, 256 mm; matrix, 256 × 256 (1-mm2, in-plane resolution); slice thickness, 1 mm; number of slices, 180].
All fMRI analyses were conducted with the afni 2.56 (Analysis of Functional Neuroimages) statistical software package (73). Motion correction and three-dimensional registration of each participant's functional images were performed with an automated alignment program (3dvolreg), which coregistered each volume in the time series to the middle volume acquired in that run using an iterative process. Brief periods of motion in nine ASD subjects that were uncorrectable by 3dvolreg were removed from analysis. The percent of the run that was removed ranged from 2.7% to 14.9% (mean, 7.13%). Images were then smoothed with a Gaussian filter (full-width half-maximum, 6 mm).
Individual data were analyzed using the program 3ddeconvolve. This program first estimates an impulse response function based on the measured fMRI signal data and input stimulus functions. These nine functions included the three word conditions (number, neutral, and emotional) and six motion parameters derived from the output of 3dvolreg (intrarun motion in the x, y, and z and roll, pitch, and yaw planes). The impulse response function was then convolved with the input stimulus time series and multiple regressions were run to determine a goodness-of-fit coefficient (or linear contrast weight). The global mean and linear trend were included in the regression to remove their effects from the analysis. For each condition, the linear contrast weight was determined for 0, 3, 6, and 9 s after stimulus presentation. These weights were summed for each condition to give an overall best fit for each condition compared with the rest condition. Additionally, an a priori contrast of emotional vs. neutral was determined for each individual.
Group Analysis.
To average data across participants, the functional images were then transformed into Talaraich space. t tests were conducted to determine whether linear contrast weights for the autism and control groups were significantly different from zero for each of the four main comparisons of interest (emotional vs. rest, number vs. rest, neutral vs. rest, and emotional vs. neutral). An additional set of t tests was then run to determine whether the contrast weights were significantly different between the autism and control groups for the four comparisons. All data were intensity-thresholded at P < 0.01 and cluster-thresholded at a voxelwise α-level of P < 0.05. The contrasts of any condition vs. rest reveal regions of activation and deactivation and are displayed together in each image shown.
Exploratory Correlation Analysis.
Because we predicted that functional activity at rest is related to social behavioral impairment in ASD, we ran a whole-brain regression analysis between the number vs. rest contrast and the social subscale score on the Autism Diagnostic Interview–Revised. Because results did not survive correction for multiple comparisons based on cluster volume, data were thresholded at P < 0.005, uncorrected, and we termed this analysis “exploratory.”
Additional Analyses.
Analyses of individual differences in deactivation and activation patterns in ASD and control subjects were carried out, and these methods are published as Supporting Text.
Acknowledgments
We thank the subjects who graciously gave of their time to participate in this study. This research was supported by National Institutes of Health Grant R01 MH36840 (to E.C.).
Abbreviations
- ASD
autism spectrum disorder
- fMRI
functional magnetic resonance imaging
- MPFC
medial prefrontal cortex
- rACC
rostral anterior cingulate cortex
- PCC
posterior cingulate cortex
- PrC
precuneus.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The fMRI data reported in this paper have been deposited with the fMRI Data Center, www.fmridc.org (accession no. Z-2006-121FQ).
References
- 1.Gusnard D. A., Raichle M. E. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 2.Maddock R. J. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 3.Northoff G., Bermpohl F. Trends Cognit. Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ochsner K. N., Beer J. S., Robertson E. R., Cooper J. C., Gabrieli J. D., Kihsltrom J. F., D'Esposito M. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 5.Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. Proc. Natl. Acad. Sci. USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjaer T. W., Nowak M., Lou H. C. NeuroImage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- 8.Mazoyer B., Zago L., Mellet E., Bricogne S., Etard O., Houde O., Crivello F., Joliot M., Petit L., Tzourio-Mazoyer N. Brain Res. Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 9.Cavanna A. E., Trimble M. R. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 10.McKiernan K. A., Kaufman J. N., Kucera-Thompson J., Binder J. R. J. Cognit. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 11.Shulman G. L., Fiez J. A., Corbetta M., Buckner R. L., Miezin F. M., Raichle M. E., Peterson S. E. J. Cognit. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 12.Menon V., Leroux J., White C. D., Reiss A. L. Proc. Natl. Acad. Sci. USA. 2004;101:3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lustig C., Snyder A. Z., Bhakta M., O'Brien K. C., McAvoy M., Raichle M. E., Morris J. C., Buckner R. L. Proc. Natl. Acad. Sci. USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greicius M. D., Srivastava G., Reiss A. L., Menon V. Proc. Natl. Acad. Sci. USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cato M. A., Crosson B., Gokcay D., Soltysik D., Wierenga C., Gopinath K., Himes N., Belanger H., Bauer R. M., Fischler I. S., et al. J. Cognit. Neurosci. 2004;16:167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- 16.Maddock R. J., Garrett A. S., Buonocore M. H. Hum. Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whalen P. J., Bush G., McNally R. J., Wilhelm S., McInerney S. C., Jenike M. A., Rauch S. L. Biol. Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 18.Iacoboni M., Lieberman M. D., Knowlton B. J., Molnar-Szakacs I., Moritz M., Throop C. J., Fiske A. P. NeuroImage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher P. C., Happe F., Frith U., Baker S. C., Dolan R. J., Frackowiak R. S., Frith C. D. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher H. L., Jack A. I., Roepstorff A., Frith C. D. NeuroImage. 2002;16:814–821. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher H. L., Frith C. D. Trends Cognit. Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 22.Vogeley K., Bussfeld P., Newen A., Herrmann S., Happe F., Falkai P., Maier W., Shah N. J., Fink G. R., Zilles K. NeuroImage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- 23.Williams J. H., Waiter G. D., Perra O., Perrett D. I., Whiten A. NeuroImage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Maddock R. J., Garrett A. S., Buonocore M. H. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 25.Pierce K., Haist F., Sedaghat F., Courchesne E. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 26.Courchesne E., Redcay E., Kennedy D. P. Curr. Opin. Neurol. 2004;17:489–496. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- 27.Castelli F., Frith C., Happe F., Frith U. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 28.Wang A. T., Dapretto M., Hariri A. R., Sigman M., Bookheimer S. Y. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Luna B., Minshew N. J., Garver K. E., Lazar N. A., Thulborn K. R., Eddy W. F., Sweeney J. A. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 30.Muller R.A., Pierce K., Ambrose J. B., Allen G., Courchesne E. Biol. Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- 31.Ring H. A., Baron-Cohen S., Wheelwright S., Williams S. C., Brammer M., Andrew C., Bullmore E. T. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 32.Eskes G. A., Bryson S. E., McCormick T. A. J. Autism Dev. Disord. 1990;20:61–73. doi: 10.1007/BF02206857. [DOI] [PubMed] [Google Scholar]
- 33.Ozonoff S., Jensen J. J. Autism Dev. Disord. 1999;29:171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- 34.Bush G., Whalen P. J., Rosen B. R., Jenike M. A., McInerney S. C., Rauch S. L. Hum. Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beversdorf D. Q., Anderson J. M., Manning S. E., Anderson S. L., Nordgren R. E., Felopulos G. J., Nadeau S. E., Heilman K. M., Bauman M. L. J. Neurol. Neurosurg. Psychiatry. 1998;65:685–692. doi: 10.1136/jnnp.65.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haznedar M. M., Buchsbaum M. S., Wei T. C., Hof P. R., Cartwright C., Bienstock C. A., Hollander E. Am. J. Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 37.Ohnishi T., Matsuda H., Hashimoto T., Kunihiro T., Nishikawa M., Uema T., Sasaki M. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- 38.D'Argembeau A., Collette F., Van der Linden M., Laureys S., Del Fiore G., Degueldre C., Luxen A., Salmon E. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 39.Fossati P., Hevenor S. J., Graham S. J., Grady C., Keightley M. L., Craik F., Mayberg H. Am. J. Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 40.Johnson S. C., Baxter L. C., Wilder L. S., Pipe J. G., Heiserman J. E., Prigatano G. P. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 41.Kelley W. M., Macrae C. N., Wyland C. L., Caglar S., Inati S., Heatherton T. F. J. Cognit. Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 42.Lou H. C., Luber B., Crupain M., Keenan J. P., Nowak M., Kjaer T. W., Sackeim H. A., Lisanby S. H. Proc. Natl. Acad. Sci. USA. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macrae C. N., Moran J. M., Heatherton T. F., Banfield J. F., Kelley W. M. Cereb. Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell J. P., Banaji M. R., Macrae C. N. NeuroImage. 2005;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J. P., Macrae C. N., Banaji M. R. NeuroImage. 2005;26:251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Adolphs R. Trends Cognit. Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 47.Vogeley K., May M., Ritzl A., Falkai P., Zilles K., Fink G. R. J. Cognit. Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 48.Critchley H. D., Melmed R. N., Featherstone E., Mathias C. J., Dolan R. J. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- 49.Nagai Y., Critchley H. D., Featherstone E., Trimble M. R., Dolan R. J. NeuroImage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Binder J. R., Frost J. A., Hammeke T. A., Bellgowan P. S., Rao S. M., Cox R. W. J. Cognit. Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 51.Andreasen N. C., O'Leary D. S., Cizadlo T., Arndt S., Rezai K., Watkins G. L., Ponto L. L., Hichwa R. D. Am. J. Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 52.Greene J. D., Sommerville R. B., Nystrom L. E., Darley J. M., Cohen J. D. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- 53.Paulus M. P., Frank L. R. NeuroReport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- 54.Hurlburt R. T., Happe F., Frith U. Psychol. Med. 1994;24:385–395. doi: 10.1017/s0033291700027367. [DOI] [PubMed] [Google Scholar]
- 55.Frith U., Happe F. Mind Lang. 1999;14:1–22. [Google Scholar]
- 56.Baron-Cohen S., Ring H. A., Wheelwright S., Bullmore E. T., Brammer M. J., Simmons A., Williams S. C. Eur. J. Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 57.Gervais H., Belin P., Boddaert N., Leboyer M., Coez A., Sfaello I., Barthelemy C., Brunelle F., Samson Y., Zilbovicius M. Nat. Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- 58.Pelphrey K. A., Morris J. P., McCarthy G. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 59.Carmichael S. T., Price J. L. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi Y., Amaral D. G. J. Comp. Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 61.Ongur D., Price J. L. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 62.Parvizi J., Van Hoesen G. W., Buckwalter J., Damasio A. Proc. Natl. Acad. Sci. USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A. L. Biol. Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Horwitz B., Rumsey J. M., Grady C. L., Rapoport S. I. Arch. Neurol. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- 65.Just M. A., Cherkassky V. L., Keller T. A., Minshew N. J. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 66.Koshino H., Carpenter P. A., Minshew N. J., Cherkassky V. L., Keller T. A., Just M. A. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greicius M. D., Krasnow B., Reiss A. L., Menon V. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartels A., Zeki S. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 70.Lord C., Rutter M., Le Couteur A. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 71.Lord C., Risi S., Lambrecht L., Cook E., Jr, Leventhal B., DiLavore P., Pickles A., Rutter M. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 72.Stroop J. R. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 73.Cox R. W. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]